Abstract
Outcomes of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for patients with adult T cell leukemia/lymphoma (ATL) are not satisfactory, particularly in patients in non-complete remission at transplantation (Pt-non-CR). We conducted a regional retrospective study in the ATL endemic area of Okinawa, Japan. Of 62 ATL patients, 21 received allo-HSCT in CR and 41 in non-CR. The 3-year overall survival (3yOS) rate and median survival time for the whole cohort was 25.6% and 7.7 months, respectively. The 3yOS of Pt-non-CR was significantly lower than that of patients in CR (Pt-CR) (16.8% vs. 43.6%, P = 0.005). Transplant-related mortality (TRM) was significantly higher in Pt-non-CR than in Pt-CR (46.3% vs. 15.7%, P = 0.025), while there was no significant difference in disease-associated mortality (DAM) between Pt-non-CR and Pt-CR. Multivariable analysis for Pt-non-CR revealed that poor performance status (poor-PS) and higher sIL-2R level (high sIL-2R) adversely affected OS. Poor-PS was associated with higher TRM, but not with higher DAM in Pt-non-CR. High sIL-2R did not affect TRM or DAM in Pt-non-CR. Overall, high TRM rates rather than DAM contribute to the poor outcomes of Pt-non-CR, suggesting that not only disease control but also management of transplant-related complications is required for allo-HSCT in ATL patients.
Similar content being viewed by others
Introduction
Adult T-cell leukemia/lymphoma (ATL) is a malignancy of peripheral T lymphocytes caused by human T-cell leukemia virus type 1 (HTLV-1), which was the first retrovirus to be isolated from a human malignant disease [1,2,3]. HTLV-1 shows a puzzling geographical distribution around the world, and southwestern Japan (Kyushu and Okinawa) is one of several areas with a high prevalence of infection [4]. The incidence of ATL is closely linked to the prevalence of HTLV-1 infection, and thus Okinawa is an endemic area of ATL [5].
ATL is divided into four clinical subtypes: acute, lymphoma, chronic, and smoldering [6]. These clinical subtypes are closely related to prognosis, which is extremely poor for the aggressive subtypes [7]. Although the best clinical results are achieved by systemic chemotherapy, the median survival time is only 12.7 months and complete response is achieved in only 40% of treated cases [8]. Most of these patients eventually relapse and have a median progression-free survival time of 5–7 months. Moreover, the treatment options are extremely limited for those who do not respond to the initial chemotherapy. New immunotherapy or immunomodulatory agents, such as mogamulizumab (anti-CCR4 monoclonal antibody) [9, 10] and lenalidomide (an oral immunomodulatory drug) [11] have recently been used as treatments for ATL in Japan and are effective in some patients. However, the long-term clinical outcomes remain unclear.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has been available for patients with aggressive ATL since 1987 [12] and it is now considered a promising treatment option for these patients [13,14,15,16]. A nationwide retrospective study of allo-HSCT for the treatment of ATL [15] demonstrated several pretransplantation factors that are associated with poor survival rates, such as poor Eastern Cooperative Oncology Group Performance Status (ECOG-PS) rating, higher age, male sex, non-complete remission (non-CR) at transplantation, and the use of unrelated cord blood as the stem cell source. In that study, non-CR at transplantation was also identified as a risk factor for disease-associated mortality (DAM). Although disease status at transplantation is known to be an important factor associated with outcome after allo-HSCT for ATL, it is often difficult to achieve CR in ATL patients. As a consequence, some ATL patients are compelled to receive allo-HSCT despite their non-CR status. Indeed, in daily practice, we often encounter patients whose ATL tumor cells become chemoresistant during their planned chemotherapy. We therefore think that it is essential to improve the allo-HSCT treatment strategy in ATL patients, especially those in non-CR.
Here, we conducted a regional retrospective study in the endemic ATL area of Okinawa Prefecture to clarify the factors affecting transplant outcomes of ATL patients, focusing on patients in non-CR at transplantation (Pt-non-CR).
