Abstract
Background
Dental caries is one of the prevalent conditions that threaten oral health. Arnebia euchroma (Royle) Johnst. root (AR) extracts exhibit anti-inflammatory, anti-cancer, and antibacterial properties. This study was designed to investigate the antibacterial impact of AR extract on Streptococcus mutans (S. mutans) UA159 and the anti-caries effect on rats.
Methods
The antibacterial activity of AR extract against S. mutans and its biofilm was determined using the bacterial sensitivity test, the biofilm sensitivity test, and the live-dead staining technique. By fluorescently tagging bacteria, the influence of bacterial adhesion rate was determined. Using a rat caries model, the anti-caries efficacy and safety of AR extract were exhaustively investigated in vivo.
Results
AR extract inhibit not only the growth of S. mutans, but also the generation of S. mutans biofilm, hence destroying and eliminating the biofilm. Moreover, AR extract were able to inhibit S. mutans’ adherence to saliva-encapsulated hydroxyapatite (HAP). Further, in a rat model of caries, the AR extract is able to greatly reduce the incidence and severity of caries lesions on the smooth surface and pit and fissure of rat molars, while exhibiting excellent biosafety.
Conclusions
AR extract exhibit strong antibacterial activity against S. mutans and can lower the incidence and severity of dental cavities in rats. These findings suggest that Arnebia euchroma (Royle) Johnst. could be utilized for the prevention and treatment of dental caries.
Similar content being viewed by others
Background
Caries is a chronic infectious disease mediated by bacterial biofilms, driven by sucrose, and influenced by various variables [1]. Currently, dental caries is the third most prevalent noncommunicable disease in the world, after cancer and cardiovascular disease. Permanent teeth caries and deciduous tooth caries were second and fifth, respectively, among the 10 most prevalent diseases in the globe according to an epidemiological survey conducted in 2016 [2]. Dental caries is still an extremely prevalent disease in the oral cavity, with rates of 62.5% for deciduous teeth in children ages 3–5 and 41.9% for permanent teeth in children ages 12 to 15, according to the 4th National Oral Health Survey conducted in the Mainland of China [3]. According to incomplete data, there are approximately 700 different types of bacteria in the oral microbial community, and they play a crucial role in maintaining dental health [4].
Among them, Streptococcus mutans (S. mutans) is recognized as the main cariogenic bacteria [5, 6]. It metabolizes sucrose and also synthesizes water-insoluble glucans, which are essential for the formation of stable biofilm matrix. It also produces the biofilm matrix that promotes bacterial adhesion and colonization on tooth surfaces while maintaining an acidic environment for the growth of cariogenic bacteria. Therefore, selective S. mutans in complex dental plaque biofilms, reflecting the anti-caries efficacy of drugs by suppressing the growth of S. mutans and its virulence factors, may be a promising method for preventing dental caries.
With an in-depth understanding of the etiology and pathogenesis of dental caries, fluoride [7], chlorhexidine (CHX), and other drugs have been developed and put into caries control one after another, among which CHX is often made into mouthwash because of its remarkable antibacterial ability [8]. In addition, researchers often use CHX at 0.12% concentration as a gold standard to assess the anti-cariogenic activity of the agent to be tested [9]. However, along with the overuse of CHX [10], side effects such as oral microecological disorders, drug-resistant bacteria, or fungal overgrowth have occurred one after another.
Consequently, ecological caries prevention approaches are increasingly coming into view, and it is required to create novel anti-caries strategies based on ecological prevention and to encourage the creation of a healthy and stable oral biofilm environment [11]. Existing ecological anti-cause approaches include antimicrobial peptides, probiotics, natural compounds, etc. [12]. Natural products have become the focus of research on caries prevention and treatment due to their abundant sources, low cost, potent antibacterial properties and good biological safety and other characteristics [13].
Arnebia euchroma (Royle) Johnst. (Xinjiang-Zicao or Ruan-Zicao in Chinese) is a commonly prescribed traditional herbal medicine in Xinjiang, Tibet, and Mongolia and was first recorded in “Shen Nong’s Materia Medica” [14]. Arnebia euchroma (Royle) Johnst. is mainly distributed in high-altitude areas in Central Asia, North Africa, Afghanistan, and the western Himalayas [15]. Arnebia euchroma (Royle) Johnst., according to “ The Pharmacopoeia of the People’s Republic of China 2020,” has the ability to detoxify the body, eliminate heat from the body, and cool the blood. Both Uygur and Traditional Chinese medicine make extensive use of it [16]. Numerous bioactive substances found in Arnebia euchroma (Royle) Johnst. [17], such as naphthoquinones, phenolic acids, and alkaloids, have been shown to have antibacterial, anti-inflammatory, antioxidant, and other properties [15, 18, 19]. Based on our previous research, Arnebia euchroma (Royle) Johnst. can inhibit the growth, acid generation, and sugar production of the major cariogenic bacteria [20], but its efficacy and safety in preventing caries against S. mutans adhesion and in vivo caries have not been investigated.
