Abstract
Treatment choice according to the individual conditions remains challenging, particularly in older patients with acute myeloid leukemia (AML) and high risk myelodysplastic syndrome (MDS). The impact of performance status, comorbidities, and physical functioning on survival is not well defined for patients treated with hypomethylating agents. Here we describe the impact of performance status (14% ECOG performance status 2), comorbidity (40% HCT-comorbidity index ≥ 2), and physical functioning (41% short physical performance battery < 9 and 17% ADL index < 6) on overall survival (OS) in 115 older patients (age ≥ 66 years) treated on a clinical trial with a 10-day decitabine schedule. None of the patient-related variables showed a significant association with OS. Multivariable analysis revealed that age > 76 years was significantly associated with reduced OS (HR 1.58; p = 0.043) and female sex was associated with superior OS (HR 0.62; p = 0.06). We further compared the genetic profiles of these subgroups. This revealed comparable mutational profiles in patients younger and older than 76 years, but, interestingly, revealed significantly more prevalent mutated ASXL1, STAG2, and U2AF1 in male compared to female patients. In this cohort of older patients treated with decitabine age and sex, but not comorbidities, physical functioning or cytogenetic risk were associated with overall survival.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease with regard to tumor biology, clinical characteristics and outcomes with the currently available treatments [1]. The optimal treatment for older AML patients in daily clinical practice remains challenging and the choice of therapy is informed by disease characteristics (cytogenetic and molecular abnormalities), patients characteristics (age, performance, comorbidity, cognitive function) and preference of the patient [2, 3]. Many of the same challenges are encountered in the treatment of patients with high-risk myelodysplastic syndrome (MDS). Until very recently the anti-leukemic treatment options consisted of either intensive chemotherapy (anthracycline combined with cytarabine, known as ‘3 + 7’), hypomethylating agents (HMA)(azacitidine, decitabine) or low dose cytarabine. The addition of venetoclax to the HMAs azacitidine and decitabine has been shown to significantly improve median OS, from 9.6 to 14.7 months, and diverse combinations of classic chemotherapeutic agents with new targeted drugs may enter the therapeutic playing field in the near future [4].
Selection of the optimal treatment for older patients requires additional attention for treatment tolerance and life expectancy, estimated by evaluation of generally accepted prognostic factors like performance status, comorbidities, physical function and cognition [2, 5]. Several studies have shown the prognostic value of performance status prior to the start of intensive chemotherapy [6,7,8,9]. In addition, comorbidity burden (Charlson comorbidity index > 1 or hematopoietic cell transplantation comorbidity index (HCT-CI ≥ 3)) has been shown to be associated with lower remission rates, increased early mortality and decreased survival for patients treated with intensive chemotherapy [10,11,12,13,14]. Limited data are available on the impact of patient related factors on outcome when patients are treated with lower intensity therapies, usually containing a hypomethylating agent. A randomized study evaluating survival in patients treated with azacitidine or conventional care regimens (including intensive chemotherapy) found performance status to be associated with survival, but did not consider other patient-related factors [15]. In addition, patients with an impaired performance were found to have improved survival when treated with decitabine compared to supportive care [16]. However, randomized data in the setting of lower-intensity therapies are limited.
To more adequately assess vulnerabilities in older patients, beyond performance status and comorbidities, geriatric assessment is attracting attention. Geriatric assessment refers to a comprehensive approach assessing multiple patient characteristics (i.e. physical function, comorbid disease(s), cognitive function, psychological state, social support, polypharmacy, and nutritional status) to help characterize individual patient complexity and aims to discriminate between fit, vulnerable and frail patients. In older patients with AML treated with intensive chemotherapy the short physical performance battery (SPPB) and the modified minimal mental scale (3MS), both generally included in geriatric assessments, appeared to predict for survival [17, 18]. Physical performance, fatigue, and cognitive impairment were also associated with survival in a sub-analysis of 107 AML and MDS patients treated with non-intensive therapies (i.e., HMA or only best supportive care) [19]. Recently, it has been shown in a subset of patients enrolled in the CALGB 11002 trial, of whom 82 out of 165 patients performed baseline geriatric assessment, that physical functioning and cognition are associated with 6-month mortality and survival among older patients with AML treated with non-intensive chemotherapy (i.e., 10-day decitabine ± bortezomib) [20]. Further randomized data that explore comprehensive patient-assessment strategies to guide treatment (low intensity therapies vs intensive therapies) are not available.
