Abstract
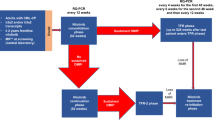
With the improving knowledge of CML and its management, the goals of therapy need to be revisited to ensure an optimal use of the BCR::ABL1 TKIs in the frontline and later-line therapy of CML. In the frontline therapy of CML in the chronic phase (CML-CP), imatinib and the three second-generation TKIs (bosutinib, dasatinib and nilotinib) are associated with comparable survival results. The second-generation TKIs may produce earlier deep molecular responses, hence reducing the time to reaching a treatment-free remission (TFR). The choice of the second-generation TKI versus imatinib in frontline therapy is based on the treatment aims (survival, TFR), the CML risk, the drug cost, and the toxicity profile with respect to the patient’s comorbidities. The TKI dosing is more flexible than has been described in the registration trials, and dose adjustments can be considered both in the frontline and later-line settings (e.g., dasatinib 50 mg frontline therapy; dose adjusted schedules of bosutinib and ponatinib), as well as during an ongoing TKI therapy to manage toxicities, before considering changing the TKI. In patients who are not candidates for TFR, BCR::ABL1 (International Scale) transcripts levels <1% are acceptable, result in virtually similar survival as with deeper molecular remissions, and need not warrant a change of TKI. For patients with true resistance to second-generation TKIs or with the T315I gatekeeper mutation, the third-generation TKIs are preferred. Ponatinib should be considered first because of the cumulative experience and results in the CML subsets, including in T315I-mutated CML. A response-based dosing of ponatinib is safe and leads to high TKI compliance. Asciminib is a third-generation TKI with possibly a better toxicity profile, but lesser activity in T315I-mutated CML. Olverembatinib is another potent third-generation TKI with early promising results.
Similar content being viewed by others
Introduction
Today, we celebrate two decades of experience with imatinib and later-generation BCR::ABL1 tyrosine kinase inhibitors (TKIs) in Philadelphia chromosome (Ph)-positive chronic myeloid leukemia (CML). With maturing data, it is important to review our established guidelines on treatment options in frontline and subsequent-line CML therapy, treatment aims, response monitoring, and the significance of the response milestones proposed by the European LeukemiaNet (ELN) and the National Comprehensive Cancer Network (NCCN) [1,2,3,4,5,6]. In this review, we discuss these issues to clarify questions about the management of CML in frontline and post-frontline TKI failure settings.
Frontline CML therapy
The primary aim of CML therapy is to improve survival so that it matches that of a normal population. A second aim, emphasized in the past decade but that benefits fewer patients, is the achievement of a durable deep molecular response (DMR), which can then allow treatment discontinuation and potentially a treatment-free remission (TFR) status [7].
Four BCR::ABL1 TKIs are approved for frontline therapy: imatinib, dasatinib, bosutinib and nilotinib. All four produce near-normal quality of life and life expectancy provided patients comply with the treatment, are monitored optimally with minimal interruptions in therapy, and are managed appropriately at the earliest sign of true treatment resistance [8,9,10,11,12,13]. Imatinib is available as a generic drug in the United States (US), and at least one of the 15 available formulations is priced at about $500/year in the US (through Cost Plus Drugs, for example) [14,15,16,17]. Imatinib generics are routinely available for $300-$3,000/year in other regions. Dasatinib will be available as a generic formulation in the US by 2024 and can be prescribed elsewhere now. The prices of patented TKIs range from $150,000 to 250,000+/year [18, 19]. Therefore, an important consideration in the frontline CML therapy is the “treatment value” or cost-benefit of a TKI if overall survival (OS) is the treatment endpoint. For this aim, generics provide the best treatment value [17, 20, 21].
The choice of frontline therapy may depend on additional factors:
-
Aim of therapy (survival or TFR): Among older patients, survival may be the primary aim, and TFR secondary. Among such patients, imatinib may be the better frontline TKI therapy. In younger patients, TFR may be pursued more aggressively by some CML experts and patients, with changes in therapy in the absence of a DMR (BCR::ABL1 transcripts on International Scale [IS] ≤ 0.01%) after 3–5 + years of frontline TKI therapy (discussed later).
-
Cost of the TKI and affordability to the patient: In the US, this may be tied to the out-of-pocket expenses, which can be as high as 25% of the price [20, 22, 23]. In such instances, generic TKIs may be the only realistically affordable option [16, 17, 19].
-
Patient co-morbidities: Some notable co-morbidities that influence the choice of a TKI include chronic lung disease, hypertension, diabetes, hepatic or renal dysfunction, pancreatitis, enterocolitis, vaso-spastic or occlusive events, and others. These will be discussed in details under “ Management of CML post TKI toxicities”.) [24].
-
CML risk category: In patients with higher-risk disease (as defined by the Sokal or other risk models), the second-generation TKIs may be favored over imatinib by some CML experts and in community practice as frontline therapy [25,26,27,28]. No advantage has been observed in lower-risk disease. Recent studies have suggested the possible adverse effects of molecular abnormalities like ASXL1 mutations, but these are not yet incorporated into the CML risk models.
What is the true incidence of resistance to optimal frontline TKI therapy?
It is often stated that the incidence of primary resistance to frontline TKI therapy is 10%, and of secondary resistance 30% [29]. This may have been true in the original TKI studies, with the suboptimal use of imatinib, and may depend on the definition of resistance. True resistance to frontline therapy may be significantly lower. In the German CML IV trial of 1551 patients treated in chronic phase (CP) with imatinib-based regimens, with a median follow-up time of 10 years, the 10-year OS rate was 82%, the relative survival rate 92%, and the cumulative incidence of blast phase (BP) only 5.8%. Only 26.5% of patients switched from imatinib to second-generation TKIs, 10% because of resistance, the others for toxicities and/or other reasons [10]. In our experience with frontline lower-dose dasatinib therapy (50 mg daily), with a median follow-up of 5 years, the incidence of true primary plus secondary resistance (defined as BCR::ABL1 transcripts [IS] > 1% any time after 12 months of therapy) was < 5%. The estimated 5-year OS rate was 98%, with only two deaths (not related to CML) and no transformation events to accelerated phase (AP) or BP [30, 31]. Similar results were reported in the randomized trial of dasatinib 100 mg daily versus imatinib in frontline CML therapy [32]. In our long-term frontline TKI therapy experience, the estimated 15-year OS rate (including any death, regardless of cause) is about 75%. The CML-specific survival rate (considering only deaths from CML or treatment complications) is >90% [33]. Thus, frontline therapy with the existing TKIs achieves the primary endpoint of survival normalization for most patients with CML.
How about the achievement of TFR?
Here, we discuss the definition of DMR and its optimal duration before considering TKI discontinuation. It is increasingly accepted that a DMR does not necessarily require negative results for BCR::ABL1 transcripts but could include levels that represent a reduction of 4 (MR4) or 4.5 logs (MR4.5). Discontinuing the TKI after a sustained DMR of >2 years results in 3-year TFR rates of 40%–50% [7, 34,35,36,37], while discontinuation after a DMR of ≥5 years results in a 5-year TFR rate of >80% [38]. Hence, in the absence of severe toxicities, more CML experts and patients are leaning toward continuing the TKI therapy for longer durations before considering stopping. The achievement of TFR has been estimated to be about 25–30% [34,35,36, 39]. This may be an underestimation of the true TFR rate. Assuming that 70%–90% of patients achieve a DMR with imatinib or second-generation TKIs, which is durable in about 80%, and also assuming that discontinuation occurs after 5 or more years of DMR, then the TFR rates should be 40%–55% of the total population treated. This assumes that all eligible patients are willing to discontinue TKI therapy, which they are often reluctant to do.
An important question that cannot be answered with objective data is the likelihood that a patient who is on imatinib or a second-generation TKI for >5 years and who has not achieved a DMR will be able to reach the goal of TFR by changing TKI therapy? The assumption that it is possible to increase the TFR rate by rotating the second-generation TKIs or changing to a third-generation option has been the rationale by many physicians (also encouraged by CML experts, and in CML reviews, advisory boards and symposia) to change the TKI in a patient who does not achieve a major molecular response (MMR; BCR::ABL1 transcripts [IS] < 0.1%) or DMR after some duration of TKI therapy. While common, this practice may not be successful and is the subject of recent discussions among CML experts.
How important is it to achieve the landmark optimal responses recommended by the ELN and NCCN?
The ELN recommendations and NCCN guidelines highlight the importance of achieving an “early molecular response” (EMR; defined as BCR::ABL1 transcripts [IS] < 10% at 3–6 months) and a complete cytogenetic response (CCyR; approximated to BCR::ABL1 transcripts [IS] < 1 %) by 12 months of therapy [5, 6].. In the early years of the TKIs, EMR was emphasized, resulting in attempts to optimize therapy by switching from imatinib to second-generation TKIs in the first few months of therapy [40, 41]. In the German CML IV experience, achieving BCR::ABL1 (IS) transcripts < 10% at 3 months was associated with a 5-year OS rate of 94%, compared with 90% if levels were >10% [10]. Indications from recent experiences are that the 6-month timepoint is more important than the 3-month timepoint for EMR, and that lack of EMR at 6 months occurs in a minority of optimally treated and compliant patients (< 5% with second-generation TKIs) [41,42,43].