In this research, the effects of AR extract on the growth and adhesion of S. mutans and their biofilm were assessed in vitro. To give theoretical and experimental support for the development of AR extract as an anti-caries agent, the extract’s safety and effectiveness against S. mutans were assessed in a rat caries model.
Materials and methods
Ethics statement
The “Ethics Review Committee of Xinjiang Medical University” gave its clearance for this research’s conduct (IACUC-JIPD-2019030). The China Ministry of Science and Technology’s Guide for the Care and Use of Laboratory Animals was followed in all experiments.
Bacterial strain and growth conditions
S. mutans UA159 (ATCC 700610, a cariogenic bacterial pathogen) provided by the Guangdong Microbial Culture Collection Center (GDMCC) was incubated in brain heart infusion (BHI; Oxoid, UK) broth containing 1% sucrose and incubated for 24 h at 37 °C. After incubation, the bacterial concentration was 107 colony forming units (CFU)/mL ([Optical Density (OD) 630 nm = 0.2)] as determined by spectrophotometry. The strains were preserved and stored in 20% glycerol at −80 °C for an extended period of time.
Plant material and chemical reagents
Dried roots of Arnebia euchroma (Royle) Johnst. were purchased from the local market of Urumqi and the species was identified and authenticated in Department of Pharmacology, Xinjiang Medical University, China. The dried roots of Arnebia euchroma (Royle) Johnst. were dried at 25 °C and ground to a fine powder in a grinder, passed through a 110-mesh stainless steel screen and stored in a desiccator. First, 500 g dried roots of Arnebia euchroma (Royle) Johnst. powder was extracted by soaking in 95% ethanol (w/v, 1:10) at room temperature and repeated three times. The extract was concentrated by evaporation under vacuum (50 °C) in a rotary evaporator and was freeze-dried. Samples were collected and stored in amber bottles at −4 °C [21].
The ingredients of AR extract were determined by Tandem Mass Spectrometry (MS/MS), in combination with an Ultra Performance Liquid Chromatography (HPLC Thermo UltiMate 3000, Thermos Fisher Scientific, Waltham, MA, USA) and a mass spectrograph (AB 5600+ Triple TOF, SCIEX, Framingham MA, USA). The collected data was initially processed and confirmed according to the exact Mass (mass error ≤30ppm), and then, Secondary mass spectrometry database (Massbank, GNPS, RIKEN PlaSMA, BMDMS-NP, mzClound, etc) was used for substance identification and analysis of the collected data. The results showed that AR extract mainly contain alkaloid, Flavonoid, phenol, coumarins, terpenoids, etc. Please refer to the appendix Table S1 for the further details.
Determination of minimal inhibitory concentrations (MIC) and minimum bactericidal concentration (MBC)
Refer to the method of Preparing Dilutions of Water-Insoluble Antimicrobial Agents to Be Used in Broth Dilution Susceptibility Tests published by the Clinical and Laboratory Standards Institute in 2022 for the determination of MIC and MBC [22]. In a nutshell, fresh BHI was used to dilute the collected S. mutans cultures into 96-well plates (NEST, Wuxi, China) at 1.0 × 107 CFU/mL. The AR extracts were diluted with BHI medium and dimethyl sulfoxide (DMSO) with reference to the double dilution method, and the final concentrations of the extracts evaluated ranged from 32 to 0.25 mg/mL. This was done to establish MIC values of AR extract for animal research and clinical study direction. DMSO with a final concentration of 1% used in the experiment has been proved to be non-toxic to bacteria [23]. Bacteria with 0.12% CHX (Yuanye, Shanghai, China) treatment were utilized as positive controls, whereas untreated bacteria and BHI broth medium were used as negative controls. The 96-well plates were grown on a rotary shaker (150 rpm) at 37 °C and the absorbance of each well at a wavelength of 595 nm was measured after 24 h using a Multiskan Spectrum (Thermo Fisher Scientific, Inc.) and the growth inhibition rate was calculated according to the formula (growth inhibition rate = [1-(A595 experimental group / A595 negative control group)] × 100%) [24]. MIC50 was established as the medication concentration that 50% inhibited growth.