In the prospective randomized HOVON135 trial (10-day decitabine vs 10-day decitabine + Ibrutinib) we evaluated in detail the impact of age, sex, performance status, comorbidity, SPPB, and activities of daily living (ADL) index on survival [21].
Methods
Study design & patient selection
This analysis was done in the context of the phase II multicenter HOVON135 clinical trial (EudraCT 2015-002855-85). The HOVON135 trial prospectively included 144 patients older than 65 years, diagnosed with AML or high-risk MDS without previous therapy, and not eligible for standard chemotherapy. Patients with secondary AML defined as AML after an antecedent hematological disease and therapy-related AML were eligible for inclusion. Patients were considered not eligible for intensive chemotherapy if they had a HCT-CI ≥ 3 or if the patients declined to receive intensive chemotherapy. Patients were randomized to receive 10-day decitabine + ibrutinib, ibrutinib given sequentially with decitabine starting the day after the last dose of decitabine until the day before the start of the next cycle, or 10-day decitabine alone [21]. Detailed information on trial design has previously been published [21]. This study was approved by the Medical Ethical committee of the University Medical Centre Groningen, and all participants provided informed consent in accordance with the Declaration of Helsinki.
Objective physical function assessment
Assessment of physical functioning was performed at inclusion of the study. It consisted of the Short Physical Performance Battery (SPPB) and the Katz Index of Independence in Activities of Daily Living (ADL index). The SPPB is a validated tool to assess lower extremity function in older populations [22]. It comprises a short walk, repeated chair stands, and a balance test. Each item is scored ranging from 0 to 4 (0=unable to complete the exercise; 4 = highest performance level), with a total ranging from 0 to 12. A cut-off score of 9 was used to differentiate between patients with impaired and unimpaired physical performance [17, 18, 23]. The ADL index is a validated assessment tool for a person’s ability to perform daily self-care tasks, with a score of 6 reflecting independence and scores below 6 suggesting dependence [24]. In addition, the Eastern Cooperative Oncology Group (ECOG) performance status was recorded and comorbidity burden was scored according to the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) [25, 26].
Molecular analyses
With real-time PCR CBFB-MYHII, RUNX1-RUNX1T1 and FLT3 internal tandem duplications were detected on bone marrow aspiration samples or peripheral blood samples taken at diagnosis. Targeted next generation sequencing (NGS) was performed for 54 frequently mutated genes in hematologic malignancies with the Illumina TruSight Myeloid Sequencing panel, as previously described [21]. Cytogenetic risk groups were defined according to the European LeukemiaNet (ELN) 2017 AML risk classification [27].
Statistical analysis
Descriptive statistics were used to characterize the study cohort. Overall survival (OS) was defined from date of study inclusion to date of death or last follow-up. Univariable Cox regression analysis was performed to calculate hazard ratios (HRs) of the SPPB score, the ADL index, HCT-CI score, and ECOG performance score at diagnosis. With multivariable Cox regression analysis the HRs were adjusted for age, sex, cytogenetic risk and AML or MDS diagnosis. Additionally, Kaplan-Meier curves were estimated for subgroups of the SPPB score, the ADL index, HCT-CI score, and ECOG performance score, and for cytogenetic risk, sex and age. Statistical comparison of mutation frequencies and clonal characteristics between sex and age subgroups was done using Fisher’s exact test and Mann-Whitney U tests. Odds ratios (OR) and 95% CI (confidence intervals) for the association between age or sex subgroups and mutation frequencies are reported.