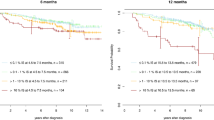
Historical experience with interferon (IFN) alpha showed that the achievement of a major cytogenetic response (roughly equivalent to BCR::ABL1 transcripts [IS] < 10%) or CCyR was associated with excellent long-term survival [44]. However, once imatinib and other highly effective TKIs became available, enthusiasm was high and the prevailing practice became to achieve the deepest responses possible (even when not aiming for TFR), regardless of the costs related to frequent changes of TKIs and their potential additional toxicities. Earlier and deeper molecular responses were assumed to be reliable surrogate endpoints for long-term survival. This did not turn to be the case, perhaps because of the availability of highly active salvage therapies for patients who progressed or lost a CCyR after >1 year of frontline TKI therapy. More recently, some studies have re-analyzed the associations between outcome and responses less than MMR. Shaya and colleagues reported that patients who did not achieve a CCyR after 2 years of TKI therapy had a significantly worse survival than those who did [45]. The estimated 10-year survival rates were 75% versus 90% (HR 0.36; p < 0.001). This was statistically and clinically significant, but showed than even patients who did not achieve a CCyR did reasonably well. The investigators did not separate the results based on transcript levels < 10% versus > 10%. In a second study of 131 patients not achieving MMR after 2 years of TKI therapy, Bidikian and colleagues observed that the 10-year CML-specific survival rate was similar (95%) among patients with BCR::ABL1 transcripts (IS) > 0.1%–1% and >1%–10% [46]. Only patients with levels > 10% at 2 years had a worse 10-year OS rate, at 80%. Thus, as with the IFN alpha experience, achieving BCR::ABL1 transcripts (IS) < 10% (≈MCyR) translated into a reasonable long-term survival. This finding, which needs to be confirmed in other studies, may be more important in the management of older patients in whom allogeneic stem cell transplantation (SCT) is considered when the BCR::ABL1 (IS) transcripts persist at levels of 1%–10%.
One of the most common questions posed in CML practice is how to treat a patient of a particular age (from 15 to 90 years old) on frontline therapy with a particular TKI (e.g. imatinib, dasatinib, bosutinib, or nilotinib) and in whom the BCR::ABL1 transcripts (IS) are anywhere from 0.01 % to 0.5%. A frequent reaction is to rotate second-generation TKIs or use a third-generation option (ponatinib, asciminib). This is driven by the emphasis in the past two decades on the paramount importance of achieving CCyR (≈ BCR::ABL1 transcripts <1%), MMR, or DMR in order to pursue a TFR. Being too aggressive in pursuing these goals may result in harmful effects, including new TKI toxicities, financial burdens and added stress for the patient [47]. Once a patient is determined to have a low probability of a TFR (i.e. no durable DMR after >5 years of TKI therapy), changing TKIs may cause more harm than potential benefit. Another course of action—which may be different from other CML experts’ opinions —would be to continue with the same TKI and monitor the patient’s BCR::ABL1 transcripts at frequent intervals (every 3 months if transcripts >0.1%; every 6 months if ≤0.1%).
Management of CML post frontline TKI therapy failure
Despite the efficacy of frontline TKI therapy in CML, treatment failure occurs in a minority of patients who then require salvage therapy with other TKIs, allogeneic SCT, non-TKI therapies (omacetaxine mepesuccinate, cytarabine, hypomethylating agents, hydroxyurea, venetoclax), or combinations.
Failure to frontline therapy can be due to 1) TKI toxicities or 2) treatment resistance [48, 49]. Poor compliance to therapy can be a major contributor to treatment failure, and can be caused by inadequately managed drug toxicities, financial burdens and other causes [50, 51]. Historically, the incidence of failure was quoted to be about 50%, with studies showing that after 5 years of frontline TKI therapy, about 40%–60% of patients were on alternative TKIs [52, 53]. This information was used to emphasize the need to develop and approve more and better TKIs, which was reasonable.
As knowledge of outcomes and experiences is gained, it appears that, in the early period of TKIs in CML, patients on a frontline TKI were often changed to other TKIs for indications that are not considered as often today. For example, many patients were advised to change from imatinib or a second-generation TKI to a different second-generation option when the BCR::ABL1 (IS) transcripts were > 10% at 3 months, > 1% after 1 year, or even 0.1% to < 1% after 1 to ≥ 5 years of therapy. Also, patients are at times advised to change TKIs even for BCR::ABL1 transcripts (IS) < 0.1%, in order to pursue TFR. In addition, when patients experienced toxicities on a TKI, they were often changed to another, regardless of the nature and severity of the toxicity, rather than attempting to lower the dose, if the symptoms were reversible or mild-moderate, and when patients were already in an acceptable state of molecular remission [54]. This was perhaps the reason for the higher rates of reported “treatment failure” than seen today. At MD Anderson, using dose reductions for toxicities and judicious changes of therapy only for clinically significant changes in molecular response, >80% of patients remain on the frontline TKI after 5 years of therapy.
Management of CML post-TKI toxicities
Some toxicities are highly TKI-specific (Table 1) [55]. Common side-effects with imatinib therapy include fluid retention, periorbital edema, bone and muscle aches, and, less commonly, weight gain. Rare events, include renal dysfunction and neurotoxicity (dementia-like; parkinsonism) [11]. Dasatinib is associated with pleural effusions, and myelosuppression; rarely, patients develop pulmonary hypertension and muscle aches [32, 56]. Bosutinib is associated with gastrointestinal (GI) toxicity (diarrhea), and hepatic and renal dysfunction [57, 58]. Nilotinib can exacerbate hyperglycemia and cause dyslipidemia [59, 60]. After a 10-year follow-up, the incidence of arterio-occlusive events(AOEs) and veno-occlusive events (VOEs) (angina, myocardial infarction, cerebrovascular accidents, transient ischemic cerebral events, peripheral arterial insufficiency) was 24.8% with nilotinib 300 mg BID and 33.4% with nilotinib 400 mg BID [24]. Imatinib and nilotinib can rarely cause pancreatitis [24, 59, 61, 62]. Ponatinib is probably the most effective but more toxic TKI when used at a dose of 45 mg daily [63,64,65]. It is associated with systemic hypertension (> 20%), AOEs (10–20%), skin rash (10–20%) and pancreatitis (5%) [65,66,67,68].
Knowing the common side effects of a TKI can help in the selection of the drug based on patient co-morbidities, as discussed earlier (under the choice of frontline therapy) [69]. While we once assumed that these toxicities were particular to certain TKIs, recent data showed a higher-than-expected rate of TKI cross-intolerances [55, 68, 70, 71]. In a registry analysis from Canada, the reason for treatment failure post frontline TKI therapy was about 57% due to intolerance and 43% due to resistance. However, with subsequent failures, intolerance became a more common cause of treatment failure and often recurred in the same patients [72].
Value of TKIs at lower dose schedules
TKI toxicities are strongly associated with the higher dose schedules. Originally, the TKIs (and many of the novel recent targeted therapies) were developed in strategies similar to the ones used for chemotherapy, at one dose lower than the maximum tolerated dose (MTD), which was based on the first 1-2 courses of therapy [73]. However, chemotherapy regimens were used for short durations of 6–12 months. In contrast, some of the targeted therapies are needed for years, and, at times, for the patient’s lifetime. This has uncovered late toxicities not observed with the shorter follow-ups (e.g. AOEs, pleural effusions, and organ dysfunctions with CML TKIs). It was also observed that efficacy could be similar, or maintained after a response is achieved, at lower dose schedules [30, 54, 74,75,76]. Thus, original experiences with the TKIs in CML highlighted a new concept in cancer therapy: developing such long-term targeted therapies at an “optimal biologic dose” (OBD), rather than at the lower-than-MTD dose [65, 77,78,79,80].
Thus, over the past decade, we have learned that dasatinib 50 mg daily is safer and as effective as 100 mg daily [30, 31, 81]. Bosutinib side-effects can be mitigated with a dose-escalation schedule: 100 mg daily x 1-2 weeks, then 200 mg daily x 2–4 weeks, then 300 mg daily x 1 month, then adjust the dose to 400 mg daily (approved dose in frontline therapy) or a lower or higher dose (500 mg daily; approved in subsequent-line therapies) depending on the response, CML status (frontline or later lines) and side-effects [82, 83]. Nilotinib can be de-escalated safely from 300–400 mg BID to 150–200 mg BID, or even 200 mg daily, if side-effects occur, or if there are safety concerns in patients who have responded optimally [84, 85]. Recent studies have also shown that dose-adjusted ponatinib schedules (e.g. starting at 45 mg daily in T315I mutated CML, 30 mg in others, and reducing the dose to 15 mg daily once BCR::ABL1 transcripts [IS] are <1%) are as effective and significantly safer than a fixed dose of 45 mg daily (reduced only if toxicities) [65, 67, 80].