The BHI agar dilution method was used to calculate the MBC. Bacterial cultures from wells with test samples having concentrations equal to or higher than MIC50 were transferred to BHI agar plates and incubated for 24 h. MBC was defined as the lowest concentration at which there were no visible bacterial colonies on the agar plates after 24 h of incubation. The assays were performed in triplicate on three different occasions.
Determination of minimum biofilm inhibition concentration (MBIC50) and minimum biofilm reduction concentration (MBRC50)
MBIC50 and MBRC50 examined the effect of AR extract on the formation and reduction of S. mutans biofilm. The method for determining MBIC50 and MBRC50 was performed as previously described [24, 25], with some modifications. MBIC50 was defined as the lowest concentration of agent showing 50% or more inhibition of biofilm formation. Briefly, the bacteria were grown in BHI broth containing 1% (w/v) sucrose [26]. With a final bacterial concentration of 1.0 × 107 CFU/mL as required for bacterial susceptibility testing; the AR extract concentrations varied from 32 to 0.25 mg/mL. The experimental groupings were consistent with those of MIC and MBC. The supernatant medium was carefully collected after the 24 h incubation period at 37 °C for static incubation, and the planktonic bacteria were then eliminated by thoroughly washing each well three times in 200 μL of PBS. The wells were stained with 0.1% (w/v) crystal violet for 5 min after being fixed with methanol for 15 min. The excess dye was removed, the plate was washed with water, and air-dried for 1 h. The 96-well plate was then shaken for 30 min at room temperature after 200 μL of 95% ethanol had been poured to each well. To calculate the biomass of the biofilm, the OD595 nm was measured using Multiskan Spectrum.
The MBRC50 was defined as the lowest concentration of agent that reduces the formed biofilm by 50% or more. Briefly, bacteria were grown in BHI broth containing 1% (w/v) sucrose. The bacteria were inoculated at a final concentration of 1.0 × 107 CFU/mL, and after 24 h of static incubation at 37 °C, the planktonic cells were removed by decanting the medium and the wells were gently washed with PBS. The AR extract concentration ranged from 32 to 0.25 mg/mL and was added to the above formed bacterial biofilm. The rest of the steps followed the same procedures as in the MBIC50 described above.
Bacteria with 0.12% CHX treatment were utilized as positive controls, whereas untreated bacteria and BHI broth medium were used as negative controls. The assays were performed in triplicate on three different occasions.
Live/dead bacteria staining
Bacterial viability was determined using the Dojindo Bacterial Viability Detection Kit-DAPI/PI (Kumamoto, Japan) [27]. According to the manufacturer’s recommendations, S. mutans was cultured on sterilized glass discs and allowed to grow for 24 h in BHI medium to produce biofilms. Planktonic bacteria were removed using 37 °C PBS after biofilm formation, and AR extract was then formulated into interventions at 8, 4, and 2 mg/mL as experimental groups, 0.12% CHX interventions as positive control groups, BHI medium interventions as blank control groups, and 1 percent DMSO interventions as solvent groups. The cultures were gently removed after 24 h of anaerobic incubation at 37 °C, washed twice with sterile water, stained with Bacstain-Bacterial Viability Detection Kit-DAPI/PI, and then left to sit at that temperature for 15 min in the dark. Under a fluorescence microscope, stained bacterial biofilms were viewed (Leica Mannheim; Wetzlar, Hessen, Germany). All bacteria can be stained with DAPI, which is membrane permeable and identifiable by blue fluorescence. Damaged bacterial membranes are stained with red fluorescence by PI, which is membrane impermeable. For each sample, three fields of vision were chosen at random under a 100× objective lens. Based on examination of the ratio between the coverage of live bacteria to total bacteria using the software Image J (National Institutes of Health, Bethesda, MD, USA).
