Abstract
FLT3 is the most frequently mutated gene in acute myeloid leukemia (AML), with FLT3 internal tandem duplication (ITD) mutations being associated with a more aggressive clinical course. While two large, randomized clinical trials have shown a survival benefit with the frontline use of an oral FLT3 inhibitor (midostaurin or quizartinib) in patients with FLT3-mutated AML, the role of FLT3 inhibitors in older adults with newly diagnosed FLT3-mutated AML remains unclear. A definitive improvement in survival has not been observed in intensively treated patients over 60 years of age receiving frontline FLT3 inhibitors. Furthermore, many patients with FLT3-mutated AML are unsuitable for intensive chemotherapy due to age and/or comorbidities, and this population represents a particular unmet need. For these older patients who are unfit for intensive approaches, azacitidine + venetoclax is a new standard of care and is used by many clinicians irrespective of FLT3 mutation status. However, FLT3-ITD mutations confer resistance to venetoclax and are a well-established mechanism of relapse to lower-intensity venetoclax-based regimens, leading to short durations of remission and poor survival. Preclinical and clinical data suggest synergy between FLT3 inhibitors and venetoclax, providing rationale for their combination. Novel strategies to safely incorporate FLT3 inhibitors into the standard hypomethylating agent + venetoclax backbone are now being explored in this older, less fit population with newly diagnosed FLT3-mutated AML, with encouraging early results. Herein, we discuss the frontline use of FLT3 inhibitors in older adults with FLT3-mutated AML, including the potential role of FLT3 inhibitors in combination with intensive chemotherapy and as part of novel, lower-intensity doublet and triplet regimens in this older population.
Similar content being viewed by others
Introduction
Since 2017, there has been a remarkable expansion of new drug approvals for the treatment of acute myeloid leukemia (AML), both in the frontline and relapsed or refractory settings [1]. These consist of novel chemotherapeutics that are largely agnostic to specific molecular mutations, as well as genomically-targeted therapies, including small molecular inhibitors of FLT3, IDH1, and IDH2 [2]. In particular, great progress has been made in the treatment of older adults with AML who are unfit for intensive chemotherapy. These patients account for a substantial proportion of new AML cases and represent a unique clinical challenge due their poorer tolerance of conventional chemotherapy and a higher incidence of adverse-risk cytomolecular features, as compared with their younger counterparts [3,4,5]. Fortunately, outcomes for this older population have substantially improved with the clinical development of the oral Bcl-2 inhibitor venetoclax [1, 6,7,8,9,10]. Compared with azacitidine alone, the combination of azacitidine and venetoclax significantly increased response rates and overall survival (OS) in older adults with newly diagnosed AML, and represents the new standard of care for this population [11]. However, fms-like tyrosine kinase 3 (FLT3) mutations—particularly FLT3-internal tandem duplication (ITD)—are a major mechanism of resistance to venetoclax-based therapies [12, 13], and novel strategies to overcome this FLT3-mediated resistance are needed. While intensive chemotherapy plus a FLT3 inhibitor is now standard of care for younger, fit patients with newly diagnosed FLT3-mutated AML [14, 15], the role of frontline FLT3 inhibitors in older, unfit patients is not well-established nor is the optimal way to integrate these agents into lower-intensity frontline regimens. In this review, we discuss the impact of FLT3 mutations in older adults with AML and the historical outcomes of FLT3-mutated AML in this population. We review the role of upfront FLT3 inhibitors and specifically highlight the ongoing efforts to develop more effective frontline therapies for older adults with FLT3-mutated AML who are unfit for intensive chemotherapy, including novel triplet regimens that integrate FLT3 inhibitors into lower-intensity backbones.
Incidence of FLT3 mutations
FLT3 mutations are identified in approximately one-third of patients with newly diagnosed AML, are enriched in AML with a normal karyotype, and are frequently co-mutated with NPM1 and/or DNMT3A mutations [16,17,18,19,20]. FLT3-ITD mutations are identified in 20–25% of patients with newly diagnosed AML, whereas point mutations in the tyrosine kinase domain (TKD) are identified in 5–10%, approximately half of which occur at D835 in the activation loop [21, 22]. However, the incidence of FLT3 mutations varies across age groups, and they are relatively less common in older patients in AML [23]. In one study of 3525 adults with newly diagnosed AML, the incidence of FLT3-ITD mutations ranged from 23% in patients <45 years of age to 15% in those >70 years of age. Similarly, the incidence of FLT3-TKD mutations ranged from 9% to 4% across these age groups, respectively [24]. However, AML is largely a disease of older age with a median age at diagnosis of 69 years [25]. Thus, while FLT3 mutations are less common in older adults in relative terms, due to the higher incidence of AML in older patients, the population-based absolute incidence of FLT3-mutated AML actually increases with age, with patients >60 years of age representing approximately 60% of all patients with FLT3-ITD mutated AML (Fig. 1) [26]. These figures help to highlight the clinical burden of FLT3-mutated AML in this older population and the unmet clinical need that it represents.
While the proportion of AML cases harboring a FLT3-ITD mutation decreases with age, the absolute incidence of FLT3-ITD-mutated AML increases with age, driven by overall higher incidences of new AML cases in older patients. Data adapted from Nagel et al. 2017, Schneider et al. 2012, and SEER database [23,24,25].