Methods
Study population
We retrospectively collected data from 62 patients with aggressive ATL who had received allogeneic transplantation at University of the Ryukyus Hospital and Heartlife Hospital in Okinawa Prefecture between September 2000 and January 2016. Since all allo-HSCT procedures are performed in these two centers in Okinawa, the patients analyzed in the current study included all patients with aggressive ATL who underwent allo-HSCT in this area. Informed consent was obtained in accordance with the Declaration of Helsinki. This study was conducted with the approval of the institutional review board of the University of the Ryukyus.
Endpoints and statistical analysis
The primary endpoint of this study was overall survival, defined as the time from the date of transplantation until the date of death from any cause. The secondary endpoints were cumulative incidences of DAM and transplant-related mortality (TRM). Reported causes of death were reviewed and categorized into disease-associated or transplant-associated deaths. Disease-associated deaths were defined as deaths from relapse or progression of ATL. Transplant-related deaths were defined as any death without relapse or progression of ATL.
Descriptive statistics were used to summarize variables related to patient demographic and transplant characteristics. Comparisons between Pt-non-CR and patients in CR at transplantation (Pt-CR) were performed with the Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables.
The probability of overall survival was estimated according to the Kaplan–Meier method, and univariable comparisons among the groups were made using the log-rank test. Data on patients who were alive at the time of last follow-up were censored. Fine and Gray’s proportional-hazards model for subdistribution of a competing risk was used to analyze the cumulative incidences of TRM and DAM. For DAM, transplant-related deaths were competing events; for TRM, disease-associated deaths were competing events. Gray’s test was used for group comparisons of cumulative incidence [17].
Cox’s proportional-hazards regression model [18] was used to evaluate variables potentially affecting overall survival. Variables considered were recipient age group (<50 years and ≥50 years); recipient sex (female and male); lines of chemotherapy prior to transplantation (1 and ≥2); donor source (related and unrelated); Human Leukocyte Antigen (HLA) matching (matched and mismatched); disease status before transplantation (CR and non-CR); type of conditioning regimen (reduced-intensity conditioning [RIC] and myeloablative conditioning [MAC]); ECOG-PS before transplantation (ECOG-PS, 0–1, and 2–4); and soluble interleukin-2 receptor (sIL-2R) level (sIL-2R < 2000 U/mL and ≥2000 U/mL). Conditioning regimens were classified as myeloablative when total-body irradiation was >8 Gy, oral busulfan was ≥9 mg/kg, intravenous busulfan was ≥7.2 mg/kg, or melphalan was >140 mg/m2, in accordance with the report by Giralt et al. [19]. HLA matching between patient and donor was defined according to the results of serological or molecular typing for HLA-A, B, and DR antigens. Results were expressed as hazard ratios with 95% confidence interval (CI). All tests were two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [20] or STATA version 13 (StataCorp LLC, College Station, TX).
Results
Patients characteristics
Table 1 shows the characteristics of the patients. Of the 62 patients, 21 (34%) received allo-HSCT while in CR and 41 (66%) while in non-CR. The patients who received transplants in non-CR had higher ECOG-PS values, higher sIL-2R levels, and shorter follow-up periods. Among the Pt-non-CR, 13 of 41 were ECOG-PS 2–4 (PS 2, n = 9; PS 3, n = 4), while there was only one patient with ECOG-PS 2–4 among the 21 Pt-CR (PS 3, n = 1). At transplantation, none of the 21 Pt-CR had sIL-2R levels of ≥2000 U/mL, while 19 of the 41 Pt-non-CR had sIL-2R levels of ≥2000 U/mL. Pt-CR had received a median 1 line (range 1–3) of chemotherapy, while Pt-non-CR had received a median 2 lines (range 1–5) of chemotherapy prior to allo-HSCT. Chemotherapy regimens prior to transplant and detailed transplant procedures are shown in Tables S1 and S2, respectively. Conditioning regimen, graft-versus-host disease (GVHD) prophylaxis, and infection prophylaxis were similar in both transplant centers. Cyclophosphamide + total-body irradiation was used as the myeloablative conditioning regimen, while fludarabine + melphalan-based or fludarabine + busulfan-based regimen was used as the RIC regimen. For GVHD prophylaxis, cyclosporine A + short-term methotrexate was used in patients transplanted from an HLA-matched related donor, while tacrolimus + short-term methotrexate was used in patients transplanted from an unrelated or HLA-mismatched related donor. Antimicrobial prophylaxis with levofloxacin, antifungal prophylaxis with fluconazole or voriconazole, varicella-zoster virus prophylaxis with acyclovir, and Pneumocystis jirovecii pneumonia prophylaxis with trimethoprim–sulfamethoxazole were standard prophylaxis for infection.