Adherence to hydroxyapatite (HAP)
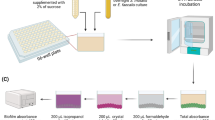
To simulate in vitro the inhibitory effect of S. mutans adhesion to the main component of dental enamel, HAP, within the oral microenvironment, we referenced the experimental method previously used [28] and made certain improvements. We used fluorescent probes to label S. mutans, which were then co-incubated with saliva and AR extract for a set period. Subsequently, the unattached bacteria from each group were removed, and the S. mutans adhered to the HAP surface were resuspended. The inhibitory effect of AR extract on the adhesion of S. mutans was assessed by measuring the fluorescence intensity of the remaining bacteria. This method allowed us to evaluate the extent of inhibition of the AR extract on the S. mutans adhesion process. Non-stimulated saliva was collected from the specific volunteer (without systemic and oral diseases) after 4 h of feeding as approved by the Ethics Review Committee of Xinjiang Medical University (IACUC-JIPD-2019030). Volunteers were asked to read the completed consent form and sign it if they agreed with its contents. Saliva samples (40 mL) were collected and centrifuged at 15000 rpm for 20 min at 4 °C before being filtered through a 0.22 μm sterile filter (Millipore Corp, Billerica, MA, USA) and stored at 4 °C. According to the steps outlined by Shahzad et al. [29], the 96-well black-walled, clear-bottomed microplates with HAP coating were created. Prepare a homogenous HAP suspension by dissolving HAP in sterile saliva (5% w/v), shaking it well, and then pouring it into the well plates. The plate was then agitated for 15 min at 37 °C and 200 rpm, dried, and then given two PBS washes. S. mutans was labeled with 2’,7’-Bis-(2-Carboxyethyl)−5-(and-6)-Carboxyfluorescein, Acetoxymethyl Ester (BCECF/AM, Beyotime, Shanghai, China). BCECF/AM-labeled S. mutans (100 µL) was added to 96-well plates along with AR extract at concentrations ranging from 4.0 to 0.25 mg/mL. The 96-well plate was incubated in the dark at 37 °C with low-speed shaking for an additional 2 h. Unbound bacteria were aspirated and washed twice with PBS. The relative fluorescence units (RFU; excitation wavelength of 495 nm; emission wavelength of 525 nm) corresponding to the level of bacterial adhesion were determined using the Victor Nivo Multimode Microplate Reader (PerkinElmer, USA). Control wells without the test drug were used to determine 100% adhesion values, while wells without bacteria were used as controls to determine basal autofluorescence. All these determinations represent the average of three independent experiments. At the same time, the adhesion ability of S. mutans to HAP was observed under a fluorescence microscope (Leica Mannheim; Wetzlar, Hessen, Germany) on the above-mentioned plates. Five fields of view were randomly selected for each sample under a 100× objective lens. A flow chart of the adherence to HAP experiment is shown in Fig. 1.
Experimental procedure for evaluating the effect of AR extract on the adhesion properties of S. mutans by saliva-coated HAP. The experiments included: (a) HAP 96-well plate fabrication; (b) saliva-coated HAP; (c) loading fluorescent probes to S. mutans; (d) Testing AR extract’s ability on the adhesion of S. mutans carrying fluorescent probes in saliva-coated HAP; the final step was to observe the adhesion of S. mutans using a fluorescence microscope.
In vivo efficacy of AR extract
Rat caries model
In total, 42 specific pathogen-free (SPF-SD) 21-day-old SD rats (only males) were purchased at the Animal Experiment Center of Xinjiang Medical University and the animal experiments were performed using the previously described methods [30]. First, to avoid the effect of endogenous oral microorganisms, 21-day-old SD rats were fed antibiotic water containing benzylpenicillin (200 μg/mL) and streptomycin sulfate (1500 μg/mL) and a diet supplemented with antibiotics (1 g/kg) for three consecutive days. Saliva from SD rats was inoculated onto Mitis Salivarius Agar Base (MSA, OXIOD, UK) to examine the therapeutic efficiency of oral endogenous antibacterial [31]. The above SD rats were randomly divided into seven groups (n = 6 for each), as reported below: (1) 0.5 mg/mL AR extract; (2) 1 mg/mL AR extract; (3) 2 mg/mL AR extract; (4) 0.12% CHX (positive control group); (5) 1% DMSO (solvent control group); (6) double distilled water (DDW, negative control group); and (7) no intervention (blank control group). For the next three consecutive days, S. mutans UA159 suspension (500 μL, 1 × 108 CFU/mL) was inoculated onto the teeth of each rat (twice daily). Each rat was fasted for 0.5 h before being inoculation and after bacterial inoculation. Then, the efficiency of bacterial inoculation was examined as described above. The above drug solution will be prepared and transferred to Eppendorf tubes (1 mL/tube), and each rat will be used independently. Sterile cotton swabs were dipped into the above solution and used to topically clean the molars and oral mucosa of the rats. The residual drug solution was then used to rinse the rats’ mouths. Water and food were abstained from for 0.5 h after treatment. Treatments were performed daily at 9 am and 9 pm for 3 weeks by the same operator. During 27–47 days, rats were fed ad libitum a cariogenic Keyes 2000 diet (Xietong Organis, Jiangsu, China) and 5% sucrose water. The body weight of the rats was also continuously recorded for 27–47 days to monitor for signs of drug toxicity [32]. A flow chart of group allocation is shown in Fig. 2.
