Abstract
Long-term proteasome inhibitor (PI) treatment can improve multiple myeloma (MM) outcomes, but this can be difficult to achieve in clinical practice due to toxicity, comorbidities, and the burden of repeated parenteral administration. US MM-6 (NCT03173092) enrolled transplant-ineligible patients with newly diagnosed MM to receive all-oral ixazomib-lenalidomide-dexamethasone (IRd; ≤39 cycles or until progression or toxicity) following three cycles of bortezomib-based induction. Primary endpoint: 2-year progression-free survival (PFS). Key secondary/exploratory endpoints included overall response rate (ORR), overall survival (OS), safety, quality of life (QoL), treatment satisfaction, and actigraphy. At datacut, in the fully accrued cohort of 140 patients, median age was 73 years with 42% aged ≥75 and 61% deemed frail; 10% of patients were ongoing on treatment. After a median follow-up of 27 months, the 2-year PFS rate was 71% (95% confidence interval: 61–78). ORR increased from 62% at the end of induction to 80% following in-class transition (iCT) to IRd for a median of 11 months. The 2-year OS rate was 86%. The overall safety profile/actigraphy levels were consistent with previous reports; QoL/treatment satisfaction scores were stable with ongoing therapy. iCT to IRd may allow prolonged PI-based therapy with promising efficacy and a tolerable safety profile, while maintaining QoL.

Similar content being viewed by others
Introduction
Long-term parenteral proteasome inhibitor (PI)-based regimens are a cornerstone of treatment among patients with multiple myeloma (MM) [1, 2]. For transplant-ineligible patients with newly diagnosed MM (NDMM), the addition of PIs to 2-drug standard of care treatment regimens has improved progression-free survival (PFS) and overall survival (OS) rates in phase 3 clinical studies [3,4,5,6]. However, prolonged therapy with parenteral PIs can be difficult to achieve in routine clinical practice due to unfavorable toxicity [7], existing comorbidities [8,9,10], the burden of repeated, clinic-based treatment administration, difficulty traveling to treatment centers, and patient preference for treatment outside of a clinic [11]. Such factors are likely to impact older and frail patients, who comprise a large proportion of all individuals with MM [12, 13]. By comparison, due to strict eligibility criteria, patient cohorts enrolled in randomized controlled trials (RCTs) are often younger and healthier versus real-world patient populations, and not always representative of the wider MM population [13].
Ixazomib is an oral PI, approved in combination with lenalidomide-dexamethasone (Rd) for the treatment of MM patients who have received ≥1 prior therapy [14, 15]. Ixazomib-lenalidomide-dexamethasone (IRd) has demonstrated prolonged PFS versus placebo-Rd and a tolerable safety profile in both relapsed/refractory MM (RRMM) and transplant-ineligible patients with NDMM [16, 17], although in the study of RRMM, no OS benefit was demonstrated at a median follow-up of 23 months, with median OS not reached in both the IRd and placebo treatment arms [16]. To facilitate continuous, long-term PI-based treatment and assess its potential benefit in a patient cohort representative of that found in routine clinical practice (including older, frail, and comorbid patients), the community-based US MM-6 study was designed to investigate in-class transition (iCT) from parenteral bortezomib-based induction to all-oral IRd therapy in patients with NDMM (NCT03173092) [18]. The objective is to increase duration of PI-based therapy and improve outcomes, while maintaining patient quality of life (QoL) and a tolerable safety profile. Here we report fully accrued data from US MM-6.
Methods
Study design and patient eligibility
Full methods have been previously published [18]. US MM-6 was designed as a prospective, open-label, single-arm, phase 4 study in a community-based population; at data accrual, enrollment was complete. Patients were enrolled at 22 US community sites, including three Veterans’ Affairs sites. Adults with NDMM who were ineligible for transplantation, or whose transplantation was delayed for ≥2 years, were eligible. Following three cycles of bortezomib-based induction, patients were administered oral IRd (planned dosages: ixazomib 4 mg on days 1, 8, and 15; lenalidomide 25 mg on days 1–21; and dexamethasone 20–40 mg on days 1, 8, 15, and 22) in 28-day cycles, for 39 cycles or until toxicity or progressive disease (PD). Patients were permitted to continue IRd beyond cycle 39 if investigators deemed a clinical benefit with further treatment. Following end-of-treatment assessment (performed within 30 days of the last ixazomib dose), patients entered a follow-up period to evaluate PFS and OS until PD or death, loss to follow-up, or study termination.
US MM-6 was conducted in accordance with the International Council on Harmonization Good Clinical Practice guidelines, the ethical principles that have their origins in the Declaration of Helsinki, and applicable regulatory requirements. Local or central institutional review boards for each study center approved the present study. All patients gave written informed consent to be included, and to the use of a wearable device to capture digital actigraphy data and a smartphone application for electronic patient reported outcomes (ePROs).
