Abstract
Allogeneic stem cell transplant (allo SCT) for multiple myeloma (MM) is potentially curative in some, while toxic in many others. We retrospectively analyzed 85 patients diagnosed with MM who underwent allo SCT as frontline or salvage therapy between 2000 and 2022 at Mayo Clinic Rochester and examined patient outcomes and prognostic markers. Overall survival (OS), progression free survival (PFS), treatment related mortality (TRM), and relapse rates (RR) were estimated using the Kaplan Meier method and competing risk models. Median follow-up was 11.5 years. Median OS and PFS were 1.7 and 0.71 years, respectively. Five-year OS and PFS were 22.2% and 15.1%, respectively. One-year TRM was 23.5%. Twelve patients demonstrated durable overall survival, living 10+ years beyond their allo SCT. This subgroup was more likely to have no or one prior auto SCT (p = 0.03) and to have been transplanted between 2000 and 2010 (p = 0.03). Outcomes were poor in this cohort with long follow-up, with few patients surviving 5 years or more, and most relapsing or dying within 2 years. We would expect better outcomes and tolerability with an expanded array of novel therapeutics and would prefer them to allo SCT.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) remains a largely incurable, clonal plasma cell disorder characterized by the presence of a monoclonal protein causing anemia, bone disease, and renal insufficiency. MM represents 1.8% of all new cancers in the United States; the median age at diagnosis is 70 years and the number of patients diagnosed annually is expected to double over the next 20 years [1]. With this increasing burden of disease, identifying an expanded set of treatment options is paramount. Initial treatment for MM depends on the patient’s eligibility for autologous stem cell transplant (auto SCT), as determined by disease stratification and patient functional status. Transplant-eligible patients often receive induction chemotherapy followed by autologous stem cell mobilization, harvest, and transplant. Transplant ineligible patients will commonly receive a variety of immunomodulatory drugs (ImID) and proteosome inhibitor-based regimens. Novel agents such as the proteasome inhibitors, in addition to monoclonal antibodies, chimeric antigen receptor T (CAR-T) cells, and bispecific T cell engagers (BiTE) have shown promising results but are not available in many countries [2, 3].
The use of allogeneic stem cell transplant (allo SCT) is controversial. For some, it offers a potentially curative modality due to a graft vs myeloma effect [4]. For many, it incurs considerable toxicity due to graft-vs-host disease (GVHD) [5]. For this reason, allo SCT is not routinely offered in MM patients, although utilization has increased over the past 30 years despite a lack of clear treatment guidelines [6, 7]. Today, Allo-SCT is generally reserved for young patients with high risk, relapsed MM [8], and has mixed efficacy overall [9,10,11,12]. With the development of modern novel therapies in the last decade, the role of allo SCT is increasingly difficult to define.
Our project aims to characterize patient outcomes and prognostic markers in patients who received allo SCT. With a median follow up of 11.5 years in a relatively large cohort of patients with multiple myeloma, we identify patients with prolonged favorable outcomes after allo SCT, which previous studies with shorter follow-up could not detect. A greater understanding of allo SCT patient outcomes and prognostic factors can help guide treatment decisions for this potentially life-prolonging and perhaps even curative therapy.
Methods
Patients’ description and data source
We retrospectively analyzed patients diagnosed with MM who underwent allo SCT between 2000 and 2022 at Mayo Clinic Rochester. Data were analyzed as of May 2022; patient data were retrieved from our institution’s continuously maintained database and patient electronic medical records. Patients received regular clinical follow-up. The analysis was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent for institution-initiated research studies. This project was approved by Mayo Clinic’s Institutional Review Board.
High risk (HR) cytogenetics were defined as the presence of del17p, t(4;14), or t(14;16) [13] and 1q+ at the time of diagnosis [14]. There was not enough available data regarding cytogenetics at the time of diagnosis or S-phase fraction to perform statistical analysis. Conditioning regimen intensities and graft-versus-host disease (GVHD) were categorized according to previously published criteria [15, 16]. The presence of chronic GVHD (cGVHD) was determined according to the 2014 NIH GVHD criteria [17]. The number of prior lines of therapy were determined according to previously proposed guidelines [18]. The International Myeloma Working Group (IMWG) criteria were used to define MM disease and response status [19]. The primary endpoints were overall survival (OS) and progression-free survival (PFS).