Asciminib was approved in 2021 for the third-line therapy of CML and for the treatment of T315I-mutated CML. Emerging experiences suggest its safety and efficacy with the short-term follow-up [86,87,88,89]. Though all grade and ≥ grade 3 AEs were lower with asciminib than bosutinib in the ASCEMBL trial, when studied at the higher 200 mg BID dose for T315I-mutated CML, asciminib showed ≥ grade 3 adverse events (AEs) in 60% of patients and AOEs in 8% of patients [87, 90]. However, longer follow-up in larger patient numbers will define better its safety [91].
Based on the above clarifications, our recommendations are as follows: For a patient on frontline imatinib therapy, if toxicities emerge in the setting of a good molecular response, the imatinib dose can be reduced to 300 mg or 200 mg daily before considering a TKI change. Response by peripheral blood BCR:ABL1 testing should be carefully monitored after dose reduction. Alternatively, a second-generation TKI can be swapped for imatinib. However, if the patient is already responding well on imatinib, then the dose of the second-generation TKI may not have to be the salvage approved dose (dasatinib 100 mg daily, nilotinib 400 mg BID, bosutinib 500 mg daily). Rather, the lower-dose schedule can be used more safely and with equal or better efficacy (dasatinib 50 mg or even 20 mg daily; nilotinib 150–300 mg BID or 200 mg daily; bosutinib 200–300 mg daily). Again, this strategy should be safe in the context of closely following the disease burden by BCR::ABL1 transcripts monitoring. If the patient has CML failure on second-line TKI therapy because of similar or new toxicities, the second-generation TKIs can be rotated (dasatinib, bosutinib, nilotinib) based on the type of toxicity and patient’s comorbidities. If toxicities occur with all the four TKIs after dose adjustments, then a third-generation TKI can be used (for example, ponatinib, not 45 mg daily, but rather 15 mg daily since most of these patients would have BCR::ABL1 transcripts (IS) < 1%). Asciminib 40 mg BID or 80 mg daily would be another third-generation TKI option. In a situation where patients start with second-generation TKIs as frontline therapy (more common today) and have toxicities with other second-generation TKIs, imatinib can be an excellent TKI option with low side effects and low cost.
While most TKI toxicities resolve with dose reductions, there are some for which this strategy might not be appropriate: 1) recurrent pleural effusions (if more than once with dasatinib after dose reductions; less commonly with other TKIs such as bosutinib); 2) pulmonary hypertension (usually on dasatinib therapy; unlike the common belief, in some patients it can resolve over time with a short course of steroids and sildenafil) [92, 93]; 3) VOE or AOE with ponatinib, nilotinib or other TKIs (bosutinib and imatinib are the safer TKIs in such instances); 4) enterocolitis with bosutinib (and less often with dasatinib; rarely patients on bosutinib with enterocolitis have undergone unnecessary bowel resections because bosutinib was not held) [58]; 5) dementia conditions, including, rarely, Lewy body-like dementia, or parkinsonism (can resolve weeks to months after discontinuing the TKI); 6) immune-mediated myocarditis, hepatitis or nephritis. In the above situations, changing to another TKI rather than dose reduction would be the safer choice. Table 1 summarizes the common toxicities with the TKIs and the recommended dose reductions.
Management of CML post-TKI resistance
In this section, we discuss the therapy of resistant CML based on BCR::ABL1 transcripts (IS) > 1% after more than one year of frontline TKI therapy, or after adequate second-line therapy at an optimal TKI dose schedule given for 3–6 months.
Among patients who develop resistance to frontline imatinib therapy, changing to a second-generation TKI is the most appropriate course. The choice of the TKI depends on the patient’s comorbidities and on the ABL1 kinase domain (KD) mutations, which should be performed on all patients with TKI resistance considered for a change of therapy (can be guiding in 50%) [94]. More than 100 ABL1 KD mutations have been reported; on asciminib therapy, new mutations involving the myristoyl pocket (site of asciminib binding) are emerging [95]. Table 2 shows the in-vitro sensitivity profiles of the TKIs to different ABL1 KD mutations. Patients who develop resistance on frontline or subsequent-line TKIs (dasatinib, bosutinib, nilotinib), should not be rotated to other second-generation TKIs unless indicated by a guiding mutation. In such patients, changing to a third-generation TKI (ponatinib, asciminib) is appropriate [96].
An alternative for younger patients is allogeneic stem cell transplantation (SCT), which remains highly curative as a one-time procedure [97,98,99]. With the choices of stem cell donor increasing (matched sibling, matched unrelated, haplo-identical, and umbilical cord), SCT is now an option for most patients if needed. In older patients, it is perhaps reasonable to forgo allogeneic SCT, with its potential morbidity, in favor of strategies that can maintain patients in CML-CP (albeit not in CCyR) for a decade or more. These include combining the most optimal TKI with other agents such as hypomethylating agents (decitabine, azacitidine), hydroxyurea, low-dose cytarabine, or omacetaxine mepesuccinate (though for 2–5 days/month rather than the approved two weeks/month schedule, which can be very myelosuppressive) [100,101,102]. In such instances, maintaining good disease control with a partial or even no cytogenetic response might be acceptable. The key to optimal therapy is to maintain a daily TKI dose schedule. As discussed above, the 10-year CML-specific survival could be as high as 90% among patient with BCR::ABL1 transcripts (IS) 1–10%, and 75% with transcripts >10% [46]. Because of its different mechanism of action, asciminib is being combined with other TKIs in ongoing investigational trials. This approach should not be carried into the standard practice since such combinations may increase the cost of care significantly and may be associated with unforeseeable longer-term synergistic toxicities.
Third-generation TKIs
The development of resistance in a subset of patients on second-generation TKIs, and of T315I mutations (resistant to imatinib and all second-generation TKIs) provided a therapeutic niche for third-generation TKIs.
Ponatinib
The first third-generation TKI to receive regulatory approval was ponatinib, which was specifically designed to bypass the ABL1-T315I KD gatekeeper mutation [103, 104]. In in-vitro studies, ponatinib had a dose-dependent inhibition of the Abl kinase activity with the highest doses required for cells with ABL1-T315I, E255V mutations and compound mutations (Table 2) [104]. Ponatinib was originally developed at a dose of 45 mg daily, based on the Phase 1 study establishing it as the Phase 2 dose [63, 64, 105]. However, recent studies showed that lower dose schedules were effective and safer [65, 67].
The Phase 2 PACE trial accrued 449 patients, including 267 patients with CML-CP (203 patients with prior TKI resistance or intolerance; 64 patients with T315I mutation), 145 patients with advanced phase CML (83 with AP; 62 with BP), and 32 patients with Ph-positive acute lymphoblastic leukemia (ALL) who received ponatinib 45 mg daily [63]. Among patients with CML, > 90% had received ≥2 prior TKIs, and 60% had received ≥3 prior TKIs. In CML-CP, among patients with prior TKI resistance or intolerance, 50% achieved CCyR and 35% an MMR. Among patients in CML-CP with a T315I mutation, 68% achieved CCyR and 56% had MMR. Eight of 14 (57%) CML-CP patients who had a compound ABL1 KD mutation that included T315I attained a CCyR. In the second, third and ≥ fourth line TKI settings in CML-CP, the CCyR rates were 74%, 56%, and 38%, and MMR rates 47%, 36% and 31%, respectively. The estimated 5-year OS was 73% for the CML-CP group. The results were also positive in CML-AP, CML-BP, and Ph-positive ALL. This led to the Food and Drug Administration (FDA) accelerated approval in 2012 of ponatinib 45 mg daily for patients with CML post resistance or intolerance to other TKIs, and for Ph-positive ALL (full approval in 2016 for CML-CP or in transformation, and Ph-positive ALL when no other TKI is indicated, and for T315I-mutated disease) [106, 107]. In this study, grade 3–4 hypertension was observed in 12%, any grade AOEs in 25% (severe AOEs in 20%). A subsequent report by an independent adjudication committee reported a lower frequency of adverse events (AEs) [108].
The OPTIC trial was a response-based dose-adjusted study of ponatinib in which 282 patients with CML (intolerant/resistant to ≥2 prior TKI or having the T315I mutation) were randomized to a starting dose of ponatinib 45 mg, 30 mg or 15 mg daily [65]. The dose in the first 2 arms was reduced to 15 mg daily upon achievement of CCyR (BCR::ABL1 transcripts [IS] < 1%). The 45 mg daily arm resulted in significantly higher response rates in T315I-mutated CML compared with the 30 mg and 15 mg doses (CCyR rate 60% versus 25% versus 10%), but less so in other CML subsets (CCyR rate 54% versus 41% versus 44%). Interestingly, the 3-year OS rates were similar with the 3 dose schedules, ≈90%. The incidence of AEs, serious AEs and AOEs were significantly less than in previous studies. However the eligibility criteria addressing the cardiovascular risk status were different in the OPTIC and PACE trials, which may explain some of the differences in toxicity patterns.