Endpoints and assessments
The primary endpoint was 2-year PFS among the intent-to-treat (ITT) population, defined as the time from IRd initiation to the first documentation of PD, or death from any cause; PD was determined based on local laboratory test results and investigator assessed modified International Myeloma Working Group (IMWG) response criteria [19]. Secondary endpoints included ixazomib and IRd treatment durations, defined as the times from the first administration of ixazomib and IRd, respectively, to the final date of administration of ixazomib or any of the drugs in the IRd regimen, respectively; response rates, also based on investigator assessed modified IMWG criteria [19] (for complete response [CR], bone marrow plasma cell percentage was measured by aspiration and/or biopsy); OS, defined as the time from IRd initiation to death from any cause; safety, assessed by Medical Dictionary for Regulatory Activities (version 22.0) preferred terms and graded according to the National Cancer institute Common Terminology Criteria for adverse events (AEs; version 4.03); and ePRO data, specifically global health status (GHS) / QoL and treatment satisfaction, which were assessed via electronic questionnaires (European Organisation for Research and Treatment of Cancer quality of life questionnaire [EORTC QLQ-C30], derived from items 29 and 30; EORTC QoL questionnaire MM module [EORTC QLQ-MY20, item 43 on peripheral neuropathy]; and Treatment Satisfaction Questionnaire for Medication-9 [TSQM-9]) using a smartphone application. Exploratory endpoints included actigraphy analyses of patient sleep duration and activity based on duration and step counts, collected automatically via digital devices worn by patients (Garmin Vivofit 3 activity tracker). Details on ePRO scoring for US MM-6 have been reported previously [18].
Statistical analysis
Planned enrollment was 160 patients, which provides 90% power with a one-sided α value of 0.05, to demonstrate a 2-year PFS rate of 62%, exceeding the 2-year PFS rate of 50% derived from historical controls [3, 20, 21]. Enrollment slowed during the COVID pandemic, leading to a decision to curtail enrollment at 140 patients.
Kaplan-Meier (KM) methodology was used to estimate the 2-year PFS rate, survival curves, and medians, alongside associated 95% confidence intervals (CIs). Median treatment durations were ‘simple’, not calculated using the KM method. For ePRO analysis, the mean change from the baseline (assessed at the end of the first cycle) over the treatment period was described among patients who had completed the initial questionnaire, as well as at ≥1 post-baseline date. To ensure analysis captured the same ePRO assessment periods across patients, only data captured between day 22 of each cycle, and day 3 of the following cycle were used for analysis to account for off-schedule launch of ePRO questionnaires that may have occurred at different sites. Actigraphy devices had to be worn for ≥14 days per treatment cycle for data collection to be compliant; compliant days were not necessarily consecutive. For each compliant day, the device had to be worn for ≥12 hours (i.e., the patient had been recorded as moving for ≥12 hours that day, assuming that even during sleep or rest, a low level of movement would have been recorded). Days for which actigraphy data were available, but during which the patient had not been recorded moving for ≥12 hours, were excluded from analyses; this allowed the exclusion of data on days the devices were likely not being worn, and hence avoided wrongful classification of these patients as ‘sedentary’. For actigraphy analysis, outlying datapoints (defined as >4 standard deviations [StDevs] from the mean) were removed; recalculation of the mean and StDev was then performed until there were no outliers. For all endpoints, subgroup analyses of outcomes by patient age (<75 years vs. ≥75 years) and frailty status (non-frail vs. frail, based on a baseline modified Charlson Comorbidity Index score, patient age at point of study enrollment, and baseline Eastern Cooperative Oncology Group performance status score) [22] were also performed. All enrolled patients were included in the ITT population; patients who received ≥1 dose of IRd were included in the safety population.
Results
Patients and treatment
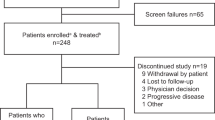
As of October 17, 2022 (the date of data accrual), 140 patients had been enrolled and treated (1 successfully screened patient was not treated) and were included in the safety and ITT populations. The median age of patients was 72.5 years; 59 patients (42.1%) were ≥75 years and 86 patients (61.4%) were considered frail (Table 1). Overall, 25 patients (17.9%) were Black or African American; 12 (8.6%) were Hispanic/Latino; 3 (2.1%) were Asian; and 1 (0.7%) was from the Pacific Islands. Of the entire cohort, 37 (26.4%), 58 (41.4%) and 44 (31.4%) patients had baseline disease stages I, II and III respectively, as defined by International Scoring System (ISS) criteria. Calculated baseline creatine clearance values among all patients were as follows: <60 mL/min, 40 patients (28.6%); ≥60 mL/min, 96 patients (68.6%); missing data, 4 patients (2.9%). At baseline, 131 patients (93.6%) had ≥1 concurrent medical condition, and 18 patients (12.9%) had peripheral neuropathy (PN). The most common induction regimens were bortezomib-lenalidomide-dexamethasone (VRd; 118 patients, 84.3%) and bortezomib-cyclophosphamide-dexamethasone (18 patients, 12.9%) (Table 1).
At data accrual, 14 patients (10.0%) were ongoing on IRd treatment, 111 (79.3%) had discontinued study treatment, and 12 (8.5%) had completed ≥39 cycles of IRd. Another 3 patients had completed 26 treatment cycles per the original study protocol. The most common reasons for treatment discontinuation were AEs (27 patients, 19.3%), patient withdrawal (26 patients, 18.6%), and PD (23 patients, 16.4%; Table 2). Overall, 3 patients (2.1%) underwent autologous stem-cell transplantation following IRd.