The ratio of Pt-non-CR to Pt-CR was higher in the University of the Ryukyus Hospital (Pt-non-CR, n = 34; Pt-CR, n = 9) than in Heartlife Hospital (Pt-non-CR, n = 7; Pt-CR, n = 12) (P = 0.002). The majority of Pt-non-CR underwent transplantation at the University of the Ryukyus Hospital before 2008 (Table S3).
Overall survival and engraftment
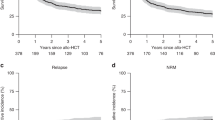
Of the 62 patients included in the study, 16 were alive after a median follow-up of 212.5 days (range, 6–4290 days). The unadjusted 3-year probability of overall survival was 25.6% (95% CI, 14.8–37.8%) and the median survival time was 7.68 months for the whole cohort (Fig. 1a). Pt-CR had a higher 3-year probability of survival than Pt-non-CR (43.6% [95% CI, 20.3–64.9%] vs. 16.8% [95% CI, 6.7–30.7%], P = 0.005) (Fig. 1b). The cumulative incidence of neutrophil engraftment within 28 days after transplantation was 100% in Pt-CR and 94.3% (95% CI, 75.5–98.8%) in Pt-non-CR (P = 0.115) (Fig. S1). Median time to neutrophil recovery in Pt-CR and Pt-non-CR was 14 days (11–22 days) and 15 days (10–28 days), respectively.
Overall survival rate and MST in this cohort study. a Overall survival rate and MST for the whole cohort. MST and 3yOS rates of the 62 patients were 7.68 months (95% CI: 4.00–20.4) and 25.6% (95% CI: 14.8–37.8), respectively. b Overall survival rate and MST according to disease status at transplantation (CR vs. non-CR). Among the CR patients, MST and 3yOS rate of the 21 patients were 21.4 months (95% CI: 10.7-NA) and 43.6% (95% CI: 20.3–64.9%), respectively. Among the non-CR patients, MST and 3yOS rate of the 41 patients were 4.00 months (95% CI: 2.62–7.68) and 16.8% (95% CI: 6.7–30.7%), respectively. The solid line shows the overall survival of CR patients and the dashed line shows that of non-CR patients. MST median survival time, 3yOS 3-year overall survival, CI confidence interval, CR complete remission, HSCT hematopoietic stem cell transplantation
Univariable analyses for the whole cohort revealed five factors that adversely affected overall survival (Table 2): age ≥50 years (hazard ratio [HR], 2.09; 95% CI, 1.07–4.08; P = 0.031), lines of chemotherapy prior to transplantation ≥2 (HR, 2.14; 95% CI, 1.12–4.08; P = 0.006), non-CR at transplantation (HR, 2.71; 95% CI, 1.33–5.52; P = 0.006), ECOG-PS 2–4 (HR, 5.70; 95% CI, 2.78–11.68; P < 0.001), and sIL-2R ≥ 2000 U/mL at transplantation (HR, 3.10; 95% CI, 1.64–5.90; P < 0.001). Because disease status at transplantation, sIL-2R level, and lines of chemotherapy prior to transplantation co-vary, sIL-2R and lines of chemotherapy were not examined in multivariable analysis. In multivariable analysis, ECOG-PS 2–4 (HR, 6.08; 95% CI, 2.76–13.37; P < 0.001), age ≥50 years (HR, 2.50; 95% CI, 1.26–4.95; P = 0.009), and non-CR status at transplantation (HR, 2.11; 95% CI, 1.01–4.42; P = 0.047) were significantly associated with worse OS (Table 2).