Prognostic impact of FLT3 mutations in older adults with AML
FLT3-ITD mutations have been consistently shown across most studies to be associated with a more aggressive phenotype and worse survival in AML, while most studies have shown no—or only minimal—prognostic impact of FLT3-TKD mutations [19, 20, 27,28,29,30]. In patients with FLT3-ITD-mutated AML, a higher allelic ratio is associated with inferior outcomes, particularly when frontline FLT3 inhibitors are not used [31]. Most of these studies have been evaluated in younger adults with AML treated intensively, and there are relatively few studies evaluating the impact of FLT3 mutations in older patients. In this older population, the prognostic impact of FLT3 mutations has been less consistent. In one analysis of 243 patients >60 years of age with newly diagnosed AML with normal karyotype treated with intensive chemotherapy, the presence of a FLT3-ITD mutation was associated with shorter disease-free survival (DFS) (hazard ratio [HR] 2.1 [95% CI, 1.36–2.23]; P < 0.001) and OS (HR 1.97 [95% CI, 1.45–2.68]; P < 0.001) in patients 60–69 years of age, but not in those patients who were >70 years of age [32]. In a larger study of patients >60 years of age treated with conventional 7 + 3 induction, FLT3-ITD mutations were independently associated with worse OS in a multivariate analysis, with high FLT3-ITD allelic ratios associated with a higher risk of death than were lower allelic ratios (HR 1.85 [95% CI, 1.31–2.62]; P = 0.0005 and HR 3.51 [95% CI, 2.03–6.08]; P < 0.0001, respectively) [33]. However, several other studies failed to show a negative impact of FLT3-ITD mutations in intensively treated older adults with newly diagnosed AML [28, 34, 35]. The reasons for the discrepant findings in these studies of intensively treated older adults is unclear but may be due at least in part to smaller patient numbers and underpowered analyses in some of the negative studies.
Studies of FLT3 mutations in older patients treated with lower-intensity therapies are even more limited. A subgroup analysis of the AZA-AML-001 study—which randomized patients >65 years of age with newly diagnosed AML to azacitidine versus conventional care regimens (CCR)—found no difference in outcomes of FLT3-mutated versus FLT3 wild type patients, although only 18 patients were included in this analysis, limiting its power [36]. In a pooled analysis of patients treated in the VIALE-A trial and the preceding phase 1b study of azacitidine + venetoclax, there was no clear difference in outcomes of patients with FLT3-mutated AML (including both ITD and TKD) versus FLT3 wild type AML when treated with the venetoclax-based combination [13]. However, when further stratified by type of FLT3 mutation, those with FLT3-ITD-mutated AML had particularly poor outcomes (median OS 9.9 months), which was shorter than the FLT3 wild type population (median OS 14.7 months) and the FLT3-TKD-mutated population (median OS 19.2 months). Patients who were FLT3-ITD-mutated but NPM1 wild type AML had the shortest duration of remission among the subgroups evaluated (median 10.1 months versus 18.4 months for FLT3 wild type AML). Together, these data suggest that FLT3-ITD mutations may be a poor prognostic factor even when azacitidine + venetoclax is given.
Frontline intensive chemotherapy + FLT3 inhibitor in FLT3-mutated AML
Outcomes in younger adults
The combination of a FLT3 inhibitor with frontline intensive chemotherapy is the standard of care for fit adults with newly diagnosed FLT3-mutated AML, based on positive data from the RATIFY study that evaluated midostaurin—and, more recently, from the QuANTUM-First study evaluating quizartinib [14, 37]. The RATIFY study was a global, randomized phase III study that randomized 717 adults <60 years of age with newly diagnosed FLT3-mutated AML (either ITD or TKD) to standard chemotherapy (i.e. induction with “7 + 3” chemotherapy and high-dose cytarabine consolidation) plus either midostaurin (50 mg orally twice daily) or placebo. Patients also received 1-year of maintenance with midostaurin or placebo. Midostaurin did not significantly improve the complete remission (CR) rate (59% versus 54% in the placebo group; P = 0.15) but was associated with a significant improvement in median OS (74.7 months versus 25.6 months; HR for risk of death 0.78 [95% CI, 0.63-0.96]; P = 0.009). The survival benefit of midostaurin was seen in both FLT3-ITD and FLT3-TKD mutations, including in patients with either high or low FLT3-ITD allelic ratios. The greatest benefit to midostaurin therapy was observed in patients who underwent allogeneic hematopoietic stem cell transplantation (HSCT) in first remission [15]. These data provided the first definitive evidence for the benefit of frontline FLT3 inhibitors in FLT3-mutated AML and led to the Food and Drug Administration (FDA) approval of midostaurin in combination with standard induction and consolidation chemotherapy in adults with newly diagnosed FLT3-mutated AML, although the FDA did not approve its use as maintenance therapy. Despite the impressive survival benefit of midostaurin in the RATIFY study, it is important to note that the study did not enroll patients 60 years of age and older, and so the generalizability of these data to this older population is uncertain.