Statistical analyses
All data were analyzed using BlueSky Statistics software version 7.4 (BlueSky Statistics LLC, Chicago, IL, USA). Descriptive statistics used the median for continuous variables and counts and percentages for categorical variables. The patient, treatment, and disease characteristics for those surviving less than 10 years ( <10 yr.) versus those surviving more than 10 years (10+ yr.) were compared for independence, and p values were generated using χ2 statistics for categorical variables and the Mann-Whitney test with a two-tailed hypothesis for continuous variables. OS and PFS were calculated as the time from allo SCT to death from any cause, and the first observation of relapse or death from any cause, respectively. Treatment-related mortality (TRM) was defined as death secondary to transplant complications without progressive disease. Patients without observation of the event of interest at the last follow-up were censored. OS and PFS rates were estimated and reported using the Kaplan-Meier method. Relapse/progression and TRM were considered to be competing risks and estimated as cumulative incidence rates using Aalen Johansen estimator [20] and compared with Fine and Gray regression models for competing risks [21], similarly to previously published studies [22]. For subgroup analysis, if 5-year OS or PFS of a variable was not available, 5-year survival was estimated using the value of the nearest timepoint. Univariate analysis was conducted using the Cox proportional hazards model.
Results
Information on 91 patients with multiple myeloma who underwent an allo SCT between 2000 and 2022 was available in our institution’s database. Six patients were excluded for having undergone allo SCT in the setting of a myelodysplastic disorder secondary to previous MM treatment, not as primary treatment for MM, leaving 85 patients available for final analysis. Baseline patient characteristics are summarized in Table 1. Median age at the time of allo SCT was 51.2 years and median time from diagnosis of MM to allo-SCT was 2.7 years. By sex, 72.6% (N = 61) were male and 27.4% (N = 24) female. Most participants were White (89.4%, N = 76). Eighty-one patients (95.3%) had undergone one or more auto SCTs, while four (4.7%) had no previous auto SCT. Most patients (56.5%, N = 48) received their allo SCT two to five years from the time of MM diagnosis. Among those with a prior auto SCT, most patients received their allo SCT within two years of their auto SCT (55.6%, N = 45). The cytogenetic risk profile at time of diagnosis was available for 94.1% of participants (N = 80), with 66.3% (N = 53) of patients categorized as standard risk and 33.8% (N = 27) of patients categorized as high risk. Approximately half of patients (N = 47, 55.3%) received a myeloablative (MA) conditioning regimen in preparation for allo SCT, while the rest (N = 38, 44.7%) received a reduced-intensity (RIC) or nonmyeloablative (NMA) regimen. Patients underwent a median of 4 prior lines of therapy before allo SCT. Median follow-up for the cohort was 11.5 years.
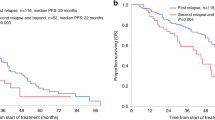
Median OS for the entire cohort was 1.7 years. 3-year OS was 37.9%, 5-year OS was 22.2%, and 10-year OS was 16.8%. Median PFS in this cohort was 0.71 years. 3-year PFS was 22.0%, 5-year PFS was 15.1%, and 10-year PFS was 10.4% (Fig. 1). TRM at 1-year post-allo transplant was 23.5%. At the time of data analysis in May 2022, 18 patients (21.2%) were alive. 5-year OS and PFS were stratified by the various patient, disease, and treatment characteristics as described above. These univariate analysis findings are outlined in Table 2. Briefly, outcome in this cohort was not significantly impacted by age, race, baseline cytogenetic risk, myeloablative regimen, or timing of allo SCT, among others. Active progression, compared to those in a complete remission at the time of allo SCT arose as a significant prognostic factor of OS (HR 3.39, p = <0.01). Development of chronic GVHD (cGVHD), (p = 0.04) and number of prior auto SCT’s, (p = 0.02) also arose as prognostic factors for OS, though some subgroups were small. Prognostic factors for PFS followed a similar pattern, with progression at the time of allo-SCT arising as the most prominent negative prognostic factor (HR 4.39, p = <0.01). The number of prior auto SCTs was also a prognostic factor (p = 0.02).