We performed subgroup analysis of the non-CR patient group to analyze the effect of pretransplantation factors on overall survival in Pt-non-CR. Univariable analysis of survival in non-CR patients identified two factors, which adversely affected overall survival: ECOG-PS 2–4 (HR, 4.27; 95% CI, 1.97–9.26; P < 0.001) and sIL-2R ≥ 2000 U/mL at transplantation (HR, 2.38; 95% CI, 1.13–5.02; P = 0.022) (Table 3). Multivariable analysis also revealed that higher ECOG-PS values (HR, 3.69; 95% CI, 1.63–8.35; P < 0.001) and sIL-2R (HR, 2.24; 95% CI, 1.05–4.81; P = 0.038) were associated with poorer overall survival (Table 3). The 1-year overall survival rates in Pt-non-CR with ECOG-PS 0–1 and those with ECOG-PS 2–4 were 42.9% (95% CI: 24.6–60.0%) and 7.7% (95% CI: 0.5–29.2%), respectively (P < 0.001) (Fig. 2a). The 1-year overall survival rates in Pt-non-CR with sIL-2R levels of <2000 U/mL and those with sIL-2R ≥ 2000 U/mL were 50.0% (95% CI: 25.9–70.1%) and 21.1% (95% CI: 6.6–41.0%), respectively (P = 0.020) (Fig. 2b).
Overall survival rate for the patients in non-CR at transplant. a Kaplan–Meier curve for non-CR patients according to ECOG-PS (ECOG-PS 0–1 vs. 2–4) at transplantation. One-year overall survival rates in patients with ECOG-PS 0–1 and those with ECOG-PS 2–4 were 42.9% (95% CI: 24.6–60.0%) and 7.7% (95% CI: 0.5–29.2%), respectively (P < 0.001). Solid and dashed lines indicate survival curves for the patients with ECOG-PS 0–1 and ECOG-PS 2–4, respectively. b Kaplan–Meier curve for non-CR patients according to sIL-2R level (sIL-2R < 2000 U/mL vs. sIL-2R ≥ 2000 U/mL) at transplantation. One-year overall survival rates in the patients with sIL-2R < 2000 U/mL and those with sIL-2R ≥ 2000 U/mL were 50.0% (95% CI: 25.9–70.1%) and 21.1% (95% CI: 6.6–41.0%), respectively (P = 0.020). Solid and dashed lines indicate survival curves for patients with sIL-2R < 2000 U/mL and sIL-2R ≥ 2000 U/mL, respectively. CR complete remission, ECOG Eastern Cooperative Oncology Group, PS performance status, CI confidence interval, sIL-2R soluble interleukin-2 receptor, HSCT hematopoietic stem cell transplantation
Transplant-related mortality and DAM
Overall, 26 (41.9%) patients died from transplant-related complications. The cumulative incidence of TRM was 44.4% (95% CI, 31–56.9%) for the whole cohort (Fig. S1). The cumulative incidence of TRM in Pt-non-CR was significantly higher than that in Pt-CR (46.3% [95% CI, 30.4–60.9%] vs. 15.7% [95% CI, 3.6–35.6%], P = 0.025) (Fig. 3a).