The randomized phase III QuANTUM-First study later evaluated the same backbone chemotherapy regimen in combination with quizartinib (40 mg orally daily) or placebo in adults up to 75 years of age with FLT3-ITD-mutated AML who were deemed suitable for intensive chemotherapy [37]. Five-hundred thirty-nine patients were randomized; the median age was 56 years, and 40% of subjects were between 60 and 75 years of age—an older population not included in the RATIFY study. The quizartinib-containing arm had significantly superior survival (median OS: 31.9 months versus 15.1 months in the placebo arm; HR 0.78, [95% CI, 0.62–0.98]; P = 0.032), which was driven largely by lower relapse rates (3-year cumulative incidence of relapse 34% [95% CI, 26%–42%] and 45% [95% CI, 37%–53%], respectively). Interestingly, in a post hoc analysis, the OS benefit of quizartinib appeared to be limited to patients <60 years of age (median OS not reached versus 23.0 months; HR 0.68 [95% CI, 0.49-0.95]) and was not observed in patients ≥60 years of age (median OS 17.5 months versus 14.2 months; HR 0.91 [95% CI, 0.66–1.26]). In July 2023, quizartinib was approved by the FDA for use in combination with standard induction and consolidation chemotherapy in patients with newly diagnosed FLT3-ITD-mutated AML; importantly, it was also approved as monotherapy maintenance for up to 3 years in patients who do not undergo subsequent HSCT. Together, the RATIFY and QuANTUM-First studies provide important clinical data showing that upfront integration of FLT3 inhibitors in patients with newly diagnosed FLT3-mutated AML improves outcomes. Specifically, both studies show that the combination of a FLT3 inhibitor with intensive chemotherapy results in improved OS in patients up to 60 years of age, although the data supporting this approach in older adults are still limited.
Outcomes in older adults
Historically, intensive chemotherapy was the only treatment option for newly diagnosed AML with a reasonable chance of durable remission, and therefore patients up to approximately 75 years of age were routinely recommended to receive intensive AML-directed therapy. However, appropriate selection of patients for intensive chemotherapy is imperative, particularly for patients ≥60 years of age, a group that can have unacceptably high rates of morbidity and mortality with this approach [38].
Patients >60 years of age were excluded from the RATIFY study [14], and therefore randomized data supporting the use of frontline midostaurin in this population is lacking. In a single-arm phase II study from the German-Austrian AML Study Group (AMLSG), adults up to 70 years of age with newly diagnosed FLT3-ITD-mutated AML received intensive chemotherapy plus midostaurin, followed by allogeneic HSCT and 1 year of midostaurin maintenance [39]. The findings were then compared to a historical cohort of patients from previous AMLSG trials using propensity score weighting and covariate adjustment. Among the 128 patients 61–70 years of age who were treated, the CR/CRi rate was 72.4% and 38% underwent allogeneic HSCT in first remission. In this older population, the median event-free survival (EFS) was 11.7 months (versus 2.53 months in the control; HR 0.42 [95% CI, 0.29-0.59]; P < 0.001) and the median OS was 22.7 months (versus 8.4 months in the control; HR 0.47 [95% CI, 0.33-0.67]; P < 0.001), suggesting benefit to adding midostaurin in this group.
As previously described, while older patients who were deemed suitable for intensive chemotherapy were included in the QuANTUM-First study, a survival benefit was not observed in the subgroup of patients. While the precise reasons for the lack of benefit in the older population is not entirely clear, the quizartinib arm had a higher rate of death in the first 3 months of therapy in this group, which may have at least in part offset a potential survival benefit. Until further data are available, these findings provide some skepticism for the use of upfront intensive chemotherapy plus a FLT3 inhibitor in older adults with FLT3-mutated AML. Data from these intensive chemotherapy-based prospective studies in older adults, as well as data from prospective studies of lower-intensity regimens are summarized in Table 1.
Lower-Intensity Doublets with FLT3 Inhibitors in FLT3-Mutated AML
As many older adults are not suitable candidates for intensive chemotherapy, several clinical trials have investigated lower-intensity therapies incorporating a FLT3 inhibitor in this population. Based on encouraging data from a phase I/II study of azacitidine + sorafenib in relapsed/refractory FLT3-mutated AML [40], this combination was then evaluated as frontline therapy for patients >60 years of age with FLT3-mutated AML [41]. Twenty-seven patients were treated with a median age of 74 years (range, 61–86 years). The overall response rate was 78% (including 26% with CR and 45% with CR with incomplete platelet recovery [CRp]). The median duration of response was 14.5 months, and median OS was 8.3 months.
Another study evaluated azacitidine or low-dose cytarabine (LDAC) in combination with quizartinib in patients with FLT3-ITD-mutated AML, including patients with relapsed/refractory disease or newly diagnosed patients who were >60 years of age and/or considered to be unfit for intensive chemotherapy [42]. Thirty-four newly diagnosed patients were treated, with a median age of 65 years (range, 58–82 years); 15 patients received azacitidine + quizartinib and 19 received LDAC + quizartinib. The composite CR (CRc) rate was 79%, and the median relapse-free survival (RFS) and OS were 8.0 months and 12.4 months, respectively. Together with the study of azacitidine + sorafenib, these 2 studies show that very high rates of response can be achieved with lower-intensity FLT3 inhibitor doublets in newly diagnosed FLT3-mutated AML, although survival remains modest with these approaches.