Results
Study population and baseline clinical characteristics
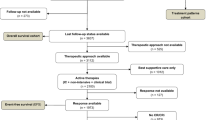
The complete cohort included 144 patients. Data regarding physical function assessment were available for 122 patients. Data on the ADL index was missing in 22 patients, and 21 patients did not have data on the SPPB (in 17 patients both the ADL index and the SPPB were missing). In addition, two patients had missing information on ELN risk classification and one patient had missing information on co-morbidities. One-hundred-fifteen fully evaluable patients were included in this analysis (Fig. 1). There was no difference in survival between the randomized treatment groups (i.e., decitabine with or without ibrutinib), therefore in the following analyses these groups were combined (Supplementary fig. 1) [21]. Median age at diagnosis was 76 years, 62% of patients were male, and the majority of patients was diagnosed with AML (Table 1). Sixty-two percent of patients had an adverse genetic risk profile according to ELN criteria. Physical function assessment scores are shown in Table 2. The majority of patients (83%) was independent in performing activities of daily living (i.e. ADL index score 6), but had an impaired performance status (ECOG score ≥ 1) (62%) or an increased comorbidity burden (HCT-CI ≥ 3) (40%). Forty-one percent of patients had a SPPB score below 9, indicating impairment in physical functioning.
Association between patient-related factors and survival
The median follow-up time was 11.3 months with a median overall survival of 11.5 months. The 30-day mortality was 5% and independent of patient-related factors. Notably, none of the patient-related variables (performance score, co-morbidities) nor variables included in physical function assessment at baseline (SPPB, ADL index) showed a significant association with survival in univariable or multivariable analysis (Table 3). Indeed, the Kaplan-Meier curves plotted for each variable were comparable between the subgroups (Fig. 2). Specifically, patients with a SPPB ≥ 9 had a median OS of 12.1 months and patients with a SPPB < 9 had a median OS of 11.6 months. When looking at ADL index, a median OS of 11.9 months was observed for patients with an ADL score of 6 versus 12.2 months for patients with an ADL score below 6. Patients with a HCT-CI < 3 had a median OS of 11.3 months (vs 12.2 months if ≥ 3) and patients with a ECOG PS 0 had a median OS of 13.4 months (vs 10.7 months if PS 1 or 2). Female patients had longer median OS compared to male patients although the difference was not significant (16.7 vs 11.3 months, respectively).
When looking specifically at the SPPB scores at baseline, a group of patients (n = 12) could be identified with very low SPPB scores ( ≤ 4), indicating severely impaired physical functioning. This subgroup did not have significantly reduced OS (p = 0.79). In addition the Kaplan-Meier survival estimates of the subgroup with SPPB score 5–8 was comparable to the subgroup with SPPB score 9–12 (Supplementary fig. 2).
Additionally, the effect of cytogenetic risk, classified by ELN 2017 criteria, on survival was evaluated. Survival was not significantly different between AML patients with a favorable, intermediate or adverse cytogenetic risk. All three ELN risk groups had worse overall survival compared to high-risk MDS patients (Fig. 2E). Consistently, cytogenetic risk was not significantly associated with survival in univariable or multivariable analysis (Table 3). Further, the effect of primary versus secondary AML on survival was evaluated and no difference was observed (Fig. 1F; Table 3). High-risk MDS patients had superior survival compared to AML patients (HR 0.53, p = 0.048).
Age and survival
Higher age (i.e., above median age of 76 years) was significantly associated with decreased survival in univariable analysis. This effect remained significant in multivariable analysis after adjusting for the patient-related factors (sex, performance score, co-morbidities, SPPB and ADL index) and genetic risk (HR 1.58, p = 0.043)(Table 3). The median OS was 13.4 months and 10.7 months for patients aged ≤ 76 or > 76 years respectively (Fig. 2H). The effect of age was not a surrogate for patient fitness or cytogenetic risk as these variables were included in the multivariable analysis and showed no effect on the risk estimate. An interaction between age and other patient-related factors was also considered but no significant interaction effect for age and SPPB or ADL index was observed.
Age, sex and molecular subgroups
Since we were unable to identify patient related factors to explain the difference in survival amongst the age subgroups below and above the median age (i.e., ≤ 76 vs > 76 years), we compared the mutational profiles. Comprehensive molecular analysis was performed on patient material obtained from all included patients at diagnosis and extensive mutational data were available for 113 patients. Six patients (5%) had no detectable gene mutations. The highest variant allele frequency (VAF) per individual, the number of mutations per individual (median 3; range 0–12) and the number of mutated genes per individual (median 3; range 0–9) did not differ between patients aged ≤ 76 or > 76 years (Fig. 3B, C). With respect to the most common molecular abnormalities, evaluated by NGS, also no difference between patients aged ≤ 76 or > 76 years could be identified (Fig. 3A). Specifically there was no difference frequency of TP53 mutations (11% vs 13%, p = 0.647) in patients aged ≤ 76 or > 76 years respectively.