In a pooled analysis of the patients on the PACE and OPTIC trials, the dose-adjusted schedules showed similar, if not better, results and significantly reduced toxicities compared with the 45 mg fixed dose (with dose reductions only for toxicities) [67]. The results with ponatinib appear to be equally promising in real-life experiences (Table 3). Breccia and colleagues reported their experience in 666 patients with CML treated with ponatinib in Italy [109]. Ninety percent of patients had prior exposure to 2 TKIs (68% to 3 and 22% to 4). Among 515 patients in CML-CP, the cumulative CCyR rate was 77%, MMR rate 65% and MR4 rate 43%. Among the 151 patients with advanced phase CML the CCyR rate was 50%, MMR rate 37% and MR4 rate 28%. With a median follow-up of 18 months, only 28/515 patients with CML-CP (5%) had died; 113 (22%) required a dose reduction from 45 mg for an AE [109].
There are no head-to-head comparisons of ponatinib versus second-generation TKIs or other third-generation TKIs (such as asciminib). To provide some context regarding the potential efficacy of ponatinib versus second-generation TKIs, two studies compared the experiences in CML third-line therapy with ponatinib versus second-generation TKIs. In a systematic review by Lipton and colleagues that included 12 clinical trials comparing ponatinib to second-generation TKI in patients resistant to ≥1 prior second-generation option, ponatinib was associated with significantly superior rates of responses [96]. In another study, 354 patients with CML-CP who received third-line therapy with ponatinib on PACE-OPTIC (n = 150) or at MD Anderson (n = 31) were compared to 173 patients from MD Anderson treated with second-generation TKIs as third-line therapy (96 patients in each TKI arm after 1:1 propensity matching) [110]. Ponatinib therapy was associated with significantly higher molecular response rates. The estimated 3-year progression-free survival (PFS) rates were 83% versus 59% (p < 0.001) and OS rates 87% versus 83% (p = 0.03) for ponatinib and second-generation TKI, respectively. On multivariate analysis, ponatinib was an independent favorable factor for survival (HR 0.45; p = 0.003). In a combined analysis of a subset of patients from the PACE and OPTIC trials who were treated with a starting ponatinib dose of 45 mg daily and had exposure to ≥1 prior second-generation TKI, the 2-year MMR rate in second-line therapy and ≥ third-line therapy for both trials were similar at 38% and ≈30%, respectively [80]. In both trials, responses were superior in patients with T315I mutation than in those who either had no mutation or other ABL1 KD mutations. In the OPTIC trial the 2-year PFS rate was 91% and OS rate 97% with ponatinib as second-line therapy while they were 73% and 88%, respectively, with ponatinib as ≥ third-line therapy. These figures were slightly inferior in the PACE trial [80]. Thus, ponatinib is highly active and a response-directed dose schedule leads to higher tolerability/safety and superior survival outcomes even in multi-TKI exposed or T315I-mutated CML.
Asciminib
The next third-generation TKI to receive regulatory approval for the treatment of CML was asciminib, in 2021 [86]. Asciminib works through a novel mechanism that involves binding to the myristoyl pocket of the ABL1 and allosterically inhibiting the overactive kinase activity [111, 112].
The asciminib FDA approval was for CML-CP with previous exposure to ≥ 2 TKI or the presence of T315I mutation [86]. It was based on the ASCEMBL Phase 3 randomized trial that compared (2:1) asciminib (40 mg twice daily, n = 157) to bosutinib 500 mg daily (no allowance for dose-adjusted bosutinib; n = 76) in patients with CML-CP and prior exposure to ≥ 2 TKIs (patients were required to have had resistance to a second-line TKI or intolerance to the most recent TKI and no T315I or V299L mutation) [87]. Asciminib was used as third-line therapy in 52% patients compared with 40% for bosutinib. Prior exposure to ponatinib was noted in 15% patients on the asciminib arm and 24% patients on bosutinib. The study demonstrated significantly higher MMR rates at 6 months (the primary study endpoint) with asciminib (25% versus 12%). The long-term follow-up showed a higher MMR rate at 2 years (38% versus 16%) but the 2-year OS was similar, 97% with asciminib and 99% with bosutinib [113]. Of note, the bosutinib results in this control arm were worse than the published bosutinib data in the third-line settings. For example, Hochhaus et al. reported a cumulative 2-year MMR rate of 75% (2-year MR4 rate 60%, and MR4.5 rate 45%) in 55 patients treated with bosutinib as third-line therapy in the BYOND study [114].
The asciminib FDA approval also covered T315I-mutated CML, but the asciminib dose-schedule for this subset is 200 mg BID, which quadruples the cost (to about $1.3 million/year). This raises the issue of the treatment value of asciminib in relation to other options, e.g. allogeneic SCT. In a recent update of 48 patients with CML-CP and T315I mutations treated on the expansion cohort of the Phase 1 trial at a dose of 200 mg BID, the 2-year MMR rate was 49%. The rate of AOEs was 8% and two patients had fatal AEs [90].
In real-world experiences from Spain, Russia, Canada, the Netherlands, Italy and the United Kingdom involving > 250 patients with CML, asciminib therapy resulted in CCyR rates of 58%–70%, MMR rates of 33–52% and MR4 rates of 16–32% [115,116,117,118,119]. Patients with prior ponatinib exposure (mostly intolerant) had significantly lower response rates. In the study from the Netherlands, only 1/10 patients (10%) with primary ponatinib resistance achieved a CCyR with asciminib [117].
There are no trials that compare head-to-head the efficacy of ponatinib and asciminib. Table 3 shows the response rates reported with ponatinib and asciminib in real-world experiences. In prospective trials, the outcomes of patients with T315I-mutated CML appear to be better with ponatinib than with asciminib (Table 4). Also, ponatinib has shown better survival in third-line CML therapy compared with the second-generation TKIs; asciminib has not shown better survival so far in studies with shorter follow-up. Trials that compare the efficacy and toxicity profiles of ponatinib (response dose-adjusted) and asciminib in second- or ≥ third-line therapy of CML are needed.
Olverembatinib
Olverembatinib (HQP1351) is a new third-generation TKI that showed in cell lines the potential capability to inhibit both wild type BCR::ABL1 and T315I-mutated BCR::ABL1 [120]. The drug is approved in China for the treatment of adults with TKI-resistant CML in CP or AP harboring the T315I mutation. Because of the approval in China (1.4 billion people), and because of the encouraging results, detailing the reported results is valuable.
In pre-clinical studies, olverembatinib has shown an exciting efficacy profile across CML mutants, compared with second- and third generation TKIs (Table 2). In a study of 101 patients with CML (CML-CP = 86, CML-AP = 15) treated with olverembatinib, 62% had T315I mutation and 83% had ≥2 TKIs. At the 5-year follow-up, in CML-CP, the CCyR rate was 71%, the MMR rate was 55%, and the estimated 4-year PFS rate 85.6% [121]. Grade 3/4 adverse cardiovascular events were noted in 12% of patients, most commonly hypertension. One patient each had a retinal vein occlusion, CNS infarction, and a myocardial infarction. In CML-AP, the CCyR rate was 40%, the MMR rate 40%, and the estimated 4-year PFS rate 50%. In another update of 64 patients with CML-CP (n = 41) or CML-AP (n = 23) and T315I mutation, the results were encouraging. In CML-CP the CCyR rate was 71%, the MMR rate 58%, the estimated 3-year PFS rate was 86% and OS rate 95% [122]. In CML-AP, the CCyR rate was 52%, the MMR rate 48%, the estimated 3-year PFS rate was 57% and OS rate 70%. A recent US study treated 30 patients with olverembatinib 30, 40 or 50 mg every other day (24/30 with ≥3 prior TKIs; prior ponatinib 21/30; ponatinib resistance 17/30). The CCyR rate in CML-CP was 69% and the MMR rate 44%. Among patients with ponatinib resistance 5/9 (56%) achieved CCyR, and 6/11 (55%) achieved MMR [123].
Table 4 shows the efficacy of the third-generation TKIs from prospective clinical trials, stratified based on T315I mutation data and the line of TKI salvage.