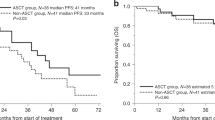
Twelve patients (14%) demonstrated durable overall survival, living more than 10 years beyond their allo SCT. Comparison of patient, treatment, and disease characteristics among those surviving <10 yrs. versus 10+ yrs. are outlined in Table 1. The number of prior auto SCTs and date of allo transplant arose as statistically significant differences among these groups, with the 10+ yr. survivor subgroup being more likely to have no or one prior auto SCT (p = 0.03) and to have been transplanted between 2000 and 2010 (p = 0.03). No difference was found among other characteristics such as baseline cytogenetic risk, myeloablation regimen, or number of previous lines of therapy. Sex approached, but did not reach, statistical significance (p = 0.07), with a higher proportion of 10+ yr. survivors being male.
Discussion
This study demonstrates overall poor survival outcomes in patients who underwent allo SCT for treatment of multiple myeloma with long-term survival ( >5 years) being achieved in less than 25% of patients. Nearly 75% of patients experienced disease progression or death by 2 years, and 1-year TRM was high at 22.2% Despite this, a subgroup of patients entered a durable remission after receiving an allo SCT, potentially constituting a cure. Those who saw particular benefit from allo SCT were more likely to have been in complete remission at time of allo SCT, had only one previous auto SCT, and to have undergone their allo transplant in the early 2000s. In all, allo-SCT as a treatment for multiple myeloma was minimally beneficial outside of a select few patients.
5-year OS following allogeneic transplant was poor at 22.2% with a cohort size of 85 patients and a long median follow-up of 11.5 years. As demonstrated by Fig. 1, there was a rapid survival decline immediately following transplant that did not plateau until roughly four years post-transplant and did not entirely stabilize until eight years post-transplant. 5-year PFS was 15.1%. Cumulative 1-year TRM was high at 23.5% (N = 20), exceeding the number of “cured” patients who survived 10+ years (N = 12). The largest study to examine allo SCT in MM was conducted by the Bone Marrow Transplant Clinical Trials Network (BMT CTN), and found 3-year OS and PFS to be 77% and 43%, respectively, with a TRM of 11% [23] for patients who underwent allo SCT as upfront therapy. Our overall cohort demonstrated considerably worse outcomes for the same timepoints with 3-year OS and PFS being 37.9% and 22.0%, respectively.
It is important to note that the findings of the BMT CTN study represent allogeneic transplant as upfront treatment (auto SCT to RIC allo SCT) in a group of individuals primarily with standard-risk cytogenetics and partial-response or better to previous therapy. Conversely our data represented a heterogenous and highly challenging population usually at a salvage point, with over 75% having at least 3 previous lines of therapy, and over 30% of patients in active progression at the time of transplant. The poorer survival outcomes in our study were not unexpected, given the prolonged disease history and more extensive prior therapies. Even when examining the few among our cohort who underwent auto SCT to RIC allo SCT as upfront therapy (N = 3), OS and PFS were 33% and 19%, respectively, considerably lower than the BMT CTN study.
As such, our findings more closely align with those from studies examining allo SCT as salvage therapy. Numerous studies have been conducted over the years with considerable variability in survival findings depending on pretransplant disease and patient characteristics, with estimated 2-year OS and PFS between 32–54% and 19–42%, respectively [12, 24, 25], and estimated 5-year OS and PFS between 14–26% and 2–20%, respectively [12, 24,25,26,27]. The largest allo SCT MM salvage study [28], with 413 patients, found a 5-year OS of 30% with a cumulative 1-year TRM of 21.5%, with no apparent plateau in the survival curves.
With a median follow-up of 11.5 years, our study provides unique insight into the long-term outcomes of allo SCT in MM patients. Extended follow-up revealed an OS plateau at eight years following allo SCT, with roughly 15% of patients (N = 12) surviving beyond 10 years, representing a potentially cured subgroup. Descriptive examination of patient and disease characteristics among this set of “long-term” survivors reveals them to have a higher proportion of individuals with no or one previous auto SCT, and allo SCT date between 2000 and 2010. Those receiving heavy pretreatment likely had a more aggressive or advanced disease at time of allo SCT. Regarding transplant dates, allo SCT was more commonly used as upfront therapy in the 2000s, whereas by the 2012 over two-thirds of allo SCT’s were performed as salvage therapy for progressive disease due to the advent and increased usage of novel therapeutics [7]. Of note, any patients in this study who underwent allo SCT in May 2012 or beyond had not been followed long-enough to reveal a 10-year survival at the time of analysis (May 2022). Six patients among the allo-SCT 2011-to-present analysis group were still alive at the time of analysis, five of whom underwent allo SCT from 2019 onward, and one who underwent allo SCT in 2014.