Duration of treatment
Median duration of IRd
The overall median duration of all PI therapy (including the 3 cycles of bortezomib-based induction) was 13.6 months, while the overall median duration of IRd therapy was 11.0 months, with a median of 11 treated cycles (Table 3). Among patient subgroups aged <75 and ≥75 years, median IRd therapy duration was 13.8 months and 9.2 months, respectively, while the median number of treatment cycles was 13 and 9, respectively. Non-frail patients were treated with IRd for a median of 11.9 months with a median of 12 cycles, while frail patients were treated for a median of 10.3 months with a median of 11 cycles.
Overall, 102 patients (72.9%) completed five or more cycles of IRd; in the subgroups aged <75 and ≥75 years, 61 (75.3%) and 41 (69.5%) patients completed five or more cycles, respectively. Among non-frail and frail subgroups, 39 (72.2%) and 63 (73.3%) patients completed five or more cycles, respectively.
Median duration of ixazomib
The median duration of ixazomib therapy among the entire cohort was 10.5 months and the median duration of all PI-based therapy (including bortezomib-based induction) was 13.6 months. For patients aged <75 and ≥75 years, median durations of therapy with ixazomib were 13.2 and 8.5 months, respectively; for all PI-based therapy, median durations of therapy were 18.0 and 11.5 months, respectively. Among subgroups of non-frail and frail patients, median durations of ixazomib treatment were 10.8 and 10.1 months, while median durations of treatment of total PI-based therapy were 14.6 and 13.3 months, respectively (Table 3).
Efficacy
After a median follow-up of 26.8 months at data accrual, 29 patients had progressed and 11 had died. In subgroups aged <75 and ≥75 years, median follow-up was 29.2 and 24.3 months, respectively; for non-frail and frail patients, median follow-up was 27.5 and 26.7 months, respectively. The overall 2-year PFS rate (KM estimate) from the start of IRd treatment was 71% (95% CI: 61–78), and median PFS had not been reached (Fig. 1A). Among subgroups of interest, for patients aged <75 and ≥75 years, 2-year PFS rates were 72% (95% CI: 61–81; Fig. 1B) and 67% (95% CI: 50–80; Fig. 1C). Median PFS values were not reached among patients aged <75 years, and 32.7 months (95% CI: 20.7–not calculable) among those aged ≥75 years. For patients defined as non-frail and frail, 2-year PFS rates were 74% (95% CI: 58–85) and 68% (95% CI: 55–78), respectively; for both subgroups, median PFS was not reached.
A ITT population (N = 140a). B Stratified by age subgroup. C Stratified by frailty subgroup. IMWG International Myeloma Working Group, IRd ixazomib-lenalidomide-dexamethasone, ITT intent-to-treat, PD progressive disease, PFS progression-free survival. aOne successfully screened patient was not treated. PFS defined as the time from first administration of IRd to the date of the first documentation of PD based on local laboratory results and the investigator’s assessment using modified IMWG response criteria, or death due to any cause, whichever occurred first; data are stratified by (A) ITT population; (B) subgroups aged <75 and ≥75 years and (C) subgroups defined as non-frail and frail.
The overall 2-year OS rate (KM estimate) from the start of IRd treatment was 86% (Fig. 2A), 86% and 87%, for patients aged <75 years and ≥75 years (Fig. 2B) and 86% and 87%, for non-frail and frail patients (Fig. 2C), respectively. At data accrual, median OS had not been reached in either the overall population or in any of the subgroups.
A ITT population (N = 140a). B Stratified by age subgroup. C Stratified by frailty subgroup. IRd ixazomib-lenalidomide-dexamethasone, ITT intent-to-treat, OS overall survival. aOne successfully screened patient was not treated. OS defined as the time from the date of the first administration of IRd to the date of death from any cause. Patients without documentation of death at the time of analysis were censored at the date last known to be alive; data are stratified by (A) ITT population; (B) subgroups aged <75 and ≥75 years and (C) subgroups defined as non-frail and frail.
Among the entire cohort, overall response rate (ORR) had increased from 62.1% (CR 7.9%; very good partial response [VGPR] 24.3%; partial response [PR] 30.0%) at the end of bortezomib-based induction to 80.0% after iCT to IRd (CR [including stringent CR and molecular CR] 37.1%; VGPR 26.4%; PR 16.4%) (Fig. 3). For patients aged <75 years and ≥75 years, ORR increased from 60.5% and 64.4%, respectively, to 80.2% and 79.7% respectively following iCT. An increase in ORR from 70.4% to 81.5% was observed among non-frail patients, while an increase from 57.0% to 79.1% was observed among frail patients.
CR complete response, iCR immunophenotypic CR, iCT in-class transition, IRd ixazomib-lenalidomide-dexamethasone, ITT intent-to-treat, mCR molecular CR, ORR overall response rate, PR partial response, sCR stringent CR, V bortezomib, VGPR very good partial response. aOne successfully screened patient was not treated. bORR = PR + VGPR + CR + sCR + iCR + mCR. cTotal CR = CR + sCR + iCR + mC.
Safety
Overall, 137 patients (97.9%) experienced any treatment emergent AE (TEAE); 96 patients (68.6%) had a grade ≥3 TEAE (Table 4). The most common TEAEs included gastrointestinal disorders and PNs. Gastrointestinal disorders were experienced by 93 patients (66.4%); of these, 60 patients (42.9%) experienced treatment-related gastrointestinal disorders. PNs were observed among 39 patients (27.9%, Table 5); 30 patients (21.4%) experienced treatment-related PNs. Overall, 36 patients (25.7%) had grades 1–2 PNs and 3 (2.1%) experienced grade 3 PNs; there were no cases of grade 4 PNs.