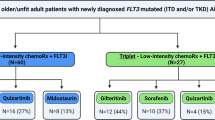
Building upon the encouraging data with a quizartinib-based doublet, a subsequent study randomized older adults with newly diagnosed AML (irrespective of FLT3 mutation status) to LDAC with or without quizartinib [43]. A total of 209 patients were randomized (median age 77 years), including 27 patients (16%) with a FLT3-ITD mutation and 6 patients (3%) with a FLT3-TKD mutation. In the global population there was no difference in response rates or survival outcomes. However, in a subgroup analysis of the 27 patients with a FLT3-ITD mutation, the quizartinib-containing arm was associated with higher rates of CR/CRi (38% versus 0%; P = 0.05) and superior OS (median OS 13.7 months versus 4.2 months; P = 0.04). While the number of patients in this subgroup analysis are relatively small, the findings are suggestive of a benefit to the addition of a FLT3 inhibitor to a lower-intensity backbone in older adults with newly diagnosed FLT3-mutated AML who are unfit for intensive chemotherapy.
Gilteritinib is a potent FLT3 inhibitor that is FDA-approved for the treatment of relapsed/refractory FLT3-mutated AML based on increased response rates and improved survival compared with chemotherapy alone observed in the randomized phase III ADMIRAL study [44]. To evaluate the possible benefit of gilteritinib in the frontline setting, the LACEWING study randomized patients >65 years of age who were unsuitable for intensive chemotherapy in a 2:1 allocation to either azacitidine + gilteritinib or azacitidine alone [45]. One hundred twenty-three patients were randomized (74 to azacitidine plus gilteritinib and 49 to azacitidine), and the median ages in each cohort were 78 years (range, 59–90 years) and 76 years (range, 61–88 years), respectively. The majority of patients in each group had a FLT3-ITD mutation, with or without a concomitant TKD mutation (81% and 86%, respectively). The azacitidine + gilteritinib doublet was associated with a significantly higher rate of CRc (58.1% versus 26.5%; P < 0.001), with an improvement in CRc rates that was particularly notable in patients with a FLT3-ITD allelic ratio >0.5 (71.4% versus 20.8%; P = 0.003). However, despite the increase in CRc rates with the doublet, there was no difference in OS (the primary endpoint of the study) between the 2 arms. The median OS was 9.8 and 8.9 months, respectively (HR 0.92 [95% CI, 0.53–1.56]; P = 0.75), and the study was terminated early due to futility. The lack of survival benefit may be explained—at least in part—by different rates of subsequent AML-directed treatment after discontinuing protocol therapy. Fifteen patients (20.3%) in the azacitidine + gilteritinib arm received subsequent therapy, 3 of whom received a subsequent FLT3 inhibitor, compared with 22 patients (44.9%) in the azacitidine arm, 14 of whom received a FLT3 inhibitor. However, it is notable that EFS was also not significantly different between the 2 arms, and therefore the lack of survival benefit cannot be explained entirely by differences in post-protocol therapy. Ultimately, the LACEWING study failed to show a significant benefit to the addition of gilteritinib to azacitidine in older patients with newly diagnosed FLT3-mutated AML and suggests that use of these doublets may not be optimal in this population.
Lower-intensity triplets with FLT3 inhibitors in FLT3-mutated AML
Outcomes of FLT3-mutated AML with hypomethylating agent (HMA)/LDAC + venetoclax
The outcomes of older adults with AML have greatly improved with the clinical development of venetoclax-based lower-intensity therapies. In the randomized phase III VIALE-A study, patients ≥75 years of age or unsuitable for intensive chemotherapy with newly diagnosed AML who received azacitidine + venetoclax had improved response rates (CRc rate: 66.4% versus 28.3%; P < 0.001) and survival (median OS: 14.7 months versus 9.6 months; P < 0.001), as compared with azacitidine alone [11]. In a similar older population, the combination of LDAC + venetoclax (as compared with LDAC alone) also significantly improved response rates and OS [46]. These regimens now represent new standards of care in this older, unfit population with newly diagnosed AML. However, despite the substantial progress that these new venetoclax-based therapies represent, the outcomes of patients with FLT3-mutated AML—as well as some other high-risk subgroups—remain suboptimal. For example, in a study of 81 patients who received either azacitidine or LDAC in combination with venetoclax, expansion of pre-existing FLT3 clones or development of new FLT3 mutations were commonly seen at the time of relapse [12]. In vitro studies have shown that FLT3-ITD mutations lead to overexpression of BCL-xL and MCL-1, both anti-apoptotic proteins shown to contribute to venetoclax resistance [47,48,49,50,51,52,53]. While response rates with HMA/LDAC + venetoclax are similar in patients with FLT3-mutated and FLT3 wild type AML, the clonal expansion of FLT3-mutated cells in response to these therapies suggest that these mutations are an important mechanism of relapse.
Given the survival benefit observed with azacitidine + venetoclax, many clinicians offer this regimen to older, unfit adults with newly diagnosed AML, regardless of FLT3 mutation status. A pooled analysis of patients with FLT3-mutated AML treated on prospective clinical trials with azacitidine + venetoclax or azacitidine alone was conducted to clarify the clinical outcomes in this population with these commonly used lower-intensity regimens [13]. In this analysis, 42 patients with FLT3-mutated AML received azacitidine + venetoclax, and 22 patients had received azacitidine alone (all of whom were treated on the control arm of the VIALE-A study). Across both groups, 41 patients had a FLT3-ITD mutation alone, 21 had a FLT3-TKD mutation alone, and 2 patients had both. This subgroup analysis confirmed that azacitidine + venetoclax improves response rates in patients with FLT3-mutated AML (CRc rates: 67% versus 36% with azacitidine alone). The combination regimen was also associated with numerically superior OS (median OS: 12.5 months versus 8.6 months with azacitidine alone). Among patients treated with azacitidine + venetoclax, there was no difference in OS between those FLT3-mutated or FLT3 wild type AML. However, there was evidence of differential outcomes between the different types of FLT3 mutations, which may have confounded this analysis. The CRc rates for patients with a FLT3-ITD or FLT3-TKD mutation were 63% and 77%, respectively. The median OS in patients with FLT3-TKD-mutated AML was 19.2 months, compared with only 9.9 months in those with FLT3-ITD-mutated AML. This relatively poor survival highlights that effective treatment of FLT3-ITD-mutated AML represents a particular unmet need even in the era of venetoclax-based lower-intensity therapies.