Finally, among the entire cohort, univariate analysis for prognostic factors in survival showed patients with disease progression at the time of allo SCT did poorly, the presence of chronic GVHD conferred a survival benefit, and the presence of two prior auto SCT’s conferred a survival detriment. Though this could represent the previously described survival advantage of mild cGVHD [6], it may indicate survival bias toward a subset of patients who survived long enough to develop cGVHD. Our study’s finding that two prior auto SCT’s is associated with worsened OS and PFS likely reflects the trend that patients who are heavily pre-treated, in general, have more aggressive or advanced disease and are at higher risk of mortality.
This study should be interpreted in the context of several strengths and limitations. The cohort was quite racially/ethnically homogenous with most participants identifying as White. Future studies should aim to include a more diverse and ethnically representative cohort. Furthermore, small numbers among some patient characteristic subgroups limited the power of statistical analyses. Notwithstanding these limitations, the long median follow-up of this study allowed for examination of the extended outcomes and the ability to rely on the higher accuracy of observed, rather than estimated, OS and PFS. Additionally, the cohort size of 85 provides a relatively large series among these patients [29].
In conclusion, allogeneic transplant poses a therapeutic dilemma for myeloma clinicians. Particularly as salvage therapy, allo SCT has poor outcomes. It is curative for a small subset of patients, however, the patient, treatment, and disease characteristics that predispose those in this subgroup to favorable long-term outcomes remain ill-defined. Unfortunately, the curative potential of allo SCT is tempered by poor OS, poor PFS, and a high TRM that exceeded the “cure” rate among the rest of the observed patients. The benefit of allo SCT may be tremendously high—a potential cure—but the risk is even higher. With an expanding array of anti-CD38, BiTE, and other novel therapeutics, we would expect better overall outcomes and tolerability of these agents compared to allo SCT and, if available, would prefer them to allo SCT.
Data availability
The data that support the findings of this study are available from the corresponding author (WMS) upon reasonable request.
References
Mateos MV, Landgren O. MGUS and smoldering multiple myeloma: diagnosis and epidemiology. Cancer Treat Res. 2016;169:3–12.
Mateos MV, San Miguel JF. Management of multiple myeloma in the newly diagnosed patient. Hematol Am Soc Hematol Educ Program. 2017;2017:498–507.
Kegyes D, Constantinescu C, Vrancken L, Rasche L, Gregoire C, Tigu B, et al. Patient selection for CAR T or BiTE therapy in multiple myeloma: Which treatment for each patient? J Hematol Oncol. 2022;15:78.
Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood 1996;87:1196–8.
Dhakal B, Vesole DH, Hari PN. Allogeneic stem cell transplantation for multiple myeloma: is there a future? Bone Marrow Transpl. 2016;51:492–500.
Yin X, Tang L, Fan F, Jiang Q, Sun C, Hu Y. Allogeneic stem-cell transplantation for multiple myeloma: a systematic review and meta-analysis from 2007 to 2017. Cancer Cell Int. 2018;18:62.
Sobh M, Michallet M, Gahrton G, Iacobelli S, van Biezen A, Schönland S, et al. Allogeneic hematopoietic cell transplantation for multiple myeloma in Europe: trends and outcomes over 25 years. A study by the EBMT Chronic Malignancies Working Party. Leukemia 2016;30:2047–54.
Giralt S, Garderet L, Durie B, Cook G, Gahrton G, Bruno B, et al. American society of blood and marrow transplantation, European Society of blood and marrow transplantation, blood and marrow transplant clinical trials network, and international myeloma working group consensus conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transpl. 2015;21:2039–51.
Gahrton G, Iacobelli S, Björkstrand B, Hegenbart U, Gruber A, Greinix H, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood 2013;121:5055–63.
Giaccone L, Storer B, Patriarca F, Rotta M, Sorasio R, Allione B, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood 2011;117:6721–7.
de Lavallade H, El-Cheikh J, Faucher C, Fürst S, Stoppa AM, Coso D, et al. Reduced-intensity conditioning allogeneic SCT as salvage treatment for relapsed multiple myeloma. Bone Marrow Transpl. 2008;41:953–60.