Patient-reported quality of life, treatment satisfaction, and global satisfaction
Of all 1875 EORTC QLQ-C30, EORTC QLQ-MY20, and TSQM-9 questionnaires that could have been completed in the entire sample, 1004 (53.5%), 1012 (54.0%), and 1012 (54.0%) had been completed by patients, respectively. Considering that only a subset of all possible questionnaires were successfully launched to patients, the overall completion percentage was re-calculated as 95.2%.
Overall, patient-reported QoL (EORTC QLQ-C30 GHS/QoL) was maintained during IRd therapy in the overall cohort (Supplementary Fig. 1A), and by age and frailty status (Supplementary Fig. 1B). Treatment satisfaction (TSQM-9 Effectiveness [Supplementary Fig. 2A], Treatment Convenience [Supplementary Fig. 2B], and Global Satisfaction with treatment [Supplementary Fig. 2C]) was also generally maintained during IRd therapy among the overall cohort, and by age and frailty status. For item 43 in the EORTC QLQ-MY20 questionnaire measuring the burden of PN [23], the mean change from ePRO baseline score was ≤0.7 during IRd treatment (Supplementary Fig. 3A), while mean changes from ePRO baseline scores in age and frailty subgroups were similarly minor (Supplementary Fig. 3B). For all ePRO outcomes, considering the relatively small sample sizes which decreased over time, results were interpreted with caution at later cycles and for all subgroups.
Actigraphy
Among 94 patients with available daily actigraphy data (a total of 26,665 days), 24,283 (91.1%) compliant days were included in the analysis. The mean number of steps per day was 3107 (StDev 2360). Mean daily active time among the whole cohort was 25.2 minutes (StDev 19.8), while for subgroups aged <75 and ≥75 years it was 25.8 (StDev 20.4) and 23.4 (StDev 17.4) minutes, respectively. Non-frail and frail patients were active for a mean of 27.0 (StDev 19.8) and 22.8 (StDev 19.2) minutes per day, respectively. These data are shown by cycle in Supplementary Fig. 4A. Mean daily sleep time for all patients was 7.6 hours (StDev 2.7). Patients aged <75 and ≥75 years slept for a mean of 7.8 (StDev 2.8) and 6.9 (StDev 2.4) hours daily, while non-frail and frail subgroups had mean daily sleep durations of 7.5 (StDev 2.5) and 7.6 (StDev 2.9) hours, respectively. These data are shown by cycle in Supplementary Fig. 4B.
Discussion
These data from the fully accrued US MM-6 community-based cohort confirm preliminary findings, including promising overall 2-year PFS and OS rates of 71% and 86% respectively, high response rates and a deepened response after iCT to IRd (overall ORR increased from 62.1% at induction to 80.0% after iCT to IRd; total CR increased from 7.9% to 37.1%), a tolerable safety profile, with maintained QoL and actigraphy data [18]. Notably, the US MM-6 2-year PFS rate appears higher than figures typically reported by both RCTs and community-based studies investigating bortezomib-based regimens among transplant-ineligible patients with NDMM. For example, the community-based UPFRONT trial, which was designed to compare eight 21-day cycles of three bortezomib-based regimens (bortezomib-dexamethasone, bortezomib-dexamethasone-thalidomide and bortezomib-melphalan-prednisone [VMP]) followed by bortezomib maintenance, reported 2-year PFS values ranging from ~25–40% [20]. Additionally, the VISTA phase 3 RCT reported 2-year PFS rates of ~50% among patients with NDMM, who were administered nine planned 6-week cycles of VMP [4]. The phase 3 SWOG S0777 study reported a 2-year PFS of ~65% for previously untreated patients without an intent for immediate transplant, who received eight planned 21-day cycles of VRd followed by lenalidomide maintenance [6]. A phase 2 trial which administered nine planned 35-day cycles of modified VRd (RVD lite), followed by lenalidomide consolidation, in patients with previously untreated NDMM (NCT01782963) demonstrated a 2-year PFS of ~80%; however, the relatively small cohort size (N = 53) limits the study relevance to US MM-6; we also note that NCT01782963 was conducted at academic and medical centers, unlike the current study which was community-based [24]. The 2-year OS rate of US MM-6 was promising at 86%, and in line with TOURMALINE-MM2 outcomes for continuous ixazomib in transplant ineligible patients [17], and similar to cohorts of patients with NDMM who were administered bortezomib-based regimens (e.g., SWOG S0777, ~90% [6]; UPFRONT, ~75–80% [20]; VISTA, ~85% [4]). At data accrual, median OS had not been reached among the overall US MM-6 population, nor among any of the subgroups. While SWOG S0777 and US MM-6 demonstrated similar overall 2-year PFS and OS rates, it is notable that, compared to US MM-6, SWOG S0777 included generally younger and healthier patients, including patients who went on to receive transplant. Therefore, the SWOG S0777 patient population was likely not representative of MM populations typically seen in routine clinical practice; indeed, 39% of patients enrolled in SWOG S0777 were aged ≥65 years in the study arm, lower than the 79.3% of patients enrolled in the same age group of this real-world study.