Addressing the most frequent question in CML management: changing TKI therapy in a patient with BCR::ABL1 transcripts (IS) < 1% but not in MMR, DMR or undetectable levels
The absence of MMR by one year of TKI therapy is considered a “warning” in the ELN recommendations. The true clinical risk of not achieving this endpoint may be over-estimated. Thus, in patients who do not have high-risk CML features (high-risk additional cytogenetic abnormality, mutations in genes such as ASXL1) and in whom TFR is not an aim, it is reasonable to continue the same TKI at the same dose, provided the patient tolerates the drug well, maintains compliance to therapy and is monitored every 3–6 months. As detailed earlier, in patients with persistent low-level BCR::ABL1 transcripts (IS) 0.1–1%, the long-term CML-specific survival is excellent (10-year OS rate about 90%). Changing to a third-generation TKI in such situations may increase the toxicities and cost, without improving the long-term outcome.
Approach to patients with T315I mutation
The treatment options in patients with ABL1-T315I mutation have evolved with the approval of ponatinib and asciminib, and the promising results from reported and ongoing trials of olverembatinib. Cross-trial comparison of data shows that ponatinib may result in better responses compared with asciminib in T315I-mutated CML and should be the preferred option in the absence of absolute contraindications. Also, as discussed earlier, the approved dose of asciminib for T315I-mutated CML has a prohibitive cost (about $1.3 million/year) and becomes a major “financial toxicity,” given that these drugs need to be continued for years.
Options beyond TKIs should be explored. In the early trials with omacetaxine mepesuccinate, the drug led to a steady reduction in T315I-mutated BCR::ABL1 transcripts, rendering these patients amenable to treatment with earlier-generation TKIs [124]. Small patient series have also reported similar activity with IFN alpha sequenced or combined with TKIs [125,126,127]. With the advent of TKIs that possess activity against T315I-mutated CML, these options are rarely used, but remain relevant in the rare patients who are unable to tolerate third-generation TKIs or who cannot access or afford them. Allogeneic SCT remains a one-time appropriate option in T315I-mutated CML, but preferably after a trial of therapy with ponatinib, as retrospective data have shown the superiority of ponatinib over SCT in CML-CP with T315I mutation [128].
Conclusion
As patients with CML have a near-normal life span on TKI therapies, it has become increasingly important to clarify the goals of therapy (survival; TFR) and the likelihood that such goals can be achieved on different TKIs, and then to revisit the treatment milestones that have been standard for the past 2 decades. It is also important to clarify the benefit versus toxicity (clinical and financial) of changing TKIs more frequently than necessary in pursuit of goals that may not be achievable (for example, changing TKIs in a patient with detectable BCR::ABL1 [IS] transcripts > 0.01 % or > 0.1% after >5 years of TKI therapy in pursuit of TFR). In patients who are not candidates for TFR, any response below BCR::ABL1 (IS) transcripts <1% is a reasonable goal. More stringent molecular goals could be considered in patients in whom a TFR is an aim. In patients with non-prohibitive TKI toxicity, dose reductions should be the first step before a TKI change since dose reductions in the right context are effective and safer, often leading to better treatment compliance. This review attempts to analyze these issues and stimulate discussions as to the most appropriate courses of action in the management of frontline and later lines of TKI therapies in CML in the community practice.
References
Kantarjian H, Cortes J, Jabbour E & O’Brien S in Mol Hematol. https://doi.org/10.1002/9781119252863.ch6. 2019:71–86.
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95:691–709.
Kantarjian H, Jabbour E, Cortes J. in Harrison’s Principles of Internal Medicine, 21e (eds J Loscalzo et al.) (McGraw-Hill Education, 2022).
Sasaki K, Jabbour E, Corte, J, Kantarjian H. in The MD Anderson Manual of Medical Oncology, 4e (eds Hagop M. Kantarjian, Robert A. Wolff, & Alyssa G. Rieber) (McGraw Hill Education, 2022).
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Deininger MW, Shah NP, Altman JK, Berman E, Bhatia R, Bhatnagar B, et al. Chronic Myeloid Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18:1385–415.
Molica M, Naqvi K, Cortes JE, Paul S, Kadia TM, Breccia M, et al. Treatment-free remission in chronic myeloid leukemia. Clin Adv Hematol Oncol. 2019;17:686–96.
Jain P, Kantarjian H, Alattar ML, Jabbour E, Sasaki K, Nogueras Gonzalez G, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from five clinical trials. Lancet Haematol. 2015;2:e118–e128.
Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J Clin Oncol. 2016;34:2851–7.
Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406.
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376:917–27.
Breccia M Imatinib improved the overall survival of chronic myeloid leukemia patients in low- and middle-income countries: A therapeutic goal has been reached. eClinicalMedicine.2020;19. https://doi.org/10.1016/j.eclinm.2020.100277.
Maas CCHM, van Klaveren D, Ector GICG, Posthuma EFM, Visser O, Westerweel PE, et al. The evolution of the loss of life expectancy in patients with chronic myeloid leukaemia: a population-based study in the Netherlands, 1989–2018. British Journal of Haematology. 2022;196:1219–24.
Chen CT, Kesselheim AS. Journey of Generic Imatinib: A Case Study in Oncology Drug Pricing. Journal of Oncology Practice. 2017;13:352–5.
Cole AL, Dusetzina SB. Generic Price Competition For Specialty Drugs: Too Little, Too Late. Health Affairs. 2018;37:738–42.
Jenei K, Lythgoe MP & Prasad V CostPlus and implications for generic imatinib. Lancet Regional Health – Americas. 2022;13. https://doi.org/10.1016/j.lana.2022.100317.
Kantarjian H, Pau S, Thakkar J, Jabbour E. The influence of drug prices, new availability of inexpensive generic imatinib, new approvals, and post-marketing research on the treatment of chronic myeloid leukaemia in the USA. The Lancet Haematology. 2022;9:e854–e861.
Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121:4439–42.
Kantarjian H, Rajkumar SV. Why Are Cancer Drugs So Expensive in the United States, and What Are the Solutions? Mayo Clinic Proceedings. 2015;90:500–4.
Kantarjian H, Steensma D, Rius Sanjuan J, Elshaug A, Light D. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract. 2014;10:e208–211.
Kenzik KM, Bhatia R, Bhatia S. Expenditures for First- and Second-Generation Tyrosine Kinase Inhibitors Before and After Transition of Imatinib to Generic Status. JAMA Oncol. 2020;6:542–6.
Kenzik KM, Bhatia R, Williams GR, Bhatia S. Medicare and patient spending among benefiiaries diagnosed with chronic myelogenous leukemia. Cancer. 2019;125:2570–8.
Talon B, Calip GS, Lee TA, Sharp LK, Patel P, Touchette DR. Trend in Tyrosine Kinase Inhibitor Utilization, Price, and Out-of-Pocket Costs in Patients With Chronic Myelogenous Leukemia. JCO Oncol Practice. 2021;17:e1811–e1820.
Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–53.
Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic. Leukemia. Blood. 1984;63:789–99.
Uz B, Buyukasik Y, Atay H, Kelkitli E, Turgut M, Bektas O, et al. EUTOS CML prognostic scoring system predicts ELN-based ‘event-free survival’ better than Euro/Hasford and Sokal systems in CML patients receiving front-line imatinib mesylate. Hematology. 2013;18:247–52.
Pfirrmann M, Clark RE, Prejzner W, Lauseker M, Baccarani M, Saussele S, et al. The EUTOS long-term survival (ELTS) score is superior to the Sokal score for predicting survival in chronic myeloid leukemia. Leukemia. 2020;34:2138–49.
Shahrin NH, Wadham C, Branford S. Defining Higher-Risk Chronic Myeloid Leukemia: Risk Scores, Genomic Landscape, and Prognostication. Curr Hematol Malig Rep. 2022;17:171–80.
Zhang WW, Cortes JE, Yao H, Zhang L, Reddy NG, Jabbour E, et al. Predictors of Primary Imatinib Resistance in Chronic Myelogenous Leukemia Are Distinct From Those in Secondary Imatinib Resistance. J Clin Oncol. 2009;27:3642–9.
Gener-Ricos G, Haddad F, Sasaki K, Issa GC, Skinner J, Takahashi K, et al. Long-Term Follow-up of Low-Dose Dasatinib (50mg Daily) As Frontline Therapy in Newly Diagnosed Chronic Myeloid Leukemia. Blood. 2022;140:1493–4.
Naqvi K, Jabbour E, Skinner J, Anderson K, Dellasala S, Yilmaz M, et al. Long-term follow-up of lower dose dasatinib (50 mg daily) as frontline therapy in newly diagnosed chronic-phase chronic myeloid leukemia. Cancer. 2020;126:67–75.
Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34:2333–40.
Kantarjian H, O’Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119:1981–7.
Radich JP, Hochhaus A, Masszi T, Hellmann A, Stentoft J, Casares MTG, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31:1525–31.
Shah NP, García-Gutiérrez V, Jiménez-Velasco A, Larson S, Saussele S, Rea D, et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. Leuk Lymphoma. 2020;61:650–9.
Gugliotta G, Castagnetti F, Breccia M, Levato L, Intermesoli T, D’Adda M, et al. Treatment-free remission in chronic myeloid leukemia patients treated front-line with nilotinib: 10-year followup of the GIMEMA CML 0307 study. Haematologica. 2022;107:2356–64.
