Abstract
Most patients with multiple myeloma (MM) undergoing autologous hematopoietic stem cell transplantation (autoHCT) eventually relapse, perhaps due to the presence of clonal plasma cells (CPC) in the autograft. We conducted a retrospective analysis to evaluate the impact of CPC in the autograft on the outcomes of high-risk chromosomal abnormalities (HRMM) patients undergoing autoHCT between 2008 and 2018. Patients were divided into CPC+ or CPC− in the autograft by next-generation flow cytometry (NGF). There were 75 CPC + autografts (18%) and 341 CPC− (82%). The CPC + group was less likely to achieve MRD-negative complete remission post-transplant (11% vs. 42%; p < 0.001). Median progression free survival (PFS) and overall survival (OS) were (12.8 vs. 32.1 months) and (36.4 vs. 81.2 months) in the CPC + and CPC− groups, respectively (both p < 0.001). Also in the subset of patients with MRD-negative ≥VGPR prior to autoHCT, those with CPC + autografts had inferior PFS (HR 4.21, p = 0.006) and OS (HR 7.04, p = 0.002) compared to CPC-. In multivariable analysis, the degree of CPC positivity in the autograft was independently predictive of worse PFS (HR 1.50, p = 0.001) and OS (HR 1.37, p = 0.001). In conclusion, both the presence and degree of CPC in the autograft were highly predictive of inferior PFS and OS.
Similar content being viewed by others
Introduction
High dose chemotherapy and autologous hematopoietic stem cell transplantation (autoHCT) is considered standard of care as part of first line therapy for patients with multiple myeloma (MM) [1]. However, patients with high-risk cytogenetic features (high risk MM, HRMM) have inferior outcomes after autoHCT compared to patients with standard risk disease. A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis showed a 3 year post-transplant progression free survival (PFS) rate of 37% vs. 49%, and overall survival (OS) rate of 72% vs. 85%, in patients with HRMM compared to those with standard risk MM, respectively [2].
The significance of aberrant clonal plasma cells (CPC) in the autografts collected for autoHCT remains a subject of debate. In a study of 76 patients reported by Vogel et al., those with CPC graft contamination of >4.5 × 105 plasma cells/kg body weight by flow cytometry had a high risk of early disease progression [3]. A follow-up report found that patients with a high degree of CPC autograft contamination had inferior OS compared to those with a low degree of contamination (median OS: 53 vs. 114 months, respectively) [4]. Several small studies provided conflicting results regarding the impact of CPC in the autograft on PFS in patients with MM [5,6,7]. More recently, a study involving 199 patients showed that autograft contamination by MM cells was associated with a higher risk of delaying or not achieving complete remission (CR) and minimal residual disease (MRD)-negativity post-transplant [8]. There was no difference in PFS or OS between the two groups, though the follow-up was relatively short.
In the current study, we conducted a single center retrospective analysis to evaluate the impact of autograft contamination by MM cells in patients with HRMM undergoing autoHCT.
Methods
Study design and participants
We searched our institution’s database for adult patients with HRMM who underwent autoHCT between 2008 and 2018. Evaluation for CPC by 6-color next-generation flow cytometry (NGF) was performed on the collected apheresis products, with a sensitivity of 0.001–0.003% depending on sample quality. Of note, 4-color flow cytometry was used before 2012, with a sensitivity of 0.05%. The patients were divided into two groups: CPC + and CPC− in the autograft by NGF. Since not all collected autograft products are infused at autoHCT, and some bags may be CPC + or CPC− for the same patient, we also evaluated the impact of infusion of CPC + autograft products on patient outcomes. MRD status in bone marrow samples was assessed using 8-color NGF. The sensitivity of our assay is 1/10−5 cells (0.001%) based on acquisition and analysis of at least 2 million events.
The University of Texas MD Anderson Cancer Center Institutional Review Board approved this retrospective study. The research was conducted in accordance with the Declaration of Helsinki and the 1996 Health Insurance Portability and Accountability Act guidelines.