Preclinical and clinical rationales for venetoclax + FLT3 inhibitor combinations
FLT3-ITD mutations have been shown preclinically to reduce BCL-2 dependence of AML cells and to lead to upregulation of BCL-xL and MCL-1, antiapoptotic proteins that are established to impart resistance to venetoclax [47,48,49,50,51,52,53]. Several studies have also shown that various FLT3 inhibitors (including midostaurin, quizartinib, and gilteritinib) increase BCL-2 dependence and reduce expression of BCL-xL and MCL-1, thereby synergistically inducing apoptosis and sensitizing FLT3-mutated AML cells to venetoclax [48, 51,52,53]. Mechanisms of FLT3-mediated resistance to venetoclax and synergy between venetoclax and FLT3 inhibitors that may lead to more durable responses in FLT3-mutated AML are highlighted in Fig. 2. Interestingly, similar synergy of venetoclax and gilteritinib has also been reported in FLT3 wild type cell lines [47]. Gilteritinib leads to proteasomal degradation of MCL-1 and increased venetoclax sensitivity, and thus, the combination of venetoclax and gilteritinib may have a possible clinical role even in non-FLT3-mutated AML.
When treated with a doublet regimen of HMA plus venetoclax (top panel), a population of the FLT3-mutated subclone persists and contributes to relapse. Sequential therapy with gilteritinib is non-curative even in responding patients, and eventually resistance mechanisms contribute to relapse. A triplet regimen of HMA plus venetoclax plus a FLT3 inhibitor (bottom panel) has added synergy that may lead to longer, more durable responses, and possibly cure. The addition of a FLT3 inhibitor not only targets the FLT3-mutated clone, but some FLT3 inhibitors have been shown to increase the sensitivity of leukemia cells to gilteritinib by promoting a more pro-apoptotic phenotype.
These preclinical observations of synergistic activity of venetoclax and FLT3 inhibition were subsequently evaluated in a phase 1b clinical trial of venetoclax + gilteritinib in patients with relapsed/refractory FLT3-mutated AML [54]. Among 56 patients with FLT3-mutated AML, 47 patients (84%) had a FLT3-ITD mutation (either alone or in combination with a TKD mutation). The modified CRc rate (including CR, CRi, CRp, and morphologic leukemia-free state [MLFS]) was 75%, and the median OS was 10.0 months. While it is challenging to compare across clinical trials, the response rates compare favorably to the ADMIRAL trial, where the CRc rate was 54.3%. The higher response rates with venetoclax + gilteritinib were observed despite this study enrolling more heavily pretreated patients than the ADMIRAL study, including 36 patients (64%) with prior FLT3 inhibitor exposure (versus only 13% in ADMIRAL) and 38% of patients having received ≥3 lines of therapy (whereas ADMIRAL only enrolled primary refractory or first salvage patients). This study therefore provides clinical rationale for the further development of venetoclax and FLT3 inhibitor combinations in FLT3-mutated AML.
Clinical experience with HMA plus venetoclax plus FLT3 inhibitor triplets
Building upon the efficacy of the HMA + venetoclax regimen in patients with newly diagnosed AML, several studies are now evaluating lower-intensity therapy + venetoclax + FLT3 inhibitor triplets in patients with newly diagnosed FLT3-mutated AML. In a single-center retrospective analysis, 87 patients with newly diagnosed FLT3-mutated AML were treated with either triplet therapy (n = 27) or doublet therapy with a lower-intensity therapy plus a FLT3 inhibitor, without venetoclax (n = 60) [55]. Patients in the triplet cohort were more likely to have received gilteritinib (44%) and those in the doublet group were more likely to have received sorafenib (60%). Triplet therapy resulted in significantly higher rates of CR (67% versus 38%; P < 0.01) and CR/CRi (93% versus 70%), which translated to superior OS with the triplets (median OS: not reached versus 9.5 months; P < 0.01), a survival benefit that was confirmed on multivariate analysis (HR 3.2 [95% CI, 1.2–9.2]; P = 0.02).
Subsequent prospective studies have suggested benefits of these triplet regimens, as compared with historical expectations with lower-intensity therapy plus either venetoclax or a FLT3 inhibitor. In a phase II study of a 10-day regimen of decitabine + venetoclax in older/unfit adults with newly diagnosed AML, a subgroup of patients with FLT3 mutations were enrolled, for whom the treating physician could add a FLT3 inhibitor [56]. Twelve patients with newly diagnosed FLT3-mutated AML were treated (8 with FLT3-ITD alone, 3 with FLT3-TKD, and 1 with both). The FLT3 inhibitor added was sorafenib in 5 patients, gilteritinib in 5 patients, and midostaurin in 2 patients. The CRc rate (CR + CRp + CRi) was 92%, and the median OS was not reached after a median follow-up of 14.5 months. Early data from an ongoing study of decitabine, venetoclax, and quizartinib have also been reported [57]. Five patients with newly diagnosed FLT3-ITD-mutated AML have been treated, all of whom responded (2 CR and 3 CRi). In both studies, no early mortality was observed, suggesting that triplet regimens can be delivered safely in this older population.