Radich JP, Hochhaus A, Masszi T, Hellmann A, Stentoft J, Casares MTG, et al. Treatment-free remission following frontline nilotinib in patients with chronic phase chronic myeloid leukemia: 5-year update of the ENESTfreedom trial. Leukemia. 2021;35:1344–55.
Haddad FG, Sasaki K, Issa GC, Garcia-Manero G, Ravandi F, Kadia T, et al. Treatment-free remission in patients with chronic myeloid leukemia following the discontinuation of tyrosine kinase inhibitors. Am J Hematol. 2022;97:856–64.
Atallah E, Schiffer CA, Radich JP, Weinfurt KP, Zhang MJ, Pinilla-Ibarz J, et al. Assessment of Outcomes After Stopping Tyrosine Kinase Inhibitors Among Patients With Chronic Myeloid Leukemia: A Nonrandomized Clinical Trial. JAMA Oncology. 2021;7:42–50.
Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232–8.
Hughes TP, Saglio G, Kantarjian HM, Guilhot F, Niederwieser D, Rosti G, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123:1353–60.
Nazha A, Kantarjian H, Jain P, Romo C, Jabbour E, Quintas-Cardama A, et al. Assessment at 6 months may be warranted for patients with chronic myeloid leukemia with no major cytogenetic response at 3 months. Haematologica. 2013;98:1686–8.
Shanmuganathan N, Hughes TP. Molecular monitoring in CML: how deep? How often? How should it influence therapy? Hematology. 2018;2018:168–76.
Kantarjian HM, O’Brien S, Cortes JE, Shan J, Giles FJ, Rios MB, et al. Complete cytogenetic and molecular responses to interferon-α-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97:1033–41.
Shaya J, Pettit K, Kandarpa M, Bixby D, Mercer J, Talpaz M. Late Responses in Patients With Chronic Myeloid Leukemia Initially Refractory to Tyrosine Kinase Inhibitors. Clin Lymphoma Myeloma Leukemia. 2022;22:17–23.
Bidikian A, Jabbour E, Issa GC, Short NJ, Sasaki K, Kantarjian H Chronic myeloid leukemia without major molecular response after 2 years of treatment with tyrosine kinase inhibitor. Am J Hematol. n/a, https://doi.org/10.1002/ajh.26836.
Hughes TP, Leber B, Cervantes F, Spector N, Pasquini R, Clementino NCD, et al. Sustained deep molecular responses in patients switched to nilotinib due to persistent BCR-ABL1 on imatinib: final ENESTcmr randomized trial results. Leukemia. 2017;31:2529–31.
Patel AB, O’Hare T, Deininger MW. Mechanisms of Resistance to ABL Kinase Inhibition in Chronic Myeloid Leukemia and the Development of Next Generation ABL Kinase Inhibitors. Hematol Oncol Clin North Am. 2017;31:589–612.
Braun TP, Eide CA, Druker BJ. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell. 2020;37:530–42.
Jabbour EJ, Kantarjian H, Eliasson L, Megan Cornelison A, Marin D. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am J Hematol. 2012;87:687–91.
Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–8.
Hehlmann R, Cortes JE, Zyczynski T, Gambacorti-Passerini C, Goldberg SL, Mauro MJ, et al. Tyrosine kinase inhibitor interruptions, discontinuations and switching in patients with chronic-phase chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol. 2019;94:46–54.
Henk HJ, Woloj M, Shapiro M, Whiteley J. Real-world analysis of tyrosine kinase inhibitor treatment patterns among patients with chronic myeloid leukemia in the United States. Clin Ther. 2015;37:124–3.
Copland M. Is There a Role for Dose Modification of TKI Therapy in CML. Curr Hematol Malig Rep. 2019;14:337–45.
Lipton JH, Brümmendorf TH, Gambacorti-Passerini C, Garcia-Gutiérrez V, Deininger MW, Cortes JE. Long-term safety review of tyrosine kinase inhibitors in chronic myeloid leukemia - What to look for when treatment-free remission is not an option. Blood Rev. 2022;56:100968.
Fox LC, Cummins KD, Costello B, Yeung D, Cleary R, Forsyth C, et al. The incidence and natural history of dasatinib complications in the treatment of chronic myeloid leukemia. Blood Adv. 2017;1:802–11.
Kantarjian HM, Cortes JE, Kim DW, Khoury HJ, Brümmendorf TH, Porkka K, et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood. 2014;123:1309–18.
Khoury HJ, Gambacorti-Passerini C, Brümmendorf TH. Practical management of toxicities associated with bosutinib in patients with Philadelphia chromosome-positive chronic myeloid leukemia. Ann Oncol. 2018;29:578–87.
Giles FJ, Rea D, Rosti G, Cross NCP, Steegmann JL, Griskevicius L, et al. Impact of age on efficacy and toxicity of nilotinib in patients with chronic myeloid leukemia in chronic phase: ENEST1st subanalysis. J Cancer Res Clin Oncol. 2017;143:1585–96.
Wang Z, Jiang L, Yan H, Xu Z, Luo P. Adverse events associated with nilotinib in chronic myeloid leukemia: mechanisms and management strategies. Exp Rev Clin Pharmacol. 2021;14:445–56
Varma MR, Mathew S, Krishnadas D, Vinayakumar KR. Imatinib-induced pancreatitis. Ind J Pharmacol. 2010;42:50–52.
Palandri F, Castagnetti F, Soverini S, Poerio A, Gugliotta G, Luatti S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758–61.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A Phase 2 Trial of Ponatinib in Philadelphia Chromosome–Positive Leukemias. N Engl J Med. 2013;369:1783–96.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome–positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404.
Cortes J, Apperley J, Lomaia E, Moiraghi B, Undurraga Sutton M, Pavlovsky C, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138:2042–50.
Chan O, Talati C, Isenalumhe L, Shams S, Nodzon L, Fradley M, et al. Side-effects profile and outcomes of ponatinib in the treatment of chronic myeloid leukemia. Blood Adv. 2020;4:530–8.
Jabbour EJ, Deininger MW, Abruzzese E, Apperley JF, Cortes JE, Chuah C, et al. Dose Modification Dynamics of Ponatinib in Patients with Chronic-Phase Chronic Myeloid Leukemia (CP-CML) from the PACE and Optic Trials. Blood. 2021;138:2550–2550.
Pasvolsky O, Leader A, Lakobishvili Z, Wasserstrum Y, Kornowski R, Raanani P. Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia. Cardio-Oncol. 2015;1:5.
Cortes J. How to manage CML patients with comorbidities. Blood. 2020;136:2507–12.
Chow EJ, Doody DR, Wilkes JJ, Becker LK, Chennupati S, Morin PE, et al. Adverse events among chronic myelogenous leukemia patients treated with tyrosine kinase inhibitors: a real-world analysis of health plan enrollees. Leuk Lymphoma. 2021;62:1203–10.
Zulbaran-Rojas A, Lin HK, Shi Q, Williams LA, George B, Garcia-Manero G, et al. A prospective analysis of symptom burden for patients with chronic myeloid leukemia in chronic phase treated with frontline second- and third-generation tyrosine kinase inhibitors. Cancer Med. 2018;7:5457–69.
Busque L, Harnois M, Szuber N, Delage R, Mollica L, Olney H, et al. S159: QUÉBEC CML RESEARCH GROUP ANALYSIS OF TREATMENT PATTERNS IN CHRONIC MYELOGENOUS LEUKEMIA: SWITCHING IS DRIVEN BY INTOLERANCE AND SIMILAR ACROSS TYROSINE KINASE INHIBITORS AND LINES OF TREATMENT. HemaSphere. 2022;6:60–61.
Sachs JR, Mayawala K, Gadamsetty S, Kang SP, de Alwis DP. Optimal Dosing for Targeted Therapies in Oncology: Drug Development Cases Leading by Example. Clin Cancer Res. 2016;22:1318–24.
Chen Y, Liu Z, Zou J, Wang D, He W, Meng L, et al. Low-dose tyrosine kinase inhibitors in patients with chronic myeloid leukemia: a retrospective study in China. Haematologica. 2022;107:1966–70.
Claudiani S, Apperley JF, Szydlo R, Khan A, Nesr G, Hayden C, et al. TKI dose reduction can effectively maintain major molecular remission in patients with chronic myeloid leukaemia. Br J Haematol. 2021;193:346–55.
Clark RE, Polydoros F, Apperley JF, Milojkovic D, Rothwell K, Pocock C, et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non-randomised, phase 2 trial. Lancet Haematol. 2019;6:e375–e383.
Fraisse J, Dinart D, Tos D, Bellera C, Mollevi C. Optimal biological dose: a systematic review in cancer phase I clinical trials. BMC Cancer. 2021;21:60.
Saglio G, Fava C, Gale RP. Precision tyrosine kinase inhibitor dosing in chronic myeloid leukemia? Haematologica. 2019;104:862–4.