Cumulative incidence of transplant-related mortality and disease-associated mortality according to disease status at transplantation. a Cumulative incidences of transplant-related mortality 1 year after transplantation among non-CR patients and CR patients were 46.3% (95% CI: 30.4–60.9%) and 15.7% (95% CI: 3.6–35.6%), respectively (P = 0.025). b Cumulative incidences of disease-associated mortality 1 year after transplantation among non-CR patients and CR patients were 22.0% (95% CI: 10.7–35.8%) and 15.7% (95% CI: 3.6–35.6%), respectively (P = 0.725). The solid line shows the cumulative incidence of patients in CR and the dashed line shows that in non-CR patients. CR complete remission, CI confidence interval, HSCT hematopoietic stem cell transplantation
Death from progression of ATL occurred in 17 (27.4%) patients. The cumulative incidence of DAM was 30.0% (95% CI, 18.4–42.5%) for the whole cohort (Fig. S2). The cumulative incidence of DAM in patients who received transplants in non-CR and in those who received transplants in CR were 31.1% (95% CI, 16.8–46.6%) and 28.2% (95% CI, 9.5–50.6%), respectively. There was no significant difference in cumulative incidence of DAM between Pt-CR and Pt-non-CR (P = 0.725) (Fig. 3b).
Among 41 Pt-non-CR, 22 patients attained CR after transplantation, while 14 patients did not. Disease status after transplantation was not evaluable for five patients in Pt-non-CR. In the non-CR group, TRM was significantly higher in patients with ECOG-PS 2–4 than in those with ECOG-PS 0–1 (PS 2–4; 69.2% [95% CI, 31.5–88.9%] vs. PS 0–1; 35.7% [95% CI, 18.4–53.7%], P = 0.027) (Fig. S3A). On the other hand, there was no significant difference in DAM between patients with ECOG-PS 2–4 and those with ECOG-PS 0–1 (PS 2–4; 23.1% [95% CI, 4.5–49.9%] vs. PS 0–1; 21.4% [95% CI, 8.4–38.3%], P = 0.971) (Fig. S3B). The sIL-2R level at transplantation did not have an effect on TRM (sIL-2R ≥ 2000 U/mL; 52.6% [95% CI, 27.3–72.8%] vs. sIL-2R < 2000 U/mL; 33.3% [95% CI, 13.1–55.3%], P = 0.206) and DAM (sIL-2R ≥ 2000 U/mL; 26.3% [95% CI, 8.9–47.9%] vs. sIL-2R < 2000 U/mL; 16.7% [95% CI, 3.8–37.5%], P = 0.687) (Fig. S4).
Causes of death after transplantation
The causes of death after transplantation in ATL patients are summarized in Table 4. Among the 41 Pt-non-CR, 12 died of the primary disease, and 21 died of transplant-related complications. Infection was the most common cause of death among the transplant-related complications (sepsis, n = 4; bacterial pneumonia, n = 2; CMV pneumonia, n = 1; HCV hepatitis, n = 1). However, 10 of 21 patients died of various transplant-related complications other than infection, such as GVHD (n = 3), interstitial pneumonia (n = 2), intracranial hemorrhage (n = 1), posttransplant encephalopathy (n = 1), acute respiratory distress syndrome (n = 1), bronchiolitis obliterans (n = 1), and veno-occlusive disease (n = 1). Notably, eight patients died of transplant-related complications before posttransplant day 50, while only one patient died of the primary disease during the same period. Among the 21 Pt-CR, 5 died of the primary disease, and 5 died of transplant-related complications. There was only one patient who died of obvious infection (sepsis), and none of these patients died before posttransplant day 50.
Discussion
This study on allo-HSCT in ATL patients demonstrated that the extremely poor outcomes in Pt-non-CR were attributable to TRM rather than DAM. Contrary to our initial expectations, the DAM rate of Pt-CR almost equaled that of Pt-non-CR. Disease status other than CR at transplantation in patients with aggressive ATL is associated with a low survival rate [15, 21], and it is widely accepted that disease progression might contribute to the poor survival rate after allo-HSCT in non-CR ATL patients. To our knowledge, however, there have been no reports focusing on the prognostic impact of disease status at transplantation on TRM following allo-HSCT in ATL patients.
