Abstract
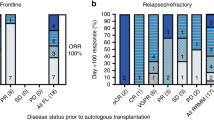
The most common preparative regimen for autologous transplantation (ASCT) in myeloma (MM) consists of melphalan 200 mg/m2 (MEL 200). Higher doses of melphalan 220–260 mg/m2, although relatively well tolerated, have not shown significant improvement in clinical outcomes. Several approaches have been pursued in the past to improve CR rates, including poly-chemotherapy preparative regimens, tandem ASCT, consolidation, and/or maintenance therapy. Since there is a steep dose–response effect for intravenous melphalan, we evaluated an alternative single ASCT strategy using higher-dose melphalan at 280 mg/m2 (MEL 280) with amifostine as a cytoprotectant as the maximum tolerated dose determined in an earlier phase I dose escalation trial. We report the final long-term outcomes of MM patients who underwent conditioning with MEL 280 with amifostine cytoprotection followed by ASCT. Although the complete response rate was quite high in the era pre-dating the routine use of novel therapies (proteasome inhibitors, immunomodulatory agents) (49%), the progression-free survival was a disappointing 22 months. The implications of this dichotomy between the excellent depth of ASCT response and progression-free survival are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. https://doi.org/10.1056/NEJMoa02234.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–7. https://doi.org/10.1056/NEJM199607113350204.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20. https://doi.org/10.1056/NEJMoa1611750
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–29. https://doi.org/10.1016/S1470-2045(15)00389-7
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905. https://doi.org/10.1056/NEJMoa1402888
Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502. https://doi.org/10.1056/NEJMoa032290.
Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma:Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–41. https://doi.org/10.1200/JCO.2006.10.2509.
Goldschmidt H. Single vs double high-dose therapy in multiple myeloma: second analysis of the GMMG-HD-2 trial. Haematologica. 2005;90 Suppl 1:38.
JP Fermand,C Alberti,J-P Marolleau. Single versus tandem high dose therapy (HDT) supported with autologous stem cell transplantation using unselected or CD-34-enriched ABSC: results of a two by two designed randomized trial in 230 young patients with multiple myeloma [abstract]. Hematol J. 2003;4 Suppl 1:559–60.
Sonneveld PvdHB, Segeren C, et al. Intensive versus double intensive therapy in untreated multiple myeloma: updated analysis of the randomized phase III study HOVON 24 MM. Blood. 2004;104:943.
Barlogie B, Attal M, Crowley J, van Rhee F, Szymonifka J, Moreau P, et al. Long-term follow-up of autotransplantation trials for multiple myeloma: update of protocols conducted by the intergroupe francophone du myelome, southwest oncology group, and university of arkansas for medical sciences. J Clin Oncol. 2010;28:1209–14. https://doi.org/10.1200/JCO.2009.25.6081
Nath CE, Shaw PJ, Trotman J, Zeng L, Duffull SB, Hegarty G, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br J Clin Pharmacol. 2010;69:484–97. https://doi.org/10.1111/j.1365-2125.2010.03638.x.
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–5.
Phillips GL, Meisenberg B, Reece DE, Adams VR, Badros A, Brunner J, et al. Amifostine and autologous hematopoietic stem cell support of escalating-dose melphalan: a phase I study. Biol Blood Marrow Transplant. 2004;10:473–83. https://doi.org/10.1016/j.bbmt.2004.03.001.
Hensley ML, Schuchter L, Lindley C, et al. American Society of Clinical Oncology clinical practice guideline for the use of chemotherapy and radiotherapy protectants. J Clin Oncol. 1999;17:3333–55.
Koukourakis MI. Amifostine: is there evidence of tumor protection. Semin Oncol Suppl. 2003;30 Suppl 18:18–30.
Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013;31:4529–35. https://doi.org/10.1200/JCO.2013.49.0086.
Iacobelli S, de Wreede LC, Schonland S, Bjorkstrand B, Hegenbart U, Gruber A, et al. Impact of CR before and after allogeneic and autologous transplantation in multiple myeloma: results from the EBMT NMAM2000 prospective trial. Bone Marrow Transplant. 2015;50:505–10. https://doi.org/10.1038/bmt.2014.310
Lee CK, Barlogie B, Munshi N, Zangari M, Fassas A, Jacobson J, et al. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003;21:2732–9. https://doi.org/10.1200/JCO.2003.01.055.
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562–8.
Blade JSD, Reece D, Apperley J, Bjorlestrand B, Gahrton G, Gertz M, Giralt S, Jagannath, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and hematopoietic stem cell transplantation. Bone Marrow Transplant. 1998;21:337–43.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. https://doi.org/10.1038/sj.leu.2404284.