As described above, ORR increased notably from the end of bortezomib-based induction to iCT to IRd. Similar increases were observed among subgroups aged <75 years and ≥75 years, as well as among non-frail and frail patients. The reported ORR, achieved with iCT among community-based US MM-6 patients, appears similar to the ORRs reported in the TOURMALINE-MM2 RCT (82.1%) [17] and the NCT01782963 (RVD lite) study (86%) [25]. The VISTA RCT reported an ORR of 70.6% among the VMP treatment arm [4], while UPFRONT reported ORRs of 69.7–79.7%, depending on the bortezomib-based regimen administered [20]. Deeper responses are key to prolonging PFS, particularly among patients with NDMM [26]. As noted, US MM-6 also demonstrated an overall deep response; total CR was 37.1% (CR 32.1%; stringent CR 3.6%; molecular CR 1.4%; immunophenotypic CR 0) in the ITT population following iCT to IRd, and was similar among subcohorts of interest. These rates are higher than corresponding rates from similar patient cohorts administered bortezomib-based treatment (SWOG S0777, CR 24.2% [6]; UPFRONT, CR 3–4% [20]; VISTA, CR 33% [4]). As opposed to many treatment regimens currently used for NDMM, US MM-6 did not include a maintenance component. Historically, maintenance was administered following transplant in MM to prolong the deep response obtained after transplantation. Maintenance treatment was later extended to transplant-ineligible patients receiving standard MM therapy. Use of a tolerable PI-based 3-drug regimen until progression results in a prolonged consolidation approach, which can facilitate maintenance of QoL and performance status. The median ixazomib treatment duration for patients aged <75 years was longer (13.2 months) than for patients aged ≥75 years (8.5 months), which could be due to the difference in study drug discontinuation rates (74.1% vs. 86.4%; including withdrawal by patient, 13.6% vs. 25.4%). However, the median duration of ixazomib treatment among the entire cohort was 10.5 months and similar durations were observed among subgroups of non-frail (10.8 months) and frail (10.1 months) patients, suggesting that long-term oral administration of IRd is viable and does not adversely impact the frail population. Furthermore, the median overall duration of PI therapy, including three cycles of bortezomib-based induction, was 13.6 months. In the SWOG S0777 study, patients were randomized to complete induction consisting of six 28-day cycles of Rd or eight 21-day cycles of VRd, followed by Rd maintenance [6]. While median cycles of study treatment have not been reported, only 55.7% (n = 131) of analyzable patients randomized to receive VRd completed induction. In addition, as mentioned above, patients tended to be younger compared with US MM-6 (39% in the study arm were aged ≥65 years vs 79.3% in US MM-6) [6]. For the current study, subgroup treatment durations generally support the tolerable safety profile of IRd in elderly and frail patients, permitting extended duration of treatment. Indeed, the overall rate of study drug discontinuation due to TEAEs in US MM-6 was 19.3%; this is lower than the equivalent rates reported in the community-based UPFRONT study (29–38%) [20]. Additionally, reviews analyzing real-world studies suggest that TEAEs are typically a leading cause of cancer therapy discontinuation (MM, ~16–39%; all cancer, ~15–32%) [12, 27] but are less prevalent in RCTs (MM: ~7–21%) [12, 28]. Of note, 18.6% of patients withdrew from treatment in US MM-6 but reasons for this were not collated. The proportion of patients choosing to withdraw is slightly lower than that reported in a retrospective analysis of 340 patients with MM who had received maintenance therapy (most commonly with bortezomib or lenalidomide) for <3 years post-autologous stem cell transplantation. In that study, 22.5% of patients discontinued due to patient preference. We acknowledge that non-collation of reasons for patient withdrawal is a limiting factor in our study and as such are currently updating the study database to enable capture of these missing data. We also demonstrate that the overall safety profile of IRd in US MM-6 was tolerable and similar to previous reports [17, 29]. Notably, 12.9% of patients had comorbid PN at baseline. Following iCT to IRd, PNs were experienced by 27.9% of patients; treatment-related PNs were experienced by 31.4%. Previous trials investigating bortezomib-based treatment among comparable transplant-ineligible patients with MM have generally demonstrated higher rates of PN occurrence (44–60%) [4, 20], suggesting that iCT to IRd could provide a tolerable treatment alternative. Mean EORTC QLQ-MY20 score was generally maintained during IRd treatment although for all ePRO outcomes, caution should be taken when interpreting later cycle data from patients aged ≥75 years, due to small cohort sizes. No adverse impact of iCT to IRd on QoL or treatment satisfaction was observed, and patients receiving IRd achieved activity levels (steps per day) and sleep durations comparable to previously published data [30, 31]. This should be considered impactful, as the overall cohort comprises a high proportion of elderly and frail patients, almost all with ≥1 comorbidity, among whom a decline in activity and QoL might be expected over the course of treatment.