Bacteriological examination of saliva
On day 47, 0.2% pilocarpine nitrate (0.4 mL/100 g) (bauschlomb, Shandong, China) was injected intraperitoneally, and 100 μL of saliva was collected from each rat and inoculated onto MSA for colony counting [33].
Keyes scoring and X-Ray
At the end of the experiment (48 days), the rats were anesthetized by Zoletil® 50 (active ingredients: tiletamine 125 mg, zolazepam 125 mg, excipients; produced by Virbac Co., France) and and Lumianning II (a new compound preparation composed of xylazine hydrochloride and other optimally proportioned drugs, manufactured by Jilin Huamu Animal Health Products Co., Ltd., approval number: Veterinary Drug (2009)070011582, production batch number: 140610) and euthanized. After decapitation of the rat, the maxilla and mandible of the rat were removed and placed in an autoclave at 121 °C for 15 min. The attached soft tissue was peeled off with a scalpel, and the molars were cleaned and dried at room temperature. Radiographic analyses were performed using an X-ray machine (Intra Oral X-ray Unit INTR, Nahkelantie 160, Finland). The X-ray tube was operated at 60 kV, 7 mA for 0.1 s, and the source-to-sensor distance was 10 cm. After photographing, all specimens were immersed in a 0.4% ammonium salt solution (Yuanye, Shanghai, China) for staining for 16 h, and protected from light. Finally, the carious areas were stained red by observation through a stereomicroscope (Leica Mannheim; Wetzlar, Hessen, Germany), and the caries condition of each rat was assessed using the Keyes’ scoring method. The smooth and occlusal surfaces of caries and their severity (E, enamel only; Ds, dentin exposed; Dm, 3/4 of affected dentin; Dx, all dentin affected) [34].
CLSM tested Dental tissue grinding plate
The rat molars were sawed sagittal with diamond wire saw and made into Dental tissue grinding plates about 300 μm thick under running water. The grinding pieces were immersed in 0.1 mmol/L Rhodamine B solution, sheltered from light, and soaked at 37 °C for 24 h. After dyeing, rinsed the excess pigment on the surface of the samples with running water and wiped dry. The samples were placed in the center of the slide, sealed with the sealing solution (Glycerin: PBS = 1:1), placed coverslip on the glass slide gently, and then observed under CLSM. Imaging principle of this experiment: In the early stage of caries, tooth demineralization leads to enlarged pores on the enamel surface, and CLSM can detect the fluorescence emitted by the fluorescent dye entering the pores [35]. The stronger the fluorescence intensity, the more serious the demineralization. The larger the fluorescence range, the more serious the caries. The continuity and integrity of enamel or dentin can also be observed. Images were analyzed using ImageJ pro plus software.
Drug safety evaluation
After the execution of the rats, the rat oral mucosa was collected and stained for H&E, while the heart, liver, spleen, lung, and kidney were harvested and weighed to evaluate the biocompatibility of the different treatments.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software (IBM Corporation, Armonk, NY, USA). Data were visualized using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA) software. The experimental data were tested for normality and homogeneity of variance. If the data met normality and homogeneity of variance, one-way ANOVA was used, and the LSD test was used for pairwise comparison. Pairwise comparisons were made using the Kruskal-Wallis Non-Parametric Hypothesis Test for non-normally distributed data. P < 0.05 was set to be statistically significant. The Image J (free and open-source software for scientific image analysis) software was used to quantify the fluorescence intensity.
Results
AR extract qualitative analysis
There has been a lot of research on the application of MS/MS in the identification of plant extracts [36]. In the chromatogram of AR extract (Fig. S1), Both positive and negative ionization chromatograms show a rich concentration of the majority compounds between 0.5 and 29 min. The primary components of Arnebia euchroma (Royle) Johnst. root extract that are potentially capable of exerting antimicrobial effects are the Shikonin, Acetylshikonin, beta,beta-Dimethylacrylshikonin, Isovalerylshikonin, Deoxyshikonin, Arnebinone, Stigmasterol and coumarins, etc. (Table S1, S2). The main compound classification within the extract is shown in Fig. S2.
AR extract inhibited the growth of S. mutans and its biofilm
We found that AR extract had a certain inhibitory effect on the growth of S. mutans. More specifically, the AR extract was able to inhibit the growth of 50% of the planktonic S. mutans at a concentration of 1.0 mg/mL, and the MBC value was 8.0 mg/mL measured by agar dilution method, as shown in Table 1. We examined the impact of AR extract on the development of S. mutans biofilms because cariogenic biofilms are crucial in the emergence of dental caries. As shown in Table 1, we measured the MBIC50 and MBRC50 of AR extract on S. mutans biofilms at 4 mg/mL and 8 mg/mL. All the above results showed that the AR extract not only has an inhibitory effect on the growth of planktonic bacteria of S. mutans but also inhibits the formation of biofilms and clears some of the formed biofilms. Meanwhile, bacterial growth was unaffected by 1% DMSO (solvent control). In this experiment, 0.12% CHX can completely inhibit the growth of planktonic bacteria and biofilm of S. mutans.
