Abstract
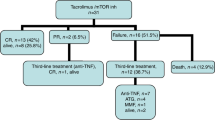
The α4ß7 integrin is upregulated on naive and memory T cell subsets in patients who subsequently develop gastrointestinal (GI) acute GVHD. Natalizumab (Tysabri®, Biogen Inc.) acts against the α4 subunit that mediates homing of lymphocytes to the GI tract. We initiated a phase II study of natalizumab with corticosteroids for initial treatment of acute GI GVHD. In total, 300 mg IV of natalizumab was given, with steroids initiated up to 3 days prior. Twenty-one subjects were treated, median age was 63 years (range 38–74), and 15 (71%) were male. Eighteen (86%) underwent RIC, 15 (71%) received MUD, and all received PBSCs. Overall GVHD at enrollment was grade II in 4 and grade III in 17. The primary endpoint, day 56 GVHD-free survival rate, was attained in 33.3%. The overall response rate at day 28 and 56 was 57% and 52%, respectively. Six of eight CRs were durable for 1 year. Five experienced toxicity possibly related to natalizumab and ten had infections before day 100. 2-year OS was 43% (95% CI 22–62%) and 2-year NRM was 52% (95% CI 29–71%). Natalizumab with corticosteroids as initial treatment of acute GI GVHD is safe, effective, and durable.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
05 October 2020
The original version of the Article stated in the Methods section that Natalizumab was provided by Genentech. In fact, it was provided by Biogen. This has now been corrected in both the PDF and HTML versions of the Article.
12 October 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41409-020-01083-y
References
Epstein FH, Ferrara JLM, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–74.
Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61.
Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119:3908–16.
Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N. Engl J Med. 2012;367:1487–96.
Hill GR, Ferrara JLM. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–9.
Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–77.
Arai S, Vogelsang GB. Management of graft-versus-host disease. Blood Rev. 2000;14:190–204.
Bruner RJ, Farag SS. Monoclonal antibodies for the prevention and treatment of graft-versus-host disease. Semin Oncol. 2003;30:509–19.
Tanaka J, Asaka M, Imamura M. T-cell co-signalling molecules in graft-versus-host disease. Ann Hematol 2000;79:283–90.
Couriel DR, Saliba R, de Lima M, Giralt S, Andersson B, Khouri I, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555–62.
Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–63.
Chen YBin, Kim HT, McDonough S, Odze RD, Yao X, Lazo-Kallanian S, et al. Up-regulation of α4β7 integrin on peripheral T cell subsets correlates with the development of acute intestinal graft-versus-host disease following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1066–76.
Chen YB, McDonough S, Chen H, Kennedy J, Illiano C, Attar EC, et al. Expression of α4β7 integrin on memory CD8 + T cells at the presentation of acute intestinal GVHD. Bone Marrow Transplant. 2013;48:598–603.
Rudick RA, Sandrock A. Natalizumab: α4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother. 2004;4:571–80.
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–23.
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
Gordon FH, Lai CWY, Hamilton MI, Allison MC, Srivastava ED, Fouweather MG, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to α4 integrin in active Crohn’s disease. Gastroenterology. 2001;121:268–74.
Gordon FH, Hamilton MI, Donoghue S, Greenlees C, Palmer T, Rowley-Jones D, et al. A pilot study of treatment of active ulcerative colitis with natalizumab, a humanized monoclonal antibody to alpha-4 integrin. Aliment Pharmacol Ther. 2002;16:699–705.
Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, et al. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348:24–32.
Sheremata WA, Vollmer TL, Stone LA, Willmer-Hulme AJ, Koller M. A safety and pharmacokinetic study of intravenous natalizumab in patients with MS. Neurology. 1999;52:1072–4.
Danylesko I, Bukauskas A, Paulson M, Peceliunas V, Gedde-Dahl DYT, Shimoni A, et al. Anti-α4β7 integrin monoclonal antibody (vedolizumab) for the treatment of steroid-resistant severe intestinal acute graft-versus-host disease. Bone Marrow Transplant. 2019;54:987–93.
Fløisand Y, Lazarevic VL, Maertens J, Mattsson J, Shah NN, Zachée P, et al. Safety and effectiveness of vedolizumab in patients with steroid-refractory gastrointestinal acute graft-versus-host disease: a retrospective record review. Biol Blood Marrow Transplant. 2019;25:720–7.
Chandar AK, Singh S, Murad MH, Peyrin-Biroulet L, Loftus EV. Efficacy and safety of natalizumab and vedolizumab for the management of Crohnʼs disease. Inflamm Bowel Dis. 2015;21:1695–708.
Przepiorka D, Weisdorf D, Martin P, Klingemann H, Beatty P, Hows J, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1994;1995:825–8.
MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NKC, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–94.
Martin PJ, Schoch G, Fisher L, Byers V, Appelbaum FR, McDonald GB, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77:1821–8.
Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med. 2008;27:3145–54.
Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 1974;69:81–86.
MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21:761–7.
Castilla-Llorente C, Martin PJ, McDonald GB, Storer BE, Appelbaum FR, Deeg HJ, et al. Prognostic factors and outcomes of severe gastrointestinal GVHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2014;49:966–71.
Chen Y-B, Shah NN, Renteria AS, Cutler C, Jansson J, Akbari M, et al. Vedolizumab for prevention of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019;3:4136–46.
Bolaños-Meade J, Logan BR, Alousi AM, Antin JH, Barowski K, Carter SL, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124:3221–7.
Incyte announces results of phase 3 study of itacitinib in patients with treatment-naive acute graft-versus-host disease. Incyte Corporation. https://investor.incyte.com/news-releases/news-release-details/incyte-announces-results-phase-3-study-itacitinib-patients. Accessed 4 September 2020.
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kekre, N., Kim, H.T., Hofer, J. et al. Phase II trial of natalizumab with corticosteroids as initial treatment of gastrointestinal acute graft-versus-host disease. Bone Marrow Transplant 56, 1006–1012 (2021). https://doi.org/10.1038/s41409-020-01049-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01049-0
This article is cited by
-
A prospective phase 2 clinical trial of a C5a complement inhibitor for acute GVHD with lower GI tract involvement
Bone Marrow Transplantation (2023)
-
The soluble VCAM-1 level is a potential biomarker predicting severe acute graft versus host disease after allogeneic hematopoietic cell transplantation
BMC Cancer (2022)