FISH analysis
Fluorescence in situ hybridization (FISH) was used to identify high-risk cytogenetic abnormalities of del17p/TP53 deletion, t(4;14)/IGH::FGFR3, t(14;16)/IGH::MAF, and 1q21/CKS1B gain (3 copies of CKS1B) or amplification (≥4 copies of CKS1B). The cut-off values established in our clinical cytogenetics laboratory were 0.4% for IGH/FGFR3 or IGH::MAF rearrangement; 4.7% for TP53 deletion and 4.2% for monosomy 17.
Statistical methods
We compared the day 100 post autoHCT response, the best response, PFS and OS between CPC+ and CPC− autograft groups. Response between CPC+ and CPC− autograft groups was evaluated using Fisher’s exact test, while response by degree of autograft CPC+ bag infused was assessed by Wilcoxon rank-sum test. PFS time was computed from autoHCT date to date of disease progression or death (if died without disease progression) or the last follow-up date. Patients who were alive and did not experience progression of disease at the last follow-up date were censored. OS time was computed from date of autoHCT to last known vital sign, and patients alive at the last follow-up date were censored. OS and PFS were estimated using the Kaplan–Meier method, and differences between groups were assessed using the log-rank test. Associations between outcomes and variables of interest were determined using univariate and multivariable Cox proportional hazards regression models.
Statistical analyses were performed using SAS 9.4 for Windows (by SAS Institute Inc., Cary, NC). All statistical tests used a significance (alpha) level of 5%. No adjustments for multiple testing were made.
Data sharing statement
The data that support the findings of this study are available on request from the corresponding author.
Results
Patients and disease characteristics
416 HRMM patients were included in the study, with a median age of 62.4 years (range 31.7–83.0), and 57% were male. Seventy-five patients (18%) had CPC + status while 341 (82%) were CPC−. A lower percentage of patients in the CPC + group received the bortezomib, lenalidomide, and dexamethasone (VRD) induction regimen prior to transplant compared to the CPC− group (24% vs. 42%, p = 0.004), yet there was no difference in the duration of induction between the two groups (p = 0.37) A lower percentage of patients in the CPC + group achieved ≥VGPR and MRD negative status after induction compared to the CPC− group (32% vs. 62%; p < 0.001 and 8% vs. 40%; p < 0.001, respectively). Del[17p] and 1q+ cytogenetic abnormalities were significantly more prevalent in patients with CPC + autografts compared to those with CPC− autografts (45% vs. 28%, p = 0.007 and 68% vs. 45%, p < 0.001). There was no significant difference in conditioning regimens used between the two groups (p = 0.73). Patient characteristics according to autograft CPC status are summarized in Table 1.
Outcomes
Median follow-up for the whole cohort was 35.7 (range 0.3–139.5) months. 100-day and best post-autoHCT CR rates in the CPC+ and CPC− groups were 8% vs. 33% (p < 0.001), and 19% vs. 54% (p < 0.001), respectively. Patients in the CPC + group were less likely to have MRD-negative CR post-transplant (11% vs. 42%; p < 0.001). Median PFS in the CPC + vs. CPC− group was 12.8 vs. 32.1 months (p < 0.001), and median OS was 36.4 vs. 81.2 months (p < 0.001).
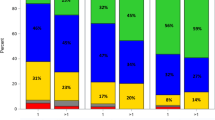
The degree of autograft CPC involvement was inversely associated with post-transplant day 100 response: the maximal autograft CPC level was 0.02% (standard deviation [SD] 0.02) for patients who achieved ≥VGPR vs. 1.04% (SD 2.59) for those who achieved ≤PR at day 100 post-transplant (p = 0.003). Similarly, the maximal autograft CPC level was inversely associated with best post-transplant response: mean maximal autograft CPC level was 0.06% (SD 0.16) for patients who achieved ≥VGPR vs. vs. 1.55% (SD 3.17) for those who achieved ≤PR (p = 0.004). Pre- and post-transplant responses for the CPC+ and CPC− groups are depicted in Fig. 1.