Effects of AR extract on S. mutans biofilm structure
Referring to the MBIC50 of AR extract, we used a 1/2 MBIC50, MBIC50 and MBIC50 (concentration range of 8-2 mg/mL) for live and dead bacteria staining. The formed biofilms were fluorescently stained using the DAPI/PI staining method to distinguish the live and dead bacteria in them, and the effect of AR extract on the biofilm structure of S. mutans, as shown in Fig. 3A(a–f). The blue fluorescence indicates the overall number of bacteria, whereas the pink fluorescence indicates the number of dead bacteria. Figure 3A contains three randomly selected fields of view. The ratio of live and dead bacteria in the area was calculated, and the Result is shown in Fig. 3B and Table 2. After 24 h of S. mutans biofilm intervention by AR extract, the percentage of live bacteria area decreased gradually with increasing drug concentration, where 8 mg/mL AR extract was greater than 0.12% CHX (P < 0.05) and 4 mg/mL AR extract was slightly greater than 0.12% CHX (no statistical difference). Even while the 2 mg/mL AR extract group had a lower percentage of living bacteria than the 0.12% CHX group, it was still possible to see that the biofilm was loose and distributed, showing a trend of destruction. The biofilms formed in the negative control and solvent groups were dense and concentrated and contained live bacteria in a large area. The results showed that the 1% DMSO (solvent control) had no impact on biomass. These findings showed that the AR extract effectively inhibited the growth of S. mutans biofilm. We first captured images containing fluorescence signals of live and dead bacterial samples using a fluorescence imaging system. Then, we normalized these images and subsequently utilized ImageJ software to separate the bacteria from the background. After segmentation, we extracted features such as fluorescent area and fluorescent intensity of the live and dead bacterial regions based on the characteristics of the fluorescence probe staining. The features, where blue fluorescence indicates live bacteria, red fluorescence denotes dead bacteria, and pink fluorescence signifies dead bacteria, were then used to classify the live and dead bacteria. Furthermore, we examined the ratio of live bacterial coverage to the total bacterial population.
A a–f shows typical fluorescence microscopy image acquisition of live, dead and merged in preformed biofilms of S. mutans after 24 h of intervention (×100). a negative control group; (b) solvent control group; (c) 2 mg/mL AR extract; (d) 4 mg/mL AR extract; (e) 8 mg/mL AR extract; (f) 0.12% CHX positive control group (n = 3). B The percentage of area occupied by live and dead bacteria was calculated for each group based on three randomly selected images. Dissimilar letters indicate significantly different values (P < 0.05).
AR extract can reduce the adhesion of S. mutans to saliva-coated HAP
One of the key elements in the development of dental caries is S. mutans’ capacity to cling to and colonize the tooth surface. Plaque is created when planktonic bacteria from the oral cavity stick to the surface of tooth enamel. In this study, S. mutans was fluorescently labeled using a BCECF/AM probe, and the effect of AR extract on the bacteria’s ability to adhere to saliva-coated HAP was assessed. Figure 4A demonstrates how the fluorescence intensity on the surface of HAP gradually decreased with increasing AR extract concentration. The fluorescence intensity of adherent bacteria around HAP was significantly reduced in the 1.0 mg/mL AR extract groups, whereas the green fluorescence around HAP was barely perceptible in the 2 and 4 mg/mL AR extract groups. The results in Fig. 4B and Table 3 also demonstrate that the concentration of the AR extract was inversely correlated with S. mutans’s capacity to adhere to saliva-coated HAP. At 0.5 mg/mL, the AR extract inhibited bacterial adhesion (P < 0.001), and at 1.0 mg/mL, it reduced S. mutans bacterial adhesion to 50.93% (P < 0.001), meaning that at least half of the bacteria were no longer being able to adhere to saliva-coated HAP.
A a–f shows the image acquisition of bacterial adherence to saliva-coated HAP under fluorescence microscopy (×100). The green fluorescence is BCECF/AM-labeled S. mutans. In the 1, 2, and 4 mg/mL AR extract intervention groups, the HAP clusters were visible in the bright field, but there was little or very little fluorescence apparent in the fluorescence field. a Negative control group; (b) 0.25 mg/mL; (c) 0.5 mg/mL; (d) 1 mg/mL; (e) 2 mg/mL; (f) 4 mg/mL. B In comparison to the S. mutans group, the adhesion rate of S. mutans on the surface of saliva-coated HAP was considerably lower following AR extract intervention (*P < 0.001). Bacterial adherence rate = fluorescence value of adherent bacteria/positive control group ×100%.