Patriarca F, Einsele H, Spina F, Bruno B, Isola M, Nozzoli C, et al. Allogeneic stem cell transplantation in multiple myeloma relapsed after autograft: a multicenter retrospective study based on donor availability. Biol Blood Marrow Transplant. 2012;18:617–26.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Schmidt TM, Barwick BG, Joseph N, Heffner LT, Hofmeister CC, Bernal L, et al. Gain of Chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood. Cancer J. 2019;9:94.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15:1628–33.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825–8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.e1.
Rajkumar SV, Richardson P, San Miguel JF. Guidelines for determination of the number of prior lines of therapy in multiple myeloma. Blood 2015;126:921–2.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46.
Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat. 1978;5:141–50.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Christine G, Monika E, Gabriele I, Katja S, Hartmut B, Reinhard M, et al. Allogeneic transplantation of multiple myeloma patients may allow long-term survival in carefully selected patients with acceptable toxicity and preserved quality of life. Haematologica 2019;104:370–9.
Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–203.
Efebera YA, Qureshi SR, Cole SM, Saliba R, Pelosini M, Patel RM, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed multiple myeloma. Biol Blood Marrow Transpl. 2010;16:1122–9.
Crawley C, Iacobelli S, Björkstrand B, Apperley JF, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood 2007;109:3588–94.
Kröger N, Shimoni A, Schilling G, Schwerdtfeger R, Bornhäuser M, Nagler A, et al. Unrelated stem cell transplantation after reduced intensity conditioning for patients with multiple myeloma relapsing after autologous transplantation. Br J Haematol. 2010;148:323–31.
Maffini E, Storer BE, Sandmaier BM, Bruno B, Sahebi F, Shizuru JA, et al. Long-term follow up of tandem autologous-allogeneic hematopoietic cell transplantation for multiple myeloma. Haematologica 2019;104:380–91.
Auner HW, Szydlo R, van Biezen A, Iacobelli S, Gahrton G, Milpied N, et al. Reduced intensity-conditioned allogeneic stem cell transplantation for multiple myeloma relapsing or progressing after autologous transplantation: a study by the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2013;48:1395–400.
Mir MA, Kapoor P, Kumar S, Pandey S, Dispenzieri A, Lacy MQ, et al. Trends and outcomes in allogeneic hematopoietic stem cell transplant for multiple myeloma at mayo clinic. Clin Lymphoma Myeloma Leuk 2015;15:349–57.e2.
Author information
Authors and Affiliations
Contributions
MAG designed the study. WMS acquired data and performed data analysis. WMS and NDP interpreted results, drafted the manuscript, and created the tables and figures. All the other authors (FKB, SRH, SKK, AD, DD, JC, MQL, PK, NL, EM, RMW, TK, MB, WIG, WJH) identified and treated the patients, acquired data, reviewed, and edited. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
SKK has received research funding from and holds membership on advisory boards for AbbVie, Celgene, Janssen, Takeda, Adaptive, MedImmune, and has received research funding from KITE, Merck, Novartis, Roche, and Sanofi. AD holds membership on advisory boards from Janssen and research funding from Alynlam, Pfizer, Takeda, and BMS. DD has performed consultancy for Alexion, Apellis, Bristol Meyers Squibb, Novartis, Roche, and Sanofi, and research funding from Regeneron. MQL has received research funding from Celgene. PK has received honoraria and research funding from Sanofi, BMS, and AbbVie, research funding from Regeneron, Amgen, Ichnos, Loxo, Karyopharma, and Takeda, and honoraria from Casma, Pharmacyclics, Imedex, GSK, Cellectar, Oncopeptides, and X4. NL Holds a membership on advisory boards for Takeda. EM has received honoraria from Janssen and performs consultancy for Protego. TK has received research funding from Novartis. MAG has received fees from Ionis/Akcea, Prothena, Sanofi, Janssen, Aptitude, Ashfield, Juno, Physician’s Education Resource, Abbvie, J&J, Research to Practice, Celgene, and Sorrento, and has developed education materials for i3Health.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmidt, W.M., Perera, N.D., Buadi, F.K. et al. Long-term outcomes of allogeneic stem cell transplant in multiple myeloma. Blood Cancer J. 13, 126 (2023). https://doi.org/10.1038/s41408-023-00900-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00900-z