Higman MA, Port JD, Beauchamp NJ Jr., Chen AR. Reversible leukoencephalopathy associated with re-infusion of DMSO preserved stem cells. Bone Marrow Transplant. 2000;26:797–800. https://doi.org/10.1038/sj.bmt.1702589.
Memon M, deMagalhaes-Silverman M, Bloom EJ, Lister J, Myers DJ, Pincus SM, et al. Reversible cyclosporine-induced cortical blindness in allogeneic bone marrow transplant recipients. Bone Marrow Transplant. 1995;15:283–6.
Blijlevens N, Schwenkglenks M, Bacon P, D’Addio A, Einsele H, Maertens J, et al. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy--European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol. 2008;26:1519–25. https://doi.org/10.1200/JCO.2007.13.6028.
Spencer A, Horvath N, Gibson J, Prince HM, Herrmann R, Bashford J, et al. Prospective randomised trial of amifostine cytoprotection in myeloma patients undergoing high-dose melphalan conditioned autologous stem cell transplantation. Bone Marrow Transplant. 2005;35:971–7. https://doi.org/10.1038/sj.bmt.1704946.
Bensinger WI, Becker PS, Gooley TA, Chauncey TR, Maloney DG, Gopal AK, et al. A randomized study of melphalan 200 mg/m2 vs 280 mg/m2 as a preparative regimen for patients with multiple myeloma undergoing auto-SCT. Bone Marrow Transplant. 2016 Jan;51(1):67-71. https://doi.org/10.1038/bmt.2015.211. Epub 2015 Sep 14.PMID:26367217.
Olivieri A, Corvatta L, Montanari M, Brunori M, Offidani M, Ferretti GF, et al. Paroxysmal atrial fibrillation after high-dose melphalan in five patients autotransplanted with blood progenitor cells. Bone Marrow Transplant. 1998;21:1049–53. https://doi.org/10.1038/sj.bmt.1701217.
Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood. 2010;115:32–7. https://doi.org/10.1182/blood-2009-06-229658.
Cavo M, Gay FM, Patriarca F, Zamagni E, Montefusco V, Dozza L, et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/HO95 study. Blood. 2017;130 Suppl 1:401–1.
Chakraborty R, Muchtar E, Kumar SK, Jevremovic D, Buadi FK, Dingli D, et al. Impact of post-transplant response and minimal residual disease on survival in myeloma with high-risk cytogenetics. Biol Blood Marrow Transplant. 2017;23:598–605. https://doi.org/10.1016/j.bbmt.2017.01.076
Lahuerta JJ, Paiva B, Vidriales MB, Cordon L, Cedena MT, Puig N, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–10. https://doi.org/10.1200/JCO.2016.69.2517
Hari P, Aljitawi OS, Arce-Lara C, Nath R, Callander N, Bhat G, et al. A phase IIb, multicenter, open-label, safety, and efficacy study of high-dose, propylene glycol-free melphalan hydrochloride for injection (EVOMELA) for myeloablative conditioning in multiple myeloma patients undergoing autologous transplantation. Biol Blood Marrow Transplant. 2015;21:2100–5. https://doi.org/10.1016/j.bbmt.2015.08.026
Qazilbash MH, Bashir Q, Thall PF, Milton DR, Shah N, Patel KK, et al. A randomized phase III trial of busulfan+melphalan vs melphalan alone for multiple myeloma. Blood. 2017;130 Suppl 1:399–9.
Rodriguez TE, Hari P, Stiff PJ, Smith SE, Sterrenberg D, Vesole DH. Busulfan, melphalan, and bortezomib versus high-dose melphalan as a conditioning regimen for autologous hematopoietic stem cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2016;22:1391–6. https://doi.org/10.1016/j.bbmt.2016.03.021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hari, P., Reece, D.E., Randhawa, J. et al. Final outcomes of escalated melphalan 280 mg/m2 with amifostine cytoprotection followed autologous hematopoietic stem cell transplantation for multiple myeloma: high CR and VGPR rates do not translate into improved survival. Bone Marrow Transplant 54, 293–299 (2019). https://doi.org/10.1038/s41409-018-0261-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0261-y
This article is cited by
-
Tandem autologous hematopoietic cell transplantation with sequential use of total marrow irradiation and high-dose melphalan in multiple myeloma
Bone Marrow Transplantation (2021)
-
Current status of autologous stem cell transplantation for multiple myeloma
Blood Cancer Journal (2019)
-
Allogeneic Hematopoietic Stem Cell Transplantation for Myeloma: Time for an Obituary or Not Just Yet!
Indian Journal of Hematology and Blood Transfusion (2019)