The triplet combination of azacitidine, venetoclax, and gilteritinib has also been evaluated in older adults and those unfit for intensive chemotherapy with newly diagnosed FLT3-mutated AML [58]. This is the largest prospective study to date evaluating a triplet approach as frontline therapy. Thirty patients were treated with a median age of 71 years (range, 18–86 years); 22 patients (73%) had a FLT3-ITD mutation, and 8 patients (27%) had a FLT3-TKD mutation. A gilteritinib dose of 80 mg daily was used in the combination based on safety established from a preceding phase I study in relapsed/refractory FLT3-mutated AML. Increased myelosuppression was observed with the addition of gilteritinib to the azacitidine + venetoclax backbone, and therefore a bone marrow was performed on day 14 of cycle 1, and the gilteritinib and venetoclax were held to allow for count recovery if it showed marrow remission or aplasia. In consolidation, azacitidine was given for 5 days and venetoclax for 7 days to minimize prolonged myelosuppression. With these mitigation strategies in place, the modified CRc rate was 100%, with 27 patients (90%) achieving CR. With a median follow-up of 17 months, the 1-year OS and 2-year OS rates were 84% and 71%, respectively. These results appear to compare favorably to historical expectations with doublet regimens in a similar older population, including with azacitidine + venetoclax, where the CRc rate was 67% and the 2-year OS in patients with FLT3-mutated AML was 20–40%. Thus, using the appropriate mitigation strategies to prevent excessive myelosuppression, the triplet regimen of azacitidine, venetoclax, and gilteritinib can be delivered safely and represents a highly effective treatment option for older or unfit adults with newly diagnosed FLT3-mutated AML.
Areas of uncertainty and future directions
The treatment of FLT3-mutated AML in older adults who are unfit for intensive chemotherapy represents a particular clinical challenge, given the lack of an approved FLT3 inhibitor for use in this setting. FLT3 mutations (particularly FLT3-ITD mutations) are associated with a more aggressive clinical course and resistance to commonly used regimens in this population, including an HMA + venetoclax. Triplet regimens of an HMA, venetoclax, and a FLT3 inhibitor have yielded promising outcomes; however, many questions still remain. Despite the encouraging response and early survival data with these regimens, the negative results from the randomized LACEWING study temper enthusiasm somewhat [45]. Thus, randomized studies and longer duration of follow-up of these ongoing single-arm studies will be needed to definitively confirm the benefit of this approach. The optimal dosing of these combinations is also uncertain. The addition of a FLT3 inhibitor to an HMA + venetoclax backbone appears to increase myelosuppression, prompting attenuation of the HMA and venetoclax dosing. While the addition of the FLT3 inhibitor may suppress the FLT3-containing subclones, it is possible that reducing doses of the other agents could predispose to FLT3-negative relapses, which have been reported to account for 40-50% of relapses with frontline regimens incorporating FLT3 inhibitors [59]. A follow-up phase I/II multicenter, randomized dose-ranging and expansion study of azacitidine, venetoclax, and gilteritinib in newly diagnosed FLT3-mutated AML will attempt to identify the optimal doses of these agents when given in combination (NCT05520567). The best FLT3 inhibitor use in the frontline setting is also not clear, and randomized studies comparing intensive chemotherapy plus midostaurin to either gilteritinib (NCT04027309) or crenolanib (NCT03258931) in younger, fit patients with newly diagnosed FLT3-mutated AML are ongoing. The findings from these studies could also inform future approaches in older and less fit patients.
Finally, the added benefit of FLT3 inhibition in patients with very low-burden FLT3 mutations and/or those with FLT3-TKD-mutated AML is not well-established in older patients. The eligibility cutoffs in the RATIFY and QuANTUM-First studies were a FLT3 allelic ratio ≥0.03 and a FLT3 variant allelic frequency ≥0.05, respectively, and thus randomized data supporting the use of FLT3 inhibitors in the context of very low-burden FLT3-mutated AML are lacking. In one retrospective analysis of patients (all ages) with newly diagnosed FLT3-mutated AML (median allelic frequency 0.03 [range, 0.01-0.09]), the best outcomes were observed in those who received a frontline FLT3 inhibitor and underwent subsequent HSCT, suggesting that this is an effective approach for fit patients with low-burden FLT3 mutations [60]. How these data might translate to older patients with low-burden FLT3 mutations who are not candidates for HSCT remains uncertain. For FLT3-TKD-mutated AML in younger, fit adults, the RATIFY study showed that this group benefitted from the addition of midostaurin; however, it is unclear whether this applies in the context of lower-intensity venetoclax-based regimens in older adults. While FLT3-ITD mutations are clearly important mechanisms of resistance to an HMA + venetoclax, these regimens result in relatively favorable outcomes in patients with FLT3-TKD-mutated AML. Thus, it remains unclear whether the potential added toxicity of a FLT3 inhibitor and the need to reduce doses of the HMA and venetoclax in order to deliver the triplet combination will ultimately improve outcomes for these patients, as compared with use of azacitidine + venetoclax alone.