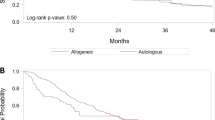
When assessing the whole cohort of patients, those with CPC + autografts had significantly worse PFS (Fig. 2A, p < 0.001) and OS (Fig. 2B, p < 0.001) compared to those with CPC- autografts. Of the patients who achieved ≥VGPR prior to autoHCT, those with CPC + autografts had inferior PFS (hazard ratio (HR) [95% CI]: 3.38 [2.05–5.58], p < 0.001; Fig. 2C) and OS (HR 2.29 [1.20–4.40], p = 0.013; Fig. 2D) compared to the CPC− group. Also in patients who achieved MRD-negative ≥VGPR prior to autoHCT, those with CPC + autografts had inferior PFS (HR 4.21 [1.50–11.81], p = 0.006) and OS (HR 7.04 [2.05–24.20], p = 0.002) compared to those of the CPC− group. In a multivariable analysis (MVA), the degree of CPC positivity in the autograft was independently predictive of worse PFS (HR 1.50 [1.17–1.90]; p = 0.001) and OS (HR 1.37 [1.13–1.67]; p = 0.001). MVA showed that infusion of CPC + autograft products was predictive of worse PFS (HR 2.72 [1.82–4.08]; p < 0.001) but not significantly worse OS (HR 1.47 [0.86–2.49]; p = 0.16). ISS stage II and III disease were associated with worse PFS compared to ISS stage I in MVA [(HR 1.47 [1.05–2.06]; p = 0.026) and (HR 1.55 [1.09–2.21]; p = 0.014), respectively]. HCT-CI score >3 was associated with worse OS (HR 1.50 [1.01–2.22]; p = 0.044), whereas post-transplant maintenance was associated with a trend toward improved OS (HR 0.68 [0.46–1.00]; p = 0.05) in MVA. MVA for PFS and OS are shown in Table 2 and Table 3, respectively. Univariate analyses for PFS and OS are shown in Supplemental Table 1 and Supplemental Table 2, respectively. Within the CPC− and CPC + groups, hematological responses prior to autoHCT and at day 100 after autoHCT mostly retained their statistical significance for both PFS and OS (Supplementary Figs. 1–5 and 7–8). However, within the CPC + group, hematological responses prior to autoHCT did not predict OS (p = 0.60, Supplementary Fig. 6).
Use of chemotherapy for stem cell mobilization did not impact the rate of CPC- in the collected autografts (83% with and 78% without chemotherapy; p = 0.39). Eighty-nine percent (n = 141) of patients who received VRD induction had CPC- autografts, whereas 78% of patients who received VCD and 83% of those who received KRD had CPC- autografts (Table 1). Use of VRD for induction was not associated with improved PFS (HR 1.14 [0.88–1.47]; p = 0.32) or OS (HR 1.08 [0.77–1.51]; p = 0.67) compared to other induction regimens. Within the subgroup of patients who received VRD induction, autograft CPC status was highly predictive of both PFS (HR 3.10 [1.75–5.49]; p < 0.001) and OS (HR 2.66 [1.33–5.33]; p = 0.006).
Since a different method of CPC detection was used between 2008–2011 (n = 55) and 2012–2018 (n = 361), we repeated the analyses while excluding patients who were transplanted in the earlier era and obtained similar results. When considering only patients transplanted between 2012 and 2018, the CPC + group had inferior median PFS (15.4 vs. 35.5 months, p < 0.001) and median OS (39.3 months vs. OS not reached, p < 0.001) compared to the CPC− group.
Discussion
To the best of our knowledge, this is the largest study to date to evaluate the impact of autograft contamination by CPC in patients with MM undergoing autoHCT. It is also the first to focus only on patients with HRMM. We found that in patients with HRMM undergoing autoHCT, presence of CPC in the autograft was associated with inferior PFS and OS. Furthermore, the degree of autograft CPC positivity was associated with worse outcomes. Through MVA, we found a significant association between autograft CPC positivity and patient outcomes, which was also observed in patients who had already achieved ≥VGPR or MRD negative ≥VGPR prior to autoHCT.
Most patients with MM eventually relapse after transplant, especially those with high-risk cytogenetic abnormalities. Infusion of CPC in the autograft is considered a potential source of relapse. Several studies, including meta-analyses, have shown that MRD detection in the bone marrow by NGF or next-generation sequencing (NGS), performed at various time points in both transplant-eligible and ineligible patients, predicts worse outcomes [9,10,11,12]. In addition, separate reports by the EBMT [13] and the CIBMTR [14] showed lower relapse rates after syngeneic hematopoietic stem cell transplants compared to autologous transplants. This could partly be due to the absence of CPC contamination in syngeneic grafts.