A Forest plot indicating the OR and 95% CIs for the association between recurrently mutated genes and age > 76 years, and a pyramid plot displaying the proportion of patients and absolute numbers with a mutation in the recurrently mutated genes per age group. B Boxplot with the distribution of the highest VAF per individual stratified by age group. C Violin plot showing the distribution of the number of mutations per individual stratified by age group.
The trend that female sex was associated with superior survival was strengthened in the multivariable analysis (HR 0.62, p = 0.061)(Table 3). Although surprising at first instance the increased significance can be explained by the modifying effect of other factors influencing survival, namely MDS diagnosis (7 females, 14 males), on sex in univariable analysis. The almost significant impact of sex on survival prompted us to compare the mutational profiles in males and females in this study. Although the VAF per individual and the number of mutations per individual did not differ between male and female patients, interestingly the prevalence of mutations in certain genes (i.e., ASXL1, STAG2, and U2AF1) was significantly more frequent in male patients compared with female patients (Fig. 4A–C). Subsequently, we evaluated whether these gene mutations determined the observed inferior OS for male patients. In univariable analysis, though hampered by low numbers, mutations detected in either ASXL1, STAG2, or U2AF1 were not significantly associated with survival (ASXL1 HR 1.14 p = 0.57; STAG2 HR 1.55 p = 0.12, U2AF1 HR 0.94 p = 0.84). Also when these three mutations were combined, they were not significantly associated with survival (HR 1.15 p = 0.53). However, when the mutations were added in the multivariable analysis, the effect of sex on survival lost its borderline significance (HR 0.66, p = 0.118), suggesting that the difference in mutational profile between males and females, may at least partly explain the difference in survival between males and females.
A Forest plot indicating the OR and 95% CIs for the association between recurrently mutated genes and male sex, and a pyramid plot displaying the proportion of patients and absolute numbers with a mutation in the recurrently mutated genes for male and female patients. B Boxplot with the distribution of the highest VAF per individual in female and male patients. C Violin plot showing the distribution of the number of mutations per individual in female and male patients.
Discussion
The role of conventional prognostic factors for survival in older patients treated with low-intensity therapies is ambiguous. This study analysed the impact of performance status, comorbidity score, SPPB score and ADL score on survival in older patients with AML or high-risk MDS (who were considered unfit for intensive treatment or who refused intensive treatment) in the context of 10-day decitabine treatment. Of 115 patients included in this analysis, 41% had an SPPB score lower than 9, indicating physical impairment, and 40% had a comorbidity score of three or higher, confirming the ‘reduced fitness’ of our study population. However we found no significant association between any of these patient-related variables and survival. Surprisingly, in multivariable analyses only age at diagnosis (significant) and sex (borderline significant) were associated with survival.
Our results are in contrast with previously published results reporting a significant association between physical functioning and survival, which overruled chronologic age in predicting survival in two cohorts of older AML patients ( > 60 years) [17, 18]. However those studies were conducted in the context of treatment with intensive chemotherapy in contrast to our cohort which was treated with a hypomethylating agent. Further, many of the well-known prognostic factors have not been validated in older patient cohorts nor in patients treated with HMAs. The disparity in the impact of physical funtioning and chronologic age in both studies might be explained by the differences in anti-leukemic mechanism of action and in treatment-related toxicities. Indeed 30-day mortality in our cohort of older patients treated with decitabine was lower compared to cohorts of older patients treated with intensive chemotherapy [6, 17, 28, 29]. Increased co-morbidity burden and decreased performance score prior to intensive chemotherapy treatment have been associated with increased treatment-related toxicity and decreased survival [6,7,8, 10,11,12, 30, 31]. The association between objective physical functioning and survival found by Klepin et al. and Min et al. in patients treated with intensive chemotherapy is in line with this [17, 18]. Although evidence on the predictive value of geriatric assessment in AML patients treated with intensive chemotherapy is growing, the data in the context of less intensive therapies as yet has remained limited. In contrast to our findings, a recent study performed in a (biased) subset of a cohort of older patients treated with decitabine found a significant association between comorbidity score and survival and between cognition and survival. Decreased physical functioning was associated with increased 6-month mortality but not with overall survival [20]. Interestingly, patients diagnosed with high-risk MDS had better survival compared to patients diagnosed with AML. This finding seems contradictory with the recently revised diagnostic criteria for myeloid neoplasms emphasizing continuum between MDS and AML diagnoses and overruling the blast threshold [32].