In this study, high ECOG-PS values and high sIL-2R levels were significantly associated with poor survival in Pt-non-CR. In ATL patients, a high level of sIL-2R (2000 U/mL or higher) at transplantation is known to be a significant risk factor for poor overall survival and disease progression after allo-HSCT [21]. In our cohort, none of the Pt-CR had high levels of sIL2-R, indicating that the level of circulating sIL-2R closely reflects the disease status of ATL. Although sIL-2R levels correlate with tumor burden in ATL [22, 23], in the current study, high sIL-2R levels at transplantation were not associated with DAM in Pt-non-CR. These findings indicate that sIL-2R levels would not provide sufficient information to enable a decision to be made on whether to proceed with additional chemotherapy and/or immunotherapy before allo-HSCT in non-CR patients because intensive therapies before transplantation can give rise to various complications after allo-HSCT.
The major causes of death in Pt-non-CR were not due to disease progression, and infection was the most common cause of death among a variety of complications. In contrast to our results, relapse and disease progression are the predominant causes of treatment failure and mortality after allo-HSCT in patients with refractory acute myeloid leukemia (AML) [24, 25]. A low number of naive T-lymphocytes may underlie the mechanism of immunodeficiency in HTLV-1 infected individuals [26]. Indeed, ATL patients are susceptible to various opportunistic infections and it has been reported that infection-related mortality is significantly higher than in patients with AML and acute lymphoblastic leukemia (ALL) [27]. Therefore, complications caused by infections may have a stronger impact on survival after allo-HSCT in patients with ATL than in patients with other hematological malignancies.
The high rate of complications among Pt-non-CR raises the question as to why transplant-related complications cause more severe problems for Pt-non-CR than for Pt-CR. Kozako et al. reported that the expression of programmed death-1 in CD8+ T-cells, including in cytomegalovirus- and Epstein–Barr virus specific cytotoxic T-cells, was significantly higher in patients with ATL than in HTLV-I carriers and control individuals [28]. It is tempting to speculate that the compromised cellular immunity in ATL patients is attributable to T-cell exhaustion induced by overexpression of programmed death-1 ligand in tumor cells [29]. Therefore, compared with those in CR, patients with ATL in non-CR may show reduced immune responses to various pathogens. We also noticed that a certain number of Pt-non-CR died of transplant-related complications other than infection, but we could not clarify whether residual ATL gave rise to these complications (Table 4).
A nationwide retrospective study of allo-HSCT in ATL patients revealed transplantation outcomes similar to that of the whole cohort in our study, that is, 3-year overall survival, cumulative incidence of TRM, and disease-associated death rates were 33%, 37%, and 21%, respectively [15]. It also showed that transplant-related events were the principal causes of early death, while disease-associated deaths were more common in the later phases [15]. We demonstrated that transplant-related deaths in the early phase of allo-HSCT in ATL patients were prominent among Pt-non-CR (Fig. 3 and Table 4). Interestingly, the cumulative incidence of disease-associated death of Pt-CR at transplantation was roughly equivalent to that of Pt-non-CR in the current study. These results suggest that disease progression after transplantation in Pt-non-CR cannot be evaluated properly due to the early deaths of the patients.
Shigematsu et al. reported that the 5-year overall survival rate of patients with aggressive ATL who received allo-HSCT in CR was more than 60% [21], while in the current study, the 3-year overall survival rate of ATL Pt-CR was only 43.6%. Patients with aggressive ATL who do not receive allo-HSCT in Okinawa Prefecture show poorer clinical outcomes than those patients in other areas of Japan [30]. The difference in clinical outcomes between patients with ATL in Okinawa and those in other areas of Japan might be partly attributed to the different distribution of the HTLV-1 tax genotype in Okinawa from mainland of Japan [31]. Further study is needed to clarify the impact of geographical factors and/or genetic backgrounds of ATL patients on transplantation outcomes after allo-HSCT in Okinawa.