In the present study, patients in the CPC+ group were more likely to have del17p and less likely to have achieved a VGPR after induction. However, when the analysis was restricted to CPC + patients who had achieved ≥VGPR or MRD-negative ≥VGPR after induction, presence of CPC in the autograft still predicted worse outcomes. Compared to MRD detection in the bone marrow, detection of CPC in the autograft is less invasive and is not limited by focal plasma cell infiltration [15]. Yet, for the most part, hematological responses prior to autoHCT and at day 100 after autoHCT within the CPC+ and CPC− groups retained their predictive impact for both PFS and OS. This is in contrast to an analysis of the PETHEMA/GEM2012MENOS65 study by Jimenez-Ubieto et al. that showed that patients with bone marrow MRD positivity had similarly poor survival outcomes, irrespective of their hematological response [16].
Bal et al. reported a trend toward higher rates of autograft CPC negativity in patients who received KRD induction, compared to VRD (81% vs. 57%, respectively; p = 0.25) [17]. In contrast, we found a higher rate of autograft CPC negativity in patients who received VRD. Differences between the study cohorts may account for these conflicting results, such as our study including only patients with high-risk cytogenetics, while only 16% of the patients in the analysis by Bal et al. had high-risk cytogenetics. There was no significant difference in the median duration of induction treatment between the CPC+ and CPC− groups, thereby ruling out the role of longer induction in the CPC− group.
Our study raises the question of whether purging of CPC could mitigate the adverse impact of CPC in the autografts. Several preclinical studies have suggested potential efficacy of various ex vivo purging methods [18,19,20]. However, clinical experience with purging in MM has had disappointing results, with several trials failing to show an advantage of ex-vivo purging [5, 21]. A phase III trial published in 2001 demonstrated that CD34 selection using a Ceprate-R Stem Cell concentrator device resulted in a 3-log reduction in MM cells in the autografts, though this did not translate into improved PFS or OS [22, 23]. Of note, 40 of the 111 enrolled patients in that study could not be assessed for autograft contamination due to various technical issues. With the availability of newer and more reliable technologies, ex-vivo purging of CPC could be revisited.
Administering chemotherapy prior to autograft collection could have an in-vivo purging effect, which may reduce CPC in the graft. However, a study comparing cyclophosphamide-based chemo-mobilization to growth factor-only mobilization showed increased toxicity with chemo-mobilization without improving EFS or OS [24]. A phase I trial examining the role of in-vivo purging by adding bortezomib to granulocyte colony-stimulating factor (G-CSF) failed to show any benefit [25]. In our study, we did not observe a difference in the rate of CPC positivity between chemo-mobilization or growth factor-only mobilization.
Our study has several limitations. First, being a retrospective analysis, it has inherent issues with patient and treatment heterogeneity, missing data, and patient selection bias. Second, since we focused on patients with HRMM, the findings may not be applicable to patients with standard risk MM. Furthermore, the detection of CPC in the autografts was limited by the sensitivity of the 6 color flow cytometry technique in most of our cohort. It is possible that a more sensitive method could identify an even smaller group of CPC− patients, with deeper response to induction and even better survival outcomes.
In conclusion, the current study shows a major impact of CPC in the autograft on post-autoHCT outcomes in HRMM. Both the presence and degree of CPC in the autograft were highly predictive of inferior PFS and OS, including in those who had achieved ≥VGPR and MRD-negative CR or VGPR prior to autoHCT. Novel strategies for purging of CPC could improve patient outcomes.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Callander NS, Baljevic M, Adekola K, Anderson LD, Campagnaro E, Castillo JJ, et al. NCCN Guidelines(R) Insights: Multiple Myeloma, Version 3.2022. J Natl Compr Canc Netw. 2022;20:8–19.
Scott EC, Hari P, Sharma M, Le-Rademacher J, Huang J, Vogl D, et al. Post-Transplant Outcomes in High-Risk Compared with Non-High-Risk Multiple Myeloma: A CIBMTR Analysis. Biol Blood Marrow Transpl. 2016;22:1893–9.
Vogel W, Kopp HG, Kanz L, Einsele H. Myeloma cell contamination of peripheral blood stem-cell grafts can predict the outcome in multiple myeloma patients after high-dose chemotherapy and autologous stem-cell transplantation. J Cancer Res Clin Oncol. 2005;131:214–8.