References
Short NJ, Konopleva M, Kadia TM, Borthakur G, Ravandi F, DiNardo CD, et al. Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov. 2020;10:506–25.
Dohner H, Wei AH, Lowenberg B. Towards precision medicine for AML. Nat Rev Clin Oncol. 2021;18:577–90.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5.
Tsai CH, Hou HA, Tang JL, Liu CY, Lin CC, Chou WC, et al. Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia. 2016;30:1485–92.
Kantarjian H, Ravandi F, O’Brien S, Cortes J, Faderl S, Garcia-Manero G, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–9.
Sekeres MA, Guyatt G, Abel G, Alibhai S, Altman JK, Buckstein R, et al. American society of hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4:3528–49.
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–9.
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol : Off J Am Soc Clin Oncol. 2012;30:2670–7.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135:2137–45.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl J Med. 2020;383:617–29.
DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135:791–803.
Konopleva M, Thirman MJ, Pratz KW, Garcia JS, Recher C, Pullarkat V, et al. Impact of FLT3 mutation on outcomes after venetoclax and azacitidine for patients with treatment-naive acute myeloid leukemia. Clin Cancer Res. 2022;28:2744–52.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl J Med. 2017;377:454–64.
Larson RA, Mandrekar SJ, Huebner LJ, Sanford BL, Laumann K, Geyer S, et al. Midostaurin reduces relapse in FLT3-mutant acute myeloid leukemia: the Alliance CALGB 10603/RATIFY trial. Leukemia. 2021;35:2539–51.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl J Med. 2016;374:2209–21.
Cancer Genome Atlas Research Network; Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New Engl J Med. 2013;368:2059–74.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl J Med. 2012;366:1079–89.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66.
Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–8.
Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–9.
Schneider F, Hoster E, Schneider S, Dufour A, Benthaus T, Kakadia PM, et al. Age-dependent frequencies of NPM1 mutations and FLT3-ITD in patients with normal karyotype AML (NK-AML). Ann Hematol. 2012;91:9–18.
Nagel G, Weber D, Fromm E, Erhardt S, Lubbert M, Fiedler W, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol. 2017;96:1993–2003.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Plus Limited-Field Data, 21 Registries, Nov 2019 Sub (2000-2017) - Linked To County Attributes - Total U.S., 1969-2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program [Internet]. Released April 2022, based on the November 2021 submission.
Bacher U, Kern W, Schnittger S, Hiddemann W, Haferlach T, Schoch C. Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica. 2005;90:1502–10.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9.
Ferrara F, Criscuolo C, Riccardi C, Izzo T, Pedata M, Copia C, et al. FLT3 mutations have no prognostic impact in elderly patients with acute myeloid leukemia and normal karyotype. Am J Hematol. 2009;84:532–5.
Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters–an analysis of 3082 patients. Blood. 2008;111:2527–37.
Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–70.
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124:3441–9.
Whitman SP, Maharry K, Radmacher MD, Becker H, Mrozek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:3622–6.
Itzykson R, Fournier E, Berthon C, Rollig C, Braun T, Marceau-Renaut A, et al. Genetic identification of patients with AML older than 60 years achieving long-term survival with intensive chemotherapy. Blood. 2021;138:507–19.
Prassek VV, Rothenberg-Thurley M, Sauerland MC, Herold T, Janke H, Ksienzyk B, et al. Genetics of acute myeloid leukemia in the elderly: mutation spectrum and clinical impact in intensively treated patients aged 75 years or older. Haematologica. 2018;103:1853–61.
Juliusson G, Jadersten M, Deneberg S, Lehmann S, Mollgard L, Wennstrom L, et al. The prognostic impact of FLT3-ITD and NPM1 mutation in adult AML is age-dependent in the population-based setting. Blood Adv. 2020;4:1094–101.
Dohner H, Dolnik A, Tang L, Seymour JF, Minden MD, Stone RM, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care. Leukemia. 2018;32:2546–57.
Erba HP, Montesinos P, Kim HJ, Patkowska E, Vrhovac R, Zak P, et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1571–83.
Short NJ, Kantarjian H. Choosing between intensive and less intensive front-line treatment approaches for older patients with newly diagnosed acute myeloid leukaemia. Lancet Haematol. 2022;9:e535–e45.
Dohner H, Weber D, Krzykalla J, Fiedler W, Wulf G, Salih H, et al. Midostaurin plus intensive chemotherapy for younger and older patients with AML and FLT3 internal tandem duplications. Blood Adv. 2022;6:5345–55.
Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121:4655–62.
Ohanian M, Garcia-Manero G, Levis M, Jabbour E, Daver N, Borthakur G, et al. Sorafenib Combined with 5-azacytidine in Older Patients with Untreated FLT3-ITD Mutated Acute Myeloid Leukemia. Am J Hematol. 2018;93:1136–41.
Swaminathan M, Kantarjian HM, Levis M, Guerra V, Borthakur G, Alvarado Y, et al. A phase I/II study of the combination of quizartinib with azacitidine or low-dose cytarabine for the treatment of patients with acute myeloid leukemia and myelodysplastic syndrome. Haematologica. 2021;106:2121–30.