The inclusion of MM patients from a real-world population in US MM-6 continues to lend credence to its findings [18]. Up to 40% of real-world patients with NDMM do not meet eligibility criteria for RCTs [32,33,34]. Eligible individuals are typically younger and have better health at baseline, and thus should not be considered entirely representative of all patients with MM. To address this, US MM-6 inclusion criteria were broader, allowing enrollment of patients who might not have access to RCTs, or who would typically be excluded due to comorbidities, making the enrolled population more representative of the US MM population. For example, Black or African American people constitute ~20% of the US population affected by MM [35], and US MM-6 included 17.9% Black or African American patients, whereas the typical RCT includes ~10% of Black or African American patients [36]. Patients had a median age of 72.5 years, 61.4% of patients were frail, and 31.4% of patients had an ISS score of III; all of these factors likely contributed to difficulties in tolerating treatment [37]. These patient baseline characteristics are similar to those reported in other community-based MM studies [20, 38, 39]. Nonetheless, in MM-6, comparable OS, PFS, and safety data between patients aged ≥75 years and frail patients versus the ITT population suggests that iCT to IRd is feasible for elderly and frail patients, in line with previous reports of the safety profile of ixazomib among elderly [40, 41] and frail NDMM patients [41].
Notably, US MM-6 did not include a comparator arm, making it difficult to evaluate the impact of iCT to IRd versus staying on bortezomib-based treatment. To address this, a comparative effectiveness analysis of the US MM-6 cohort versus a real-world bortezomib-based cohort from the multi-center, prospective, observational INSIGHT MM study was conducted [42]. The analysis indicated increased ORR, duration of treatment, and 2-year PFS for the iCT to IRd arm versus the continuous bortezomib-based cohort [42]. Real-world outcomes cannot be directly compared to RCT data; however, assessment of outcomes reported here, in the context of RCTs and community-based studies, suggests a clinical benefit for patients who undergo iCT to IRd and a manageable safety profile, which may be more practical for patients treated in the community.
US MM-6 may encourage reconsideration of patient eligibility criteria, as well as the use of the clinical standard of care at a given medical center for disease management and follow-up. It might also inform whether community-based oncology centers and patients are important to include, alongside academic centers, in meeting new Food and Drug Administration enrollment guidelines on diversity, equity, and inclusion in RCTs [43].
While iCT has rarely been studied in an RCT setting, it allows rapid disease reduction via a short-term regimen of parenteral bortezomib-based therapy, followed by long-term tolerable consolidation with an all-oral regimen. US MM-6 has demonstrated potential for sequential deepening of response following iCT, without seriously impacting QoL. This is important among community-based patients, for whom many therapeutics demonstrate reduced effectiveness and tolerability compared with published RCT data, likely due to the inherent differences in patient demographics described in this report and patient-specific environmental factors typically unaccounted for in RCTs.
US MM-6 enrollment lasted from November 2017 to May 2021; during the COVID pandemic, enrollment continued more slowly and enrolled patients continued according to study protocol. Many clinical studies were suspended during this period due to lack of cancer center access, but the US MM-6 oral triplet regimen, along with ePRO capture and teleconsultations, were amenable to uninterrupted treatment. Various guidelines suggested that patients should be transitioned to oral regimens during this period to continue therapy [44,45,46,47].
Current iCT trials are underway to investigate additional ixazomib-based treatment combinations among patients with MM; these include an RCT (NCT03942224) and others among patients with RRMM (NCT03416374, NCT03763162). It is expected that iCT will prove useful for other therapeutic approaches where long-term consolidation is important, with a focus on tolerable TEAE management.
In conclusion, updated outcomes of the fully accrued study cohort from the US MM-6 phase 4 study indicate promising PFS and OS data for community-based NDMM patients who undergo iCT from bortezomib-based induction to IRd treatment. ORRs were elevated following iCT, while actigraphy and PRO results suggest no adverse impact or decline in activity or QoL with continued treatment, and safety data suggest IRd is generally well tolerated. Furthermore, iCT from parenteral bortezomib-based induction to all-oral IRd permits long-term PI-based therapy translating into improved efficacy and outcomes in these underserved patients who are elderly, comorbid, who may not have access to an RCT or are unable to travel to an academic center or treatment site.
Data availability
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available from the completed study within three months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization.
References
Mateos MV, Richardson PG, Dimopoulos MA, Palumbo A, Anderson KC, Shi H, et al. Effect of cumulative bortezomib dose on survival in multiple myeloma patients receiving bortezomib-melphalan-prednisone in the phase III VISTA study. Am J Hematol. 2015;90:314–9.
Jimenez-Zepeda VH, Duggan P, Neri P, Tay J, Bahlis NJ. Bortezomib-containing regimens (BCR) for the treatment of non-transplant eligible multiple myeloma. Ann Hematol. 2017;96:431–9.
Durie BG, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–27.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. New Engl J Med. 2008;359:906–17.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol. 2012;31:448–55.
Durie BG, Hoering A, Sexton R, Abidi MH, Epstein J, Rajkumar SV, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10:53.
Velcade® Prescribing Information. 2022. https://www.velcade.com/files/pdfs/VELCADE_PRESCRIBING_INFORMATION.pdf. Accessed 01 March 2023.
Richardson PG, Delforge M, Beksaç M, Wen P, Jongen J, Sezer O, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608.
Zweegman S, Engelhardt M, Larocca A. Elderly patients with multiple myeloma: towards a frailty approach? Curr Opin Oncol. 2017;29:315–21.
Plummer C, Driessen C, Szabo Z, Mateos M-V. Management of cardiovascular risk in patients with multiple myeloma. Blood Cancer J. 2019;9:26.