In contrast to patients with AML treated with intensive chemotherapy, the association between the ‘traditional’ prognostic factors and HMA therapy is also less strong. Indeed in accordance with our results several studies have shown that patients classified as having ‘adverse’ risk AML based on ELN 2017 cytogenetic risk criteria don’t have significantly inferior survival after HMA treatment compared to ELN 2017 favorable and intermediate risk groups [15, 16, 33]. In line with this, the ELN 2017 classification did not provide prognostic infromation in the VIALE-A trial (comparing azacitidine with azacitidine + venetoclax) [34]. The median OS in our cohort, of which the majority had adverse cytogenetic characteristics, was comparable to the median OS reported for older patients after both intensive chemotherapy or HMA therapy [4, 15, 29, 35, 36]. Apparently the ELN 2017 risk classification, which was largely built using chemotherapy-treated patient datasets doesn’t maintain its prognostic impact in this patient cohort treated with a HMA. Our data suggest that all older patients, independent of patient-related factors (co-morbidity, physical functioning) or disease-related factors (ELN risk groups) can benefit of treatment with 10-day decitabine.
The age-effect in AML has been a widely discussed topic for many years. It has been hypothesized that age acts a surrogate marker for both patient-related factors and disease-related factors. Indeed it has been reported that the mutational profile differs between older and younger AML patients, with older patients harbouring more mutations associated with treatment-resistance and poor survival [5, 37,38,39,40]. Older patients also tend to have more comorbidities and impaired physical performance, which have been independently associated with treatment outcome [11, 12, 19, 31]. In line with this, some studies have shown the overruling effect of physical performance and cytogenetic risk on chronologic age when they were evaluated together in multivariable analyses [8, 17, 18]. However, in our analysis this was not the case, as age remained the variable with the strongest association with survival in our multivariable model, which also included sex, performance status, co-morbidities, SPPB score, ADL index and ELN risk. Apparently, in our cohort of patients treated with 10 days decitabine, the age-effect could not be explained by these patient derived variables. The availaibility of detailed molecular characterization of our cohort allowed us to investigate the possibility that the “age-effect” could be explained by a different mutational profile in the oldest patients. Therefore, we evaluated the mutational spectrum, number of mutations in patients, and number of mutated genes aged above or below 76 years and found no difference. Thus what comprises the effect of age, a mere number, in this cohort of AML patients treated with decitabine remains to be determined. We speculate that epigenetic mechanisms might play a role and may partly explain this age-effect.
Although it is widely known that the incidence of AML and MDS is higher in males compared to females, little is known about sex as a prognostic factor for survival. Our data show that older female patients tend to have a superior survival compared to older male patients after treatment with 10-day decitabine which is in accordance with previously published data [41]. In contrast to age, sex was associated with specific molecular abnormalities. Our data confirm recent manuscripts demonstrating that ASXL1, U2AF1 and STAG2 (encoded on the X-chromosome) are significantly more frequently mutated in male AML and MDS patients compared to female AML patients [38, 42,43,44]. Previously we have shown in the same cohort that patients with mutations in STAG2, IDH2, and ASXL1 showed significantly reduced CR/CRi rates within 3 cycles of decitabine (12% for STAG2, 17% for IDH2, and 20% for ASXL1) [21]. Apparently, men more frequently have a mutational profile with lower chance of response to decitabine, which may explain the worse outcome of male patients in our study. Indeed, although hampered by low numbers, our data suggests that the difference in mutational profiles between males and females, at least partly, can explain the difference in survival between males and females. A recent paper published by the GenoMed consortium found some of the same sex-dependent mutations in MDS patients and showed that these mutations had a prognostic impact [44]. Furthermore, analyses of sex-specific differences in mutational spectrum of clonal hematopoiesis in a large population-based cohort showed enrichment for ASXL1, SF3BI, SRSF2 and U2AF1 in males [45].