Kopp HG, Yildirim S, Weisel KC, Kanz L, Vogel W. Contamination of autologous peripheral blood progenitor cell grafts predicts overall survival after high-dose chemotherapy in multiple myeloma. J Cancer Res Clin Oncol. 2009;135:637–42.
Galimberti S, Morabito F, Guerrini F, Palumbo GA, Azzara A, Martino M, et al. Peripheral blood stem cell contamination evaluated by a highly sensitive molecular method fails to predict outcome of autotransplanted multiple myeloma patients. Br J Haematol. 2003;120:405–12.
Gertz MA, Witzig TE, Pineda AA, Greipp PR, Kyle RA, Litzow MR. Monoclonal plasma cells in the blood stem cell harvest from patients with multiple myeloma are associated with shortened relapse-free survival after transplantation. Bone Marrow Transpl. 1997;19:337–42.
Wuilleme S, Lok A, Robillard N, Dupuis P, Stocco V, Migne H, et al. Assessment of tumoral plasma cells in apheresis products for autologous stem cell transplantation in multiple myeloma. Bone Marrow Transpl. 2016;51:1143–5.
Kostopoulos IV, Eleutherakis-Papaiakovou E, Rousakis P, Ntanasis-Stathopoulos I, Panteli C, Orologas-Stavrou N, et al. Aberrant Plasma Cell Contamination of Peripheral Blood Stem Cell Autografts, Assessed by Next-Generation Flow Cytometry, Is a Negative Predictor for Deep Response Post Autologous Transplantation in Multiple Myeloma; A Prospective Study in 199 Patients. Cancers (Basel). 2021;13:4047.
Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, Garcia-Sanchez O, Bottcher S, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017;31:2094–103.
Korthals M, Sehnke N, Kronenwett R, Bruns I, Mau J, Zohren F, et al. The level of minimal residual disease in the bone marrow of patients with multiple myeloma before high-dose therapy and autologous blood stem cell transplantation is an independent predictive parameter. Biol Blood Marrow Transpl. 2012;18:423–31.e3.
Munshi NC, Avet-Loiseau H, Anderson KC, Neri P, Paiva B, Samur M, et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020;4:5988–99.
Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017;3:28–35.
Gahrton G, Svensson H, Bjorkstrand B, Apperley J, Carlson K, Cavo M, et al. Syngeneic transplantation in multiple myeloma - a case-matched comparison with autologous and allogeneic transplantation. European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 1999;24:741–5.
Bashey A, Perez WS, Zhang MJ, Anderson KC, Ballen K, Berenson JR, et al. Comparison of twin and autologous transplants for multiple myeloma. Biol Blood Marrow Transpl. 2008;14:1118–24.
Cengiz Seval G, Beksac M. Is Quantification of Measurable Clonal Plasma Cells in Stem Cell Grafts (gMRD) Clinically Meaningful? Front Oncol. 2022;12:800711.
Jimenez-Ubieto A, Paiva B, Puig N, Cedena MT, Martinez-Lopez J, Oriol A, et al. Validation of the International Myeloma Working Group standard response criteria in the PETHEMA/GEM2012MENOS65 study: are these times of change? Blood 2021;138:1901–5.
Bal S, Landau HJ, Shah GL, Scordo M, Dahi P, Lahoud OB, et al. Stem Cell Mobilization and Autograft Minimal Residual Disease Negativity with Novel Induction Regimens in Multiple Myeloma. Biol Blood Marrow Transpl. 2020;26:1394–401.
Bartee E, Chan WM, Moreb JS, Cogle CR, McFadden G. Selective purging of human multiple myeloma cells from autologous stem cell transplantation grafts using oncolytic myxoma virus. Biol Blood Marrow Transpl. 2012;18:1540–51.
Lee AJ, Kim SG. Selective purging of human multiple myeloma cells from peripheral blood mononuclear cells: a preliminary study. J Blood Med. 2019;10:105–9.
Yang H, Robinson SN, Nieto Y, Jones RJ, Gocke CD, Lu J, et al. Ex vivo graft purging and expansion of autologous blood progenitor cell products from patients with multiple myeloma. Cancer Res. 2011;71:5040–9.