Dennis M, Thomas IF, Ariti C, Upton L, Burnett AK, Gilkes A, et al. Randomized evaluation of quizartinib and low-dose ara-C vs low-dose ara-C in older acute myeloid leukemia patients. Blood Adv. 2021;5:5621–5.
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-Mutated AML. N. Engl J Med. 2019;381:1728–40.
Wang ES, Montesinos P, Minden MD, Lee JH, Heuser M, Naoe T, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140:1845–57.
Wei AH, Strickland SA Jr., Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II Study. J Clin Oncol : Off J Am Soc Clin Oncol. 2019;37:1277–84.
Janssen M, Schmidt C, Bruch PM, Blank MF, Rohde C, Waclawiczek A, et al. Venetoclax synergizes with gilteritinib in FLT3 wild-type high-risk acute myeloid leukemia by suppressing MCL-1. Blood. 2022;140:2594–610.
Zhu R, Li L, Nguyen B, Seo J, Wu M, Seale T, et al. FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation. Signal Transduct Target Ther. 2021;6:186.
Choudhary GS, Al-Harbi S, Mazumder S, Hill BT, Smith MR, Bodo J, et al. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015;6:e1593.
Lin KH, Winter PS, Xie A, Roth C, Martz CA, Stein EM, et al. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci Rep. 2016;6:27696.
Singh Mali R, Zhang Q, DeFilippis RA, Cavazos A, Kuruvilla VM, Raman J, et al. Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models. Haematologica. 2021;106:1034–46.
Brinton LT, Zhang P, Williams K, Canfield D, Orwick S, Sher S, et al. Synergistic effect of BCL2 and FLT3 co-inhibition in acute myeloid leukemia. J Hematol Oncol. 2020;13:139.
Ma J, Zhao S, Qiao X, Knight T, Edwards H, Polin L, et al. Inhibition of Bcl-2 synergistically enhances the antileukemic activity of midostaurin and gilteritinib in preclinical models of FLT3-mutated acute myeloid leukemia. Clin Cancer Res. 2019;25:6815–26.
Daver N, Perl AE, Maly J, Levis M, Ritchie E, Litzow M, et al. Venetoclax plus gilteritinib for FLT3-mutated relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2022;40:4048–59.
Yilmaz M, Kantarjian H, Short NJ, Reville P, Konopleva M, Kadia T, et al. Hypomethylating agent and venetoclax with FLT3 inhibitor “triplet” therapy in older/unfit patients with FLT3 mutated AML. Blood Cancer J. 2022;12:77.
Maiti A, DiNardo CD, Daver NG, Rausch CR, Ravandi F, Kadia TM, et al. Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood. Cancer J. 2021;11:25.
Yilmaz M, Muftuoglu M, Kantarjian H, DiNardo C, Kadia T, Borthakur G, et al. S127: quizartinib with decitabine and venetoclax (triplet) is active in patients with flt3-itd mutated acute myeloid leukemia - a phase I/II study. HemaSphere. 2022;6.
Short N, DiNardo CD, Daver N, Macaron W, Yilmaz M, Borthakur G, et al. Updated results from a Phase I/II study of the triplet combination of azacitidine, venetoclax and gilteritinib for patients with FLT3-mutated acute myeloid leukemia. Blood. 2022;140:2007–9.
Schmalbrock LK, Dolnik A, Cocciardi S, Strang E, Theis F, Jahn N, et al. Clonal evolution of acute myeloid leukemia with FLT3-ITD mutation under treatment with midostaurin. Blood. 2021;137:3093–104.
Yilmaz M, Daver N, Borthakur G, Kadia T, DiNardo C, Kanagal-Shamanna R, et al. FLT3 inhibitor based induction and allogeneic stem cell transplant in complete remission 1 improve outcomes in patients with newly diagnosed Acute Myeloid Leukemia with very low FLT3 allelic burden. Am J Hematol. 2021;96:E275–e9.
Sierra J, Montesinos P, Thomas X, Griškevičius L, Cluzeau T, Caillot D, et al. Phase 3b study assessing the safety and efficacy of midostaurin in younger and older patients with newly diagnosed, FLT3-mutated acute myeloid leukemia (AML) who are eligible for 7+3 or 5+2 chemotherapy. Blood. 2020;136:23–4. Supplement 1.
Funding
Supported by an MD Anderson Cancer Center Support Grant (CA016672) and SPORE.
Author information
Authors and Affiliations
Contributions
NJS and DN conceptualized the review and wrote the first draft of the manuscript. FR critically reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
NJS has served as consultant for Pfizer Inc., GSK, NKARTA, and Sanofi, reports receiving research grants from Takeda Oncology, Astellas Pharma Inc., Xencor, Stemline Therapeutics, and NextCure, and has received honoraria from Novartis, Amgen, Takeda Oncology, Astellas Pharma Inc., Sanofi, and BeiGene. FR has served as a consultant for AbbVie, reports receiving research grants from Astellas Pharma Inc. and Celgene/BMS, and has received honoraria from Astellas Pharma Inc. and Celgene/BMS. The other authors report no relevant conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Short, N.J., Nguyen, D. & Ravandi, F. Treatment of older adults with FLT3-mutated AML: Emerging paradigms and the role of frontline FLT3 inhibitors. Blood Cancer J. 13, 142 (2023). https://doi.org/10.1038/s41408-023-00911-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00911-w