Since this study was an observational retrospective study and included a small patient population, we cannot draw definitive conclusions about factors affecting the outcomes of allo-HSCT in all ATL patients. However, our study included all patients with aggressive ATL who received allo-HSCT in Okinawa Prefecture during the study period. Therefore, the results of our study reflected actual conditions of allo-HSCT for ATL in Okinawa. Because patients received transplantation without strict transplant eligibility criteria, patients with poor ECOG-PS were included in this study. Indeed, there was one patient with an ECOG-PS of three in Pt-CR and four patients with an ECOG-PS of three in Pt-non-CR. No patients had ECOG-PS 4 in either group. Even after excluding patients with ECOG-PS of three, similar results were seen in these patients (Fig. S5 and Table S4). In this study, 41 Pt-non-CR included 20 patients with partial response, 6 patients with stable disease, and 15 patients with progressive disease at transplantation (Table 1). Worse disease status was associated with lower overall survival and higher TRM, but did not affect DAM (Fig. S6).
Intensive chemotherapy for patients with ATL is effective for the first several courses of treatments. However, it is difficult to complete planned treatments because of toxicity and/or loss of effectiveness of the chemotherapy [8]. In cases of dismal outcomes after intensive chemotherapy in ATL patients, hematologists often consider allo-HSCT as a treatment option for patients with aggressive ATL in non-CR. Indeed, in the current study, and in other retrospective studies of allo-HSCT for aggressive ATL, the number of Pt-non-CR tends to be more than double that of Pt-CR [15, 27]. Since the early application of allo-HSCT is considered to reduce TRM and improve overall survival in patients with aggressive ATL, we should consider both the disease status at transplantation and the optimal timing of allo-HSCT. Furthermore, we should also consider more effective treatment strategies to reduce disease progression and relapses after allo-HSCT.
In conclusion, we revealed that high TRM rates in the early posttransplantation phase contribute considerably to the poor survival rate of patients with ATL who received allo-HSCT while in non-CR. Even after overcoming complications in the early phase, disease progression and relapse remain important problems in patients with ATL both in non-CR and in CR at transplantation. Our findings suggest that not only treatment for disease control but also intensive management to prevent transplant-related complications is required in order to improve the success rate of transplantation in ATL patients who cannot achieve CR before allo-HSCT. Furthermore, more effective therapeutic strategies for ATL are required to attain CR in patients undergoing allo-HSCT.
References
Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–92.
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–9.
Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–80.
Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J Med Virol. 2012;84:327–35.
Statistical analyses of clinico-pathological, virological and epidemiological data on lymphoid malignancies with special reference to adult T-cell leukemia/lymphoma: a report of the second nationwide study of Japan. The T- and B-Cell Malignancy Study Group. Jpn J Clin Oncol. 1985;15:517–35.
Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79:428–37.
Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453–9.
Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25:5458–64.
Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28:1591–8.
Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837–42.
Ishida T, Fujiwara H, Nosaka K, Taira N, Abe Y, Imaizumi Y, et al. Multicenter phase II study of lenalidomide in relapsed or recurrent adult T-cell leukemia/lymphoma: ATLL-002. J Clin Oncol. 2016;34:4086–93.
Sobue R, Yamauchim T, Miyamura K, Sao H, Tahara T, Yoshikawa H, et al. Treatment of adult T cell leukemia with mega-dose cyclophosphamide and total body irradiation followed by allogeneic bone marrow transplantation. Bone Marrow Transplant. 1987;2:441–4.
Utsunomiya A, Miyazaki Y, Takatsuka Y, Hanada S, Uozumi K, Yashiki S, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:15–20.
Fukushima T, Miyazaki Y, Honda S, Kawano F, Moriuchi Y, Masuda M, et al. Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia. 2005;19:829–34.
Hishizawa M, Kanda J, Utsunomiya A, Taniguchi S, Eto T, Moriuchi Y, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood. 2010;116:1369–76.