The method of data collection, in the context of a prospective multicenter study, is a strength of this study since scores on objective physical performance tests did not influence the treatment decision. In addition, detailed molecular screening was available for the majority of included patients. Further, this trial confirms, what has been suggested by others, that older patients with AML and reduced fitness can be safely included in a clinical trial [46]. However, the number of patients in molecular subgroups was limited and objective physical performance assessment was not performed on all patients at baseline. It remains unclear whether patients declined to participate, weren’t able to due to impairment, or physical function assessment was simply forgotten.
In conclusion, not performance status, comorbidities or physical functioning but age and sex had impact on survival in patients treated with 10-day decitabine. Treatment with 10-day decitabine can benefit patients with ‘traditionally’ poor risk AML and MDS such as those with severe co-morbidities, low physical performance and adverse cytogenetic risk profile. Future research should continue to identify criteria that can enable the prediction of treatment response and treatment tolerance also for patients candidate for less intensive therapies as the therapeutic field moves towards personalized care.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to patient privacy concerns but are available from the corresponding author on reasonable request.
References
Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52.
Ossenkoppele G, Lowenberg B. How I treat the older patient with acute myeloid leukemia. Blood 2015;125:767–74.
Huls G. Azacitidine in AML: A treatment option? Blood 2015;126:283–4.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–29.
Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol. 2014;32:2541–52.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood 2006;107:3481–5.
Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 2006;106:1090–8.
Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145:598–605.
Ostgard LS, Norgaard JM, Sengelov H, Severinsen M, Friis LS, Marcher CW, et al. Comorbidity and performance status in acute myeloid leukemia patients: A nation-wide population-based cohort study. Leukemia 2015;29:548–55.
Etienne A, Esterni B, Charbonnier A, Mozziconacci MJ, Arnoulet C, Coso D, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer 2007;109:1376–83.
Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukemia. Br J Haematol. 2007;136:624–7.
Savic A, Kvrgic V, Rajic N, Urosevic I, Kovacevic D, Percic I, et al. The hematopoietic cell transplantation comorbidity index is a predictor of early death and survival in adult acute myeloid leukemia patients. Leuk Res. 2012;36:479–82.
Della Porta MG, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, Zipperer E, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica 2011;96:441–9.
Naqvi K, Garcia-Manero G, Sardesai S, Oh J, Vigil CE, Pierce S, et al. Association of comorbidities with overall survival in myelodysplastic syndrome: development of a prognostic model. J Clin Oncol. 2011;29:2240–6.
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–9.
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–7.
Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013;121:4287–94.
Min GJ, Cho BS, Park SS, Park S, Jeon YW, Shin SH, et al. Geriatric assessment predicts nonfatal toxicities and survival for intensively treated older adults with AML. Blood 2022;139:1646–58.
Deschler B, Ihorst G, Platzbecker U, Germing U, Marz E, de Figuerido M, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica 2013;98:208–16.
Ritchie EK, Klepin HD, Storrick E, Major B, Le-Rademacher J, Wadleigh M, et al. Geriatric assessment for older adults receiving less-intensive therapy for acute myeloid leukemia: report of CALGB 361101. Blood Adv. 2022;6:3812–20.
Huls G, Chitu DA, Pabst T, Klein SK, Stussi G, Griskevicius L, et al. Ibrutinib added to 10-day decitabine for older patients with AML and higher risk MDS. Blood Adv. 2020;4:4267–77.
Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016;14:215.
da Camara SM, Alvarado BE, Guralnik JM, Guerra RO, Maciel AC. Using the short physical performance battery to screen for frailty in young-old adults with distinct socioeconomic conditions. Geriatr Gerontol Int. 2013;13:421–8.
Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–55.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47.
Kantarjian H, Ravandi F, O’Brien S, Cortes J, Faderl S, Garcia-Manero G, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010;116:4422–9.
Lowenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl J Med. 2009;361:1235–48.
Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica 2012;97:1916–24.
Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood 2009;113:4179–87.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International consensus classification of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022;140:1200–28.
Ritchie EK, Feldman EJ, Christos PJ, Rohan SD, Lagassa CB, Ippoliti C, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54:2003–7.