Gupta D, Bybee A, Cooke F, Giles C, Davis JG, McDonald C, et al. CD34+-selected peripheral blood progenitor cell transplantation in patients with multiple myeloma: tumour cell contamination and outcome. Br J Haematol. 1999;104:166–77.
Bourhis JH, Bouko Y, Koscielny S, Bakkus M, Greinix H, Derigs G, et al. Relapse risk after autologous transplantation in patients with newly diagnosed myeloma is not related with infused tumor cell load and the outcome is not improved by CD34+ cell selection: long term follow-up of an EBMT phase III randomized study. Haematologica 2007;92:1083–90.
Stewart AK, Vescio R, Schiller G, Ballester O, Noga S, Rugo H, et al. Purging of autologous peripheral-blood stem cells using CD34 selection does not improve overall or progression-free survival after high-dose chemotherapy for multiple myeloma: results of a multicenter randomized controlled trial. J Clin Oncol. 2001;19:3771–9.
Tuchman SA, Bacon WA, Huang LW, Long G, Rizzieri D, Horwitz M, et al. Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. J Clin Apher. 2015;30:176–82.
Ghobadi A, Fiala MA, Rettig M, Schroeder M, Uy GL, Stockerl-Goldstein K, et al. A Phase I Study of the Safety and Feasibility of Bortezomib in Combination With G-CSF for Stem Cell Mobilization in Patients With Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2019;19:e588–e93.
Funding
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Author information
Authors and Affiliations
Contributions
OP and MHQ conceived and designed the study. MR, SG, AM, AHM, and PL collected and assembled the data. OP, MHQ, and DRM analyzed and verified the data. MRT, QB, SS, NS, PL, JR, YN, GT, HCL, KKP, PK, SKT, DMW, RZO, KR, RC, and EJS verified and interpreted the data. All authors wrote and approved of the paper and are accountable for publication.
Corresponding author
Ethics declarations
Competing interests
MHQ has research funding from Janssen, NexImmune, and Angiocrine. QB has research funding from Stemline, GlaxoSmithKline, Takeda, and Acrotech, and consulting with Purdue, Amgen, Kite, and Wolters Kluwer. PL has a license and research agreement with Takeda Pharmaceuticals. YN has research funding from Affimed, AstraZeneca, and Securabio and consulting from Affimed. HCL has consulting with Bristol Myers Squibb, Celgene, Genentech, Karyopharm, Legend Biotech, GlaxoSmithKline, Sanofi, Pfizer, Monte Rosa Therapeutics, Oncopeptides, and Takeda Pharmaceuticals and research funding from Amgen, Bristol Myers Squibb, Janssen, GlaxoSmithKline, Regeneron, and Takeda Pharmaceuticals. KKP has research funding from Abbvie, Bristol Myers Squibb, Celgene, Takeda, Precision Bio, Cellectis, Allogene, and Nektar and consulting with Bristol Myers Squibb, Celgene, Janssen, Pfizer, Oncopeptides, Arcellx, Cellectis, and Karyopharm. PK has research funding from Amgen and Ziopharm and consulting with Jazz, Kite, and Pfizer. SKT has research funding from Bristol Myers Squibb, Genentech, X4 Pharma, Cellectar, and Ascentage. RZO is founder of Asylia Therapeutics, has research funding from Biotheryx, Asylia Therapeutics, CARsgen, Celgene, Exelixis, and Heidelberg Pharma and consulting with Biotheryx, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Karyopharm, Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Therapeutics, Oncopeptides, Regeneron, Sanofi-Aventis, and Takeda. KR, EJS, and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical and Affimed GmbH. KR participates on the Scientific Advisory Board for GemoAb, AvengeBio, Virogin Biotech, GSK, Caribou Biosciences, Navan Technologies and Bayer. EJS has consulting with Bayer, Novartis, Navan, Magenta, Adaptimmune, Mesoblast, and Axio. The other authors have not declared a competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pasvolsky, O., Milton, D.R., Rauf, M. et al. Impact of clonal plasma cells in autografts on outcomes in high-risk multiple myeloma patients. Blood Cancer J. 13, 68 (2023). https://doi.org/10.1038/s41408-023-00842-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00842-6