Iurlo A, Cattaneo D, Bucelli C, Breccia M. Dose Optimization of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: A New Therapeutic Challenge. J Clin Med. 2021;10:515.
Kantarjian HM, Jabbour E, Deininger M, Abruzzese E, Apperley J, Cortes J, et al. Ponatinib after failure of second-generation tyrosine kinase inhibitor in resistant chronic-phase chronic myeloid leukemia. Am J Hematol. 2022;97:1419–26.
Jabbour E, Sasaki K, Haddad FG, Issa GC, Skinner J, Dellasala S, et al. Low-dose dasatinib 50 mg/day versus standard-dose dasatinib 100 mg/day as frontline therapy in chronic myeloid leukemia in chronic phase: A propensity score analysis. Am J Hematol. 2022;97:1413–8.
Kota V, Brümmendorf TH, Gambacorti-Passerini C, Lipton JH, Kim DW, An F, et al. Efficacy and safety following bosutinib dose reduction in patients with Philadelphia chromosome‒positive leukemias. Leuk Res. 2021;111:106690.
Cortes JE, Apperley JF, DeAngelo DJ, Deininger MW, Kota VK, Rousselot P, et al. Management of adverse events associated with bosutinib treatment of chronic-phase chronic myeloid leukemia: expert panel review. J Hematol Oncol. 2018;11:143.
Rea D, Cayuela J-M, Dulucq S, Etienne G. Molecular Responses after Switching from a Standard-Dose Twice-Daily Nilotinib Regimen to a Reduced-Dose Once-Daily Schedule in Patients with Chronic Myeloid Leukemia: A Real Life Observational Study (NILO-RED). Blood. 2017;130:318.
Stagno F, Abruzzese E, Iurlo A, Pane F, Attolico I, Sportoletti P, et al. Treatment-Free Remission Outcome in Patients with Chronic Myeloid Leukemia in Chronic Phase Following One Year of Nilotinib De-Escalation: 96-Week Update of Dante Study. Blood. 2022;140:9614–6.
US, F. D. A. FDA approves asciminib for Philadelphia chromosome-positive chronic myeloid leukemia. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-asciminib-philadelphia-chromosome-positive-chronic-myeloid-leukemia.
Réa D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138:2031–41.
Garcia-Gutiérrez V, Luna A, Alonso-Dominguez JM, Estrada N, Boque C, Xicoy B, et al. Safety and efficacy of asciminib treatment in chronic myeloid leukemia patients in real-life clinical practice. Blood Cancer Journal. 2021;11:16.
Yeung DT, Shanmuganathan N, Hughes TP. Asciminib: a new therapeutic option in chronic-phase CML with treatment failure. Blood. 2022;139:3474–9.
Hughes T, Cortes JE, Rea D, Mauro MJ, Hochhaus A, Kim DW, et al. P704: ASCIMINIB PROVIDES DURABLE MOLECULAR RESPONSES IN PATIENTS (PTS) WITH CHRONIC MYELOID LEUKEMIA IN CHRONIC PHASE (CML-CP) WITH THE T315I MUTATION: UPDATED EFFICACY AND SAFETY DATA FROM A PHASE I TRIAL. HemaSphere.2020;6:599−600.
Hughes T, Cortes JE, Takahashi N, Larson RA, Issa CG, Bombaci F. et al.ASC4FIRST: A Phase III Study of Asciminib vs Investigator-Selected Tyrosine Kinase Inhibitor in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP).Blood. 2022;140:6767–8.
Weatherald J, Chaumais MC, Savale L, Jaïs X, Seferian A, Canuet M, et al. Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: a population-based study. Eur Resp J. 2017;50:1700217.
Özgür Yurttaş N, Eşkazan AE. Dasatinib-induced pulmonary arterial hypertension. Br J Clin Pharmacol. 2018;84:835–45.
Hochhaus A, Breccia M, Saglio G, García-Gutiérrez V, Réa D, Janssen J, et al. Expert opinion—management of chronic myeloid leukemia after resistance to second-generation tyrosine kinase inhibitors. Leukemia. 2020;34:1495–502.
Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibitor Failure. N Engl J Med. 2019;381:2315–26.
Lipton JH, Bryden P, Sidhu MK, Huang H, McGarry LJ, Lustgarten S, et al. Comparative efficacy of tyrosine kinase inhibitor treatments in the third-line setting, for chronic-phase chronic myelogenous leukemia after failure of second-generation tyrosine kinase inhibitors. Leukemia Res. 2015;39:58–64.
Craddock CF. We do still transplant CML, don’t we? Hematology. 2018;2018:177–84.
Chalandon Y, Sbianchi G, Gras L, Koster L, Apperley J, Byrne J, et al. Allogeneic hematopoietic cell transplantation in patients with chronic phase chronic myeloid leukemia in the era of third generation tyrosine kinase inhibitors: A retrospective study by the chronic malignancies working party of the EBMT. Am J Hematol n/a https://doi.org/10.1002/ajh.26764.
Radich J. Allogeneic transplantation for chronic myeloid leukemia: I’m not dead yet. Am J Hematol. 2023;98:4–5.
Kantarjian HM, O’Brien SM, Keating M, Beran M, Estey E, Giralt S, et al. Results of decitabine therapy in the accelerated and blastic phases of chronic myelogenous leukemia. Leukemia. 1997;11:1617–20.
Cortes J, Lipton JH, Rea D, Digumarti R, Chuah C, Nanda N, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120:2573–80.
Cortes JE, Kantarjian HM, Rea D, Wetzler M, Lipton JH, Akard L, et al. Final analysis of the efficacy and safety of omacetaxine mepesuccinate in patients with chronic- or accelerated-phase chronic myeloid leukemia: Results with 24 months of follow-up. Cancer. 2015;121:1637–44.
Zhou T, Parillon L, Li F, Wang Y, Keats J, Lamore S, et al. Crystal Structure of the T315I Mutant of Abl Kinase. Chem Biol Drug Design. 2007;70:171–81.
O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12.
Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in Refractory Philadelphia Chromosome–Positive Leukemias. N Engl J Med. 2012;367:2075–88.
Food and Drug Administration, U. S. ICLUSIG® (ponatinib) tablets for oral use (2012). Accessed May 24, 2021. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203469lbl.pdf.
Pulte ED, Chen H, Price LSL, Gudi R, Li H, Okusanya OO, et al. FDA Approval Summary: Revised Indication and Dosing Regimen for Ponatinib Based on the Results of the OPTIC Trial. Oncologist. 2022;27:149–57.
Dorer DJ, Knickerbocker RK, Baccarani M, Cortes JE, Hochhaus A, Talpaz M, et al. Impact of dose intensity of ponatinib on selected adverse events: Multivariate analyses from a pooled population of clinical trial patients. Leukemia Res. 2016;48:84–91.
Breccia M, Olimpieri PP, Celant S, Olimpieri O, Pane F, Iurlo A, et al. Management of chronic myeloid leukaemia patients treated with ponatinib in a real-life setting: A retrospective analysis from the monitoring registries of the Italian Medicines Agency (AIFA). Br J Haematol. 2022;198:965–73.
Jabbour EJ, Sasaki K, Haddad FG, Issa GC, Garcia-Manero G, Kadia TM. et al. The outcomes of patients with chronic myeloid leukemia treated with third-line BCR::ABL1 tyrosine kinase inhibitors. Am J Hematol. 2023;98:658–665.
Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, et al. Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1. J Med Chem. 2018;61:8120–35.
Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR–ABL1. Nature. 2017;543:733–7.
Rea D, Hochhaus A, Mauro MJ, Minami Y, Lomaia E, Voloshin S, et al. S155: EFFICACY AND SAFETY RESULTS FROM ASCEMBL, A PHASE 3 STUDY OF ASCIMINIB VS BOSUTINIB IN PATIENTS WITH CHRONIC MYELOID LEUKEMIA IN CHRONIC PHASE AFTER ≥2 PRIOR TYROSINE KINASE INHIBITORS: WK 96 UPDATE. HemaSphere. 2022;6:56–57.
Hochhaus A, Gambacorti-Passerini C, Abboud C, Gjertsen BT, Brümmendorf TH, Smith BD, et al. Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: primary results of the phase 4 BYOND study. Leukemia. 2020;34:2125–37.
Luna A, Pérez-Lamas L, Boque C, Giraldo P, Xicoy B, Ruiz Nuño C, et al. Real-life analysis on safety and efficacy of asciminib for ponatinib pretreated patients with chronic myeloid leukemia. Ann Hematol. 2022;101:2263–70.
Khadadah F, Turkina AG, Lomaia E, Morozova EV, Shukjov OA, Petrova A, et al. P708: CANADIAN AND RUSSIAN EXPERIENCES OF ASCIMINIB IN CHRONIC MYELOID LEUKEMIA (CML) PATIENTS WHO FAILED MULTIPLE LINES OF TYROSINE KINASE INHIBITOR (TKI) THERAPY. HemaSphere 2022;6:603–304 (2022).