Döhner H, Pratz KW, DiNardo CD, Jonas BA, Pullarkat VA, Thirman MJ, et al. ELN Risk Stratification Is Not Predictive of Outcomes for Treatment-Naïve Patients with Acute Myeloid Leukemia Treated with Venetoclax and Azacitidine. Blood 2022;140:1441–4.
Maiti A, Qiao W, Sasaki K, Ravandi F, Kadia TM, Jabbour EJ, et al. Venetoclax with decitabine vs intensive chemotherapy in acute myeloid leukemia: A propensity score matched analysis stratified by risk of treatment-related mortality. Am J Hematol. 2021;96:282–91.
Ossenkoppele GJ, Breems DA, Stuessi G, van Norden Y, Bargetzi M, Biemond BJ, et al. Lenalidomide added to standard intensive treatment for older patients with AML and high-risk MDS. Leukemia 2020;34:1751–9.
Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): Analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood 2001;98:1312–20.
Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Gorlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 2016;128:686–98.
Eisfeld AK, Kohlschmidt J, Mrozek K, Blachly JS, Walker CJ, Nicolet D, et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: an analysis of Alliance studies. Leukemia 2018;32:1338–48.
de Jonge HJ, de Bont ES, Valk PJ, Schuringa JJ, Kies M, Woolthuis CM, et al. AML at older age: age-related gene expression profiles reveal a paradoxical down-regulation of p16INK4A mRNA with prognostic significance. Blood 2009;114:2869–77.
DeZern AE, Zeidan AM, Barnard J, Hand W, Al Ali N, Brown F, et al. Differential response to hypomethylating agents based on sex: A report on behalf of the MDS Clinical Research Consortium (MDS CRC). Leuk Lymphoma. 2017;58:1325–31.
De-Morgan A, Meggendorfer M, Haferlach C, Shlush L. Male predominance in AML is associated with specific preleukemic mutations. Leukemia 2021;35:867–70.
Bose P, Nazha A, Komrokji RS, Patel KP, Pierce SA, Al-Ali N, et al. Mutational landscape of myelodysplastic/myeloproliferative neoplasm-unclassifiable. Blood 2018;132:2100–3.
GenoMed4All c. A sex-informed approach to improve the personalised decision making process in myelodysplastic syndromes: A multicentre, observational cohort study. Lancet Haematol. 2022;10:E117–E128.
Kamphuis P, van Zeventer IA, de Graaf AO, Salzbrunn JB, van Bergen M, Dinmohamed AG, et al. Sex differences in the spectrum of clonal hematopoiesis. Hemasphere. 2023;7:e832.
Montalban-Bravo G, Huang X, Naqvi K, Jabbour E, Borthakur G, DiNardo CD, et al. A clinical trial for patients with acute myeloid leukemia or myelodysplastic syndromes not eligible for standard clinical trials. Leukemia 2017;31:318–24.
Acknowledgements
This investigator-sponsored trial was financially supported by Janssen Pharmaceutics, and they provided the decitabine and ibrutinib used in the trial free of charge.
Author information
Authors and Affiliations
Consortia
Contributions
The study was designed by the Leukemia Working Group of the HOVON; the HOVON Data Center was responsible for the central data management; and JRH, DAC, and IAvZ performed the analysis of the data; TP, SKK, GS, LG, PJMV, JC, AAvdL, DB, DvL-V, RB, MJ-L, MF, MH, MGM, MS, RvMK, DD, MWMvdP, MCL, LT, YC, EA, SB, and GJO collected and interpreted the data. The decision to publish was made by the cooperative group. JH and GH and subsequently IAvZ, BL, and GO produced the first version of the manuscript, which was circulated for comments to the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hilberink, J.R., van Zeventer, I.A., Chitu, D.A. et al. Age and sex associate with outcome in older AML and high risk MDS patients treated with 10-day decitabine. Blood Cancer J. 13, 93 (2023). https://doi.org/10.1038/s41408-023-00850-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00850-6
This article is cited by
-
Clinical data mining: challenges, opportunities, and recommendations for translational applications
Journal of Translational Medicine (2024)
-
Real-world data of AML in Japan: results of JALSG clinical observational study-11 (JALSG-CS-11)
International Journal of Hematology (2024)