Evaluation of the dental caries in rat model
AR extract significantly reduces the number of salivary S. mutans in the oral cavity of rats
This study used a rat caries model for in vivo tests to further explore the antibacterial effect of AR extract on dental caries in light of its antibacterial action on S. mutans and its biofilm in vitro. We discovered that AR extract could significantly lower the number of S. mutans in the oral cavity by counting the S. mutans in the rats’ saliva. Figure 5 and Table 4 show that there were considerably less S. mutans in the oral cavities of rats in the 0.5 mg/mL, 1 mg/mL, 2 mg/mL AR extract, and 0.12% CHX groups compared to the caries model group (P < 0.01). All of the aforementioned findings suggested that topical application of AR extract could successfully lessen S. mutans colonization and proliferation in the oral cavity of rats.
Keyes scoring and X-ray manifestations
We used a well-established animal model of dental caries to test whether topical treatment of AR extract can prevent the development and severity of dental caries in vivo. The molar dental caries of rats was observed using body vision microscopy and X-ray photography, the findings were displayed in Fig. 6A, while the above results were scored by the Keyes method, as shown in Fig. 6B and Table 5. In the caries model group and solvent control group, widespread pit, fissure, and smooth surface caries lesions were identified, as represented by arrows. In the AR extract treatment group, the Keyes scores of surfaces, enamel only (E), and dentin exposed (Ds) levels in the 0.5 mg/mL AR extract treatment group were significantly different from those in the caries model group (P < 0.01). The Keyes scores at the surface, E, Ds, 3/4 of affected dentin (Dm), and all dentin affected (Dx) levels in the 1 mg/mL and 2 mg/mL AR extract treatment groups were significantly different from those of the caries model group (P < 0.001), and the results were similar to the 0.12% CHX treatment group. According to the aforementioned findings, topical applications of 1 mg/mL and 2 mg/mL AR extract effectively reduced the frequency and severity of caries lesions at all levels on smooth and pitted surfaces. The number of smooth surfaces, E, and Ds caries lesions can be decreased with topical application of 0.5 mg/mL AR extract, but the depth of deep caries developed cannot be decreased. According to the aforementioned findings, topical applications of 1 mg/mL and 2 mg/mL AR extract effectively reduced the frequency and severity of caries lesions at all levels on smooth and pitted surfaces. The number of smooth surfaces, E and Ds caries lesions can be decreased with topical application of 0.5 mg/mL AR extract, but the depth of deep caries developed cannot be decreased.
A Smooth surface and sagittal section of the rat mandibular dentition under the stereomicroscope, arrows represent carious lesions, different color arrows represent different degrees of carious lesions. X-ray photography: X-rays of rat molars taken at the end of the AR extract treatment, where red arrows are marked as carious lesions. B The Keyes score in the molar teeth of SPF-SD rats. Surface: Surface caries; E: enamel caries; Ds: dentin exposed; Dm: three-fourths of the dentin affected; Dx: 4/3 or full thickness of dentin depth; (Surface, E, Ds) Data are expressed as mean ± standard error (n = 6); (Dm, Dx). Data are expressed as mean ± standard error (n = 6), Dissimilar letters indicate significantly different values (P < 0.05).
Observations by CLSM (Confocal Laser Scanning Microscope)
Enamel demineralization causes the surface pores to widen in the early stages of caries development, which allows the fluorescent staining solution to enter the enamel pores. Under CLSM, the fluorescence is visible, and the more intense the fluorescence, the more severe the demineralization occurrence. Figure 7 and Table 6 show the results of our use of CLSM to observe the onset and severity of dental caries in rats. We discovered that the enamel was continuous and regular in the 2.0 mg/mL AR extract treatment group and the 0.12% CHX treatment group. No obvious enamel fissures were found, and the fluorescence intensity was significantly lower than it was in the caries model group. In the 0.5 and 1.0 mg/mL AR extract treatment groups, the fluorescence intensity and the number of cracks in the enamel were between the caries model group and the 2.0 mg/mL AR extract treatment group.
A Representative CLSM images after AR extract treatment, blue arrows indicate the increase in enamel surface porosity due to caries damage. B AF is the average amount of fluorescence per unit area of lesion area after fluorescence staining, data are expressed as mean ± standard error (n = 4). Different superscript letters indicate significant differences. P < 0.05.