Kockerols CCB, Janssen JJWM, Blijlevens NMA, Klein SK, Van Hussen-Daenen LGM, Van Gorkom GGY. et al. Treatment patterns and clinical outcomes of asciminib in a real-world multi-resistant chronic myeloid leukemia patient population. Haematologica. 2022;108:240–244.
Breccia M, Russo Rossi AV, Martino B, Abruzzese E, Annunziata M, Binotto G, et al. P712: ASCIMINIB ITALIAN MANAGED ACCESS PROGRAM: EFFICACY PROFILE IN HEAVILY PRE-TREATED CML PATIENTS. HemaSphere. 2022;6:607–8.
Innes AJ, Hayden C, Orovboni V, Rees D, Claudiani S, Fernando F, et al. Real-World Experience of Asciminib: Factors Associated with Response. Blood. 2022;140:6796–7.
Ren X, Pan X, Zhang Z, Wang D, Lu X, Li Y, et al. Identification of GZD824 as an Orally Bioavailable Inhibitor That Targets Phosphorylated and Nonphosphorylated Breakpoint Cluster Region–Abelson (Bcr-Abl) Kinase and Overcomes Clinically Acquired Mutation-Induced Resistance against Imatinib. J Med Chem. 2013;56:879–94.
Jiang Q, Zongru L, Qin YZ, Zhao T, Liu B, Chen Z, et al. A Five-Year Follow-up on Safety and Efficacy of Olverembatinib (HQP1351), a Novel Third-Generation BCR-ABL Tyrosine Kinase Inhibitor (TKI), in Patients with TKI-Resistant Chronic Myeloid Leukemia (CML) in China. Blood. 2022;140:198–9.
Jiang Q, Li Z, Hou Y, Hu Y, Li W, Liu X, et al. Updated Results of Pivotal Phase 2 Trials of Olverembatinib (HQP1351) in Patients (Pts) with Tyrosine Kinase Inhibitor (TKI)-Resistant Chronic- and Accelerated-Phase Chronic Myeloid Leukemia (CML-CP and CML-AP) with T315I Mutation. Blood. 2022;140:203–4.
Jabbour E, Koller PB, Oehler VG, Jamy OH, Mukherjee S, Hunter AM, et al. Olverembatinib (HQP1351) Overcomes Ponatinib Resistance in Patients with Heavily Pretreated/Refractory Chronic Myeloid Leukemia (CML) and Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL). Blood. 2022;140:200–2.
Nicolini FE, Chomel JC, Roy L, Legros L, Chabane K, Ducastelle S, et al. The Durable Clearance of the T315I BCR-ABL Mutated Clone in Chronic Phase Chronic Myelogenous Leukemia Patients on Omacetaxine Allows Tyrosine Kinase Inhibitor Rechallenge. Clin Lymphoma Myeloma Leukemia. 2010;10:394–9.
Cornelison AM, Welch MA, Koller C, Jabbour E. Dasatinib combined with interferon-alfa induces a complete cytogenetic response and major molecular response in a patient with chronic myelogenous leukemia harboring the T315I BCR-ABL1 mutation. Clin Lymphoma Myeloma Leuk. 2011;11:S111–113.
Johnson-Ansah H, Naguib D, Mittre H, Kadi S, Troussard XA. T315I Gate Keeper Mutation Responding To Pegylated Interferon Alfa-2a (Peg-IFN) Monotherapy With Major Molecular Response (MMR4) At 12 Months In An Imatinib-Resistant Chronic Myeloid Leukemia (CML) Patient. Blood. 2013;122:5179.
Polivkova V, Rohon P, Klamova H, Cerna O, Divoka M, Curik N, et al. Interferon-α Revisited: Individualized Treatment Management Eased the Selective Pressure of Tyrosine Kinase Inhibitors on BCR-ABL1 Mutations Resulting in a Molecular Response in High-Risk CML Patients. PLoS ONE. 2016;11:e0155959.
Nicolini FE, Basak GW, Kim DW, Olavarria E, Pinilla-Ibarz J, Apperley JF, et al. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer. 2017;123:2875–80.
Heiblig M, Rea D, Chrétien ML, Charbonnier A, Rousselot P, Coiteux V, et al. Ponatinib evaluation and safety in real-life chronic myelogenous leukemia patients failing more than two tyrosine kinase inhibitors: the PEARL observational study. Exp Hematol. 2018;67:41–48.
Shacham-Abulafia A, Raanani P, Lavie D, Volchek Y, Ram R, Helman I, et al. Real-life Experience With Ponatinib in Chronic Myeloid Leukemia: A Multicenter Observational Study. Clin Lymphoma Myeloma Leukemia. 2018;18:e295–e301.
Devos T, Havelange V, Theunissen K, Meers S, Benghiat FS, Gadisseur A, et al. Clinical outcomes in patients with Philadelphia chromosome-positive leukemia treated with ponatinib in routine clinical practice-data from a Belgian registry. Ann Hematol. 2021;100:1723–32.
Sacha T, Szczepanek E, Dumnicka P, Góra-Tybor J, Niesiobędzka-Krężel J, Prejzner W, et al. The Outcomes of Ponatinib Therapy in Patients With Chronic Myeloid Leukemia Resistant or Intolerant to Previous Tyrosine Kinase Inhibitors, Treated in Poland Within the Donation Program. Clin Lymphoma Myeloma Leuk. 2022;22:405–15.
Jain P, Kantarjian H, Jabbour E, Gonzalez GN, Borthakur G, Pemmaraju N, et al. Ponatinib as first-line treatment for patients with chronic myeloid leukaemia in chronic phase: a phase 2 study. Lancet Haematol. 2015;2:e376–e383.
Jiang Q, Li Z, Qin Y, Li W, Xu N, Liu B, et al. Olverembatinib (HQP1351), a well-tolerated and effective tyrosine kinase inhibitor for patients with T315I-mutated chronic myeloid leukemia: results of an open-label, multicenter phase 1/2 trial. J Hematol Oncol. 2022;15:113.
Author information
Authors and Affiliations
Contributions
HK contributed in design, conception, collection and assembly of data, data analysis and interpretation, manuscript writing and final approval. JS, KS, GCI, JHL, JPR, EJ assisted in manuscript writing, data analysis and interpretation and final approval.
Corresponding author
Ethics declarations
Conflict of interest
Jayastu Senapati -- no conflict of interest. Koji Sasaki -- research funding and honoraria for advisory boards from Novartis. Ghayas Issa-- consulting or advisory role: Novartis, Kura OncologyResearch; research funding: Novartis, Syndax, Kura Oncology. Jeffrey H Lipton—consulting for Bristol Myers Squibb, Novartis, Pfizer, and Takeda; research funding from Bristol Myers Squibb, Novartis, Pfizer, and Takeda; received honorarium from Bristol Myers Squibb, Pfizer, and Takeda. Jerry Radich -- grants from the National Cancer Institute during the conduct of the study; consulting for Novartis, Bristol Myers Squibb, Takeda, Amgen, Cepheid, Bio-Rad, Adaptive, and SeaGen. Elias Jabbour – research grants from AbbVie, Adaptive Biotechnologies, Amgen, Pfizer, and Takeda; consulting fees from AbbVie, Adaptive Biotechnologies, Amgen, Bristol Myers Squibb, Genentech, Incyte, Novartis, Pfizer, and Takeda. Hagop Kantarjian -- grants from AbbVie, Amgen, Ascentage, Bristol Myers Squibb, Daiichi Sankyo, ImmunoGen, Jazz, Novartis, and Pfizer; honoraria from AbbVie, Amgen, Aptitude Health, Ascentage, Astellas Health, AstraZeneca, Ipsen Biopharmaceuticals, KAHR Medical Ltd, NOVA Research, Novartis, Pfizer, Precision BioSciences, and Taiho Pharma.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Senapati, J., Sasaki, K., Issa, G.C. et al. Management of chronic myeloid leukemia in 2023 – common ground and common sense. Blood Cancer J. 13, 58 (2023). https://doi.org/10.1038/s41408-023-00823-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00823-9
This article is cited by
-
Erythroid variant evolving from chronic myeloid leukemia resistant to multiple tyrosine kinase inhibitors: a rare case report
Diagnostic Pathology (2024)
-
Are there new relevant therapeutic endpoints in the modern era of the BCR::ABL1 tyrosine kinase inhibitors in chronic myeloid leukemia?
Leukemia (2024)
-
Exploring the therapeutic efficacy of crocetin in oncology: an evidence-based review
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
What is the impact of failing to achieve TKI therapy milestones in chronic myeloid leukemia
Leukemia (2023)
-
Identification of multivariable microRNA and clinical biomarker panels to predict imatinib response in chronic myeloid leukemia at diagnosis
Leukemia (2023)