Terpos E, Mikhael J, Hajek R, Chari A, Zweegman S, Lee HC, et al. Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer J. 2021;11:40.
Richardson PG, San Miguel JF, Moreau P, Hajek R, Dimopoulos MA, Laubach JP, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8:1–12.
Bertamini L, Bertuglia G, Oliva S. Beyond Clinical Trials in Patients With Multiple Myeloma: A Critical Review of Real-World Results. Front Oncol. 2022;12:844779.
Ninlaro® Prescribing Information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208462lbl.pdf. Accessed 01 March 2023.
Ninlaro® Summary of Product Characteristics. 2016. https://www.ema.europa.eu/en/documents/product-information/ninlaro-epar-product-information_en.pdf. Accessed 01 March 2023.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. New Engl J Med. 2016;374:1621–34.
Facon T, Venner CP, Bahlis NJ, Offner F, White DJ, Karlin L, et al. Oral ixazomib, lenalidomide, and dexamethasone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood. 2021;137:3616–28.
Manda S, Yimer HA, Noga SJ, Girnius S, Yasenchak CA, Charu V, et al. Feasibility of Long-term Proteasome Inhibition in Multiple Myeloma by in-class Transition From Bortezomib to Ixazomib. Clin Lymphoma Myeloma Leuk. 2020;20:e910–e925.
Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587.
Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B, et al. Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. J Clin Oncol. 2015;33:3921–9.
Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503–12.
Facon T, Dimopoulos MA, Meuleman N, Belch A, Mohty M, Chen W-M, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2020;34:224–33.
Sully K, Trigg A, Bonner N, Moreno-Koehler A, Trennery C, Shah N, et al. Estimation of minimally important differences and responder definitions for EORTC QLQ‐MY20 scores in multiple myeloma patients. Eur J Haematol. 2019;103:500–9.
O’Donnell EK, Laubach JP, Yee AJ, Chen T, Huff CA, Basile FG, et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant‐ineligible multiple myeloma. Br J Haematol. 2018;182:222–30.
O’Donnell EK, Laubach JP, Yee AJ, Redd R, Huff CA, Basile F, et al. Updated results of a phase 2 study of modified lenalidomide, bortezomib, and dexamethasone (RVd-lite) in transplant-ineligible multiple myeloma. Blood. 2019;134:3178.
Lahuerta J-J, Paiva B, Vidriales M-B, Cordón L, Cedena M-T, Puig N, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900.
Naser AY, Ofori-Asenso R, Al Awawdeh S, Qadus S, Alwafi H, Liew D. Real world adherence to and persistence with oral oncolytics in multiple myeloma: A systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2022;22:760–37.
Hentzen S, Meirson T, Koehn K, Goodman AM, Chakraborty R, Prasad V, et al. Attrition and withdrawal amongst patients with multiple myeloma participating in randomized clinical trials: A systematic review. Blood. 2022;140:7950–1.
Richardson PG, Kumar SK, Masszi T, Grzasko N, Bahlis NJ, Hansson M, et al. Final overall survival analysis of the TOURMALINE-MM1 phase III trial of ixazomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2021;39:2430–42.
Coleman EA, Goodwin JA, Coon SK, Richards K, Enderlin C, Kennedy R, et al. Fatigue, sleep, pain, mood and performance status in patients with multiple myeloma. Cancer Nurs. 2011;34:219–27.
Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, et al. How many steps/day are enough? For adults. Int J Behav Nutr Phys Act. 2011;8:1–17.
Shah JJ, Abonour R, Gasparetto C, Hardin JW, Toomey K, Narang M, et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin Lymphoma Myeloma Leuk. 2017;17:575–83.e572.
Fiala M, Dukeman J, Stockerl-Goldstein K, Tomasson M, Wildes T, Vij R. The real-world characteristics and outcomes of newly diagnosed myeloma patients ineligible for clinical trials. Clin Lymphoma Myeloma Leuk. 2017;17:e55–e56.
Hungria VT, Lee HC, Abonour R, Rifkin RM, Terpos E, Leleu X, et al. Real-world (RW) multiple myeloma (MM) patients (Pts) remain under-represented in clinical trials based on standard laboratory parameters and baseline characteristics: analysis of over 3,000 Pts from the Insight MM Global, Prospective, Observational Study. Blood. 2019;134:1887.
Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD. 2020.
Turner BE, Steinberg JR, Weeks BT, Rodriguez F, Cullen MR. Race/ethnicity reporting and representation in US clinical trials: A cohort study. The Lancet Regional Health - Americas. 2022;11:100252.
Chari A, Romanus D, Palumbo A, Blazer M, Farrelly E, Raju A, et al. Randomized clinical trial representativeness and outcomes in real-world patients: comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20:8–17.e16.
Hájek R, Minařík J, Straub J, Pour L, Jungova A, Berdeja JG, et al. Ixazomib-lenalidomide-dexamethasone in routine clinical practice: effectiveness in relapsed/refractory multiple myeloma. Future Oncol. 2021;17:2499–512.
Li J, Bao L, Xia Z, Wang S, Zhou X, Ding K, et al. Ixazomib-based frontline therapy in patients with newly diagnosed multiple myeloma in real-life practice showed comparable efficacy and safety profile with those reported in clinical trial: a multi-center study. Ann Hematol. 2020;99:2589–98.
Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, et al. Ixazomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma: long-term follow-up including ixazomib maintenance. Leukemia. 2019;33:1736–46.
Bringhen S, Pour L, Benjamin R, Grosicki S, Min C-K, De Farias DLC, et al. Progression-free survival (PFS) benefit demonstrated and quality of life (QoL) maintained across age and frailty subgroups with the oral proteasome inhibitor (PI) ixazomib vs placebo as post-induction maintenance therapy in non-transplant newly diagnosed multiple myeloma (NDMM) patients (Pts): analysis of the TOURMALINE-MM4 phase 3 trial. Blood. 2020;136:30–31.
Rifkin RM, Costello CL, Birhiray RE, Kambhampati S, Richter J, Abonour R, et al. P947: COMPARATIVE EFFECTIVENESS OF ORAL IXAZOMIB-LENALIDOMIDE-DEXAMETHASONE (IRD) AFTER INITIAL BORTEZOMIB (V)-BASED INDUCTION VS PARENTERAL V-BASED THERAPY IN NEWLY DIAGNOSED MULTIPLE MYELOMA (NDMM). HemaSphere. 2022;6:837–8.
FDA. Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry - DRAFT GUIDANCE. 2022. https://www.fda.gov/media/157635/download. Accessed 15 March 2023.
Al Saleh AS, Sher T, Gertz MA. Multiple myeloma in the time of COVID-19. Acta Haematol. 2020;143:410–6.
Malard F, Mohty M. Management of patients with multiple myeloma during the COVID-19 pandemic. Lancet Haematol. 2020;7:e435–e437.
Hungria V, Garnica M. Crusoé EdQ, Magalhaes Filho RJPd, Martinez G, Bittencourt R, et al. Managing patients with multiple myeloma during the COVID-19 pandemic: recommendations from an expert panel-ABHH monoclonal gammopathies committe. Hematol Transfus Cell Ther. 2020;42:200–5.
You B, Ravaud A, Canivet A, Ganem G, Giraud P, Guimbaud R, et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21:619–21.
Acknowledgements
This study was funded by Takeda Development Center Americas, Inc. (TDCA), Lexington, MA, USA. The authors would like to thank all patients and their families, as well as all investigators for their valuable contributions to these studies. The authors would like to acknowledge Kim Tran, Kimberly Bogard, Jyoti Arora, and Alex Savell for their input and support in developing the manuscript. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Christian Jones, PhD, and Philippa Lloyd, BSc, of Ashfield MedComms, an Inizio Company, funded by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA and complied with the Good Publication Practice (GPP) guidelines (De Tora LM, et al. Ann Intern Med 2022;175:1298-304).
Author information
Authors and Affiliations
Contributions
RMR, SJN, SK, DC, PW, JR designed the research; RMR, SKG, SJN, REB, SK, SM, RML, HAY performed the research; RMR, SKG, SK, SM, RML, PW provided study material or patients; SK provided vital new reagents or analytical tools; RMR, SKG, SJN, REB, RML, EL contributed to data collection and assembly; RMR, SKG, SJN, REB, SK, RML, HAY, DC, EL, PW, JR contributed to data analysis/interpretation; EL performed the statistical analysis; RMR wrote the manuscript; RMR, SKG, SJN, REB, SK, SM, RML, HAY, DC, PW, JR reviewed and revised the manuscript; all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
RMR: Member of board of directors/advisory committee: Amgen, Bristol Myers Squibb (Celgene), Coherus, Fresenius-Kabi, Sanofi, Takeda, and Karyopharm; Current employee and equity holder: McKesson. SKG: Honoraria: BMS, Genentech, and GSK; Speakers bureau: BMS, Celgene, GSK, Takeda, and Beigene; Member of board of directors/advisory committee: Takeda. SJN: Current employee: Takeda. REB: Consultancy: Alexion, AbbVie, Novartis, Janssen, Pharmacyclics, and Puma; Honoraria: AbbVie, Novartis, Bayer, Seattle Genetics, Coheris, Kyte Pharma, Celgene, Helsin, and Amgen; Research funding: Puma, Takeda, and Incyte; Speakers bureau: Novartis, Janssen, Pharmacyclics, Puma, Takeda, Incyte, Amgen, Pfizer, BMS, Tessaro, AstraZeneca, Genomic Health, Sanofi, Clovis, Exelexis, and Lilly. SK: nothing to disclose. S.M.: Honoraria: Morphosys; Current equity holder: Genmab. RML: nothing to disclose. HAY: Speakers bureau: Karyopharm, AstraZeneca, Janssen, Beigene, GSK, Sanofi, Amgen, Pharmacyclics, and Takeda; Current stockholder: Karyopharm; Current employee: Texas Oncology. DC: Current employee: Takeda; Ownership of stock/shares with Takeda. EL: Current employee: Takeda. PW: Current employee: Takeda. JR: Consulting fees from Takeda and BMS/Celgene.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rifkin, R.M., Girnius, S.K., Noga, S.J. et al. In-class transition (iCT) of proteasome inhibitor-based therapy: a community approach to multiple myeloma management. Blood Cancer J. 13, 147 (2023). https://doi.org/10.1038/s41408-023-00912-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00912-9