Ishida T, Hishizawa M, Kato K, Tanosaki R, Fukuda T, Taniguchi S, et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood. 2012;120:1734–41.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Cox D. Regression model and life tables. J R Stat Soc B. 1972;34:187–200.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Shigematsu A, Kobayashi N, Yasui H, Shindo M, Kakinoki Y, Koda K, et al. High level of serum soluble interleukin-2 receptor at transplantation predicts poor outcome of allogeneic stem cell transplantation for adult T cell leukemia. Biol Blood Marrow Transpl. 2014;20:801–5.
Yasuda N, Lai PK, Ip SH, Kung PC, Hinuma Y, Matsuoka M, et al. Soluble interleukin 2 receptors in sera of Japanese patients with adult T cell leukemia mark activity of disease. Blood. 1988;71:1021–6.
Kamihira S, Atogami S, Sohda H, Momita S, Yamada Y, Tomonaga M. Significance of soluble interleukin-2 receptor levels for evaluation of the progression of adult T-cell leukemia. Cancer. 1994;73:2753–8.
Koh H, Nakamae H, Hagihara K, Nakane T, Manabe M, Hayashi Y, et al. Factors that contribute to long-term survival in patients with leukemia not in remission at allogeneic hematopoietic cell transplantation. J Exp Clin Cancer Res. 2011;30:36.
Hemmati PG, Terwey TH, Na IK, Jehn CF, le Coutre P, Vuong LG, et al. Allogeneic stem cell transplantation for refractory acute myeloid leukemia: a single center analysis of long-term outcome. Eur J Haematol. 2015;95:498–506.
Yasunaga J, Sakai T, Nosaka K, Etoh K, Tamiya S, Koga S, et al. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood. 2001;97:3177–83.
Itonaga H, Taguchi J, Fukushima T, Tsushima H, Sato S, Ando K, et al. Distinct clinical features of infectious complications in adult T cell leukemia/lymphoma patients after allogeneic hematopoietic stem cell transplantation: a retrospective analysis in the Nagasaki transplant group. Biol Blood Marrow Transplant. 2013;19:607–15.
Kozako T, Yoshimitsu M, Fujiwara H, Masamoto I, Horai S, White Y, et al. PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia. 2009;23:375–82.
Kataoka K, Shiraishi Y, Takeda Y, Sakata S, Matsumoto M, Nagano S, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016;534:402–6.
Nishi Y, Fukushima T, Nomura S, Tomoyose T, Nakachi S, Morichika K, et al. Characterization of patients with aggressive adult T-cell leukemia-lymphoma in Okinawa, Japan: a retrospective analysis of a large cohort. Int J Hematol. 2016;104:468–75.
Sakihama S, Saito M, Kuba-Miyara M, Tomoyose T, Taira N, Miyagi T, et al. Human T-cell leukemia virus type I Tax genotype analysis in Okinawa, the southernmost and remotest islands of Japan: different distributions compared with mainland Japan and the potential value for the prognosis of aggressive adult T-cell leukemia/lymphoma. Leuk Res. 2017;61:18–24.
Acknowledgements
The authors thank Masumi Shimoji for technical assistance with data management. This work was supported by JSPS KAKENHI Grant Number 17K09934 and MEXT KAKENHI Grant Number 17H05797 to SM.
Author information
Authors and Affiliations
Contributions
ST, SM, YN, and HM participated in the design of the study; ST, SM, YN, SN, KT, KM, IT, NS, TH, SK, SU, RM, TM, K.Karimata, MO, AY, TT, K.Karube, and TF collected clinical data; ST and SM performed statistical data analysis; and ST, SM, and HM wrote the paper. All authors checked the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tomori, S., Morishima, S., Nishi, Y. et al. Transplant-related complications are impediments to the success of allogeneic hematopoietic stem cell transplantation for adult T cell leukemia patients in non-complete remission. Bone Marrow Transplant 55, 233–241 (2020). https://doi.org/10.1038/s41409-019-0669-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0669-z