Good biocompatibility of AR extract in rats
By measuring body weight, examining the oral mucosa’s histology, and using organ coefficient, we further assessed the biocompatibility of AR extract in vivo. All of the rat groups survived the whole trial in good health, with normal vitality and no dead rats. During the treatment period, the body weight of the rats increased continuously, and we found that except for the normal group fed with normal food, the body weight of each treatment group fed with Diet 2000 food and 5% sucrose water increased steadily, and there was no significant difference between the groups, and the results are shown in Fig. 8B. To demonstrate the safety of AR extract treatment on oral mucosal tissues, we performed HE staining on buccal mucosal tissues, and the results are shown in Fig. 8A. The epithelial layer of the oral mucosa was intact in all groups of rats, the stratum corneum was smooth and coloration was uniform, the basal cell layer was continuous and had a normal shape, the rete pegs extended toward the basal layer, and no pathological changes like erosion, ulceration, or inflammation were seen. In addition, we collected and weighed the heart, liver, spleen, lungs, and kidneys to determine the organ coefficient (organ coefficient = organ weight / body weight × 100%), and the results are shown in Table 7 and Fig. 8C. We discovered that the kidney coefficient did not differ significantly from the caries model group, and the liver coefficient did differ slightly between the treatment groups and the caries model group, but the difference was not statistically significant. All of these findings show that using AR extract to prevent and treat dental caries progression is a biocompatible strategy.
Discussion
The AR, a naturally occurring plant with significant medicinal value, are abundant in a number of secondary metabolites, such as naphthoquinone pigments, meroterpenoids, and arnebinols [37]. Ethanol can be used as a solvent to extract a number of active components, including shikonins and hydroxynaphthoquinone pigments [38].
Shikonins, a collective word for a variety of naturally occurring naphthoquinone compounds that can be extracted using ethanol, are one of them. According to ongoing research [39], at least 10 different naphthoquinone components have been found in the AR. Damianakos et al. found that different chemical components of the Arnebia euchroma (Royle) Johnst. have different antibacterial abilities against different types of bacteria, and Shikonin, Acetyl Shikonin, β-Hydroxyisovalerylshikonin, and Isobutyryl shikonin were the most active antibacterial components. Shikonin was more efficient against gram-positive bacteria and inhibited five common pathogenic microorganisms in a test tube, including Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Streptococcus agalactiae (S. agalactiae), for gram-positive bacteria, the inhibitory impact was more potent [21]. Other components of AR included butyryl alkannin and isovalerylshikonin, both of which demonstrated excellent antibacterial potential against highly drug-resistant Enterococcus faecalis (E. faecalis) (MIC = 3.16–6.26 μg/mL) and drug-resistant S. aureus (MIC = 16 μg/mL) respectively [40].
The main antibacterial mechanism of the Arnebia euchroma (Royle) Johnst. extract is that it alters the morphology and structure of bacteria [41], leading to the destruction of their cell walls and membranes, thereby altering cell permeability. Shikonin possesses the ability to disrupt the physiological properties regulated by N-acyl-L-homoserine lactones in the Gram-negative bacterium Chromobacterium violaceum, thus inducing quorum sensing (QS) inhibition. Furthermore, acetyl-shikonins are effective only in inhibiting biofilm formation. This demonstrates the diverse functions of shikonin and acetyl-shikonins in their interactions with bacteria [42]. Quorum sensing is a communication process in bacteria that involves the secretion of autoinducer molecules, thereby facilitating the regulation of biofilm formation through the synthesis of extracellular polysaccharides and intercellular polysaccharide adhesins. This mechanism plays a crucial role in microbial communities by influencing the interactions among bacterial populations. Moreover, S. mutans utilizes quorum sensing to competitively thrive within the oral cavity and to exert pathogenic effects. By responding to environmental cues through quorum sensing, S. mutans can effectively adapt to and manipulate its ecological niche in the oral microbiome [43]. One of the potential anti-biofilm mechanisms of Shikonin is its inhibition of S. mutans’s quorum sensing. Furthermore, Shikonin demonstrated inhibitory effects on Listeria monocytogenes biofilm formation, altered biofilm morphology, and attenuated adhesion. Additionally, it downregulated the expression of virulence factors associated with Listeria monocytogenes [44]. In this experiment, ethanol was used as the extraction solvent to specifically extract the naphthoquinones, the active ingredients from AR, for the purpose of evaluating their anti-caries properties in both in vivo and in vitro studies. Neuraminidase (NA), a significant factor in causing infections, is widely known for its involvement in various biological processes and is deemed as a major virulence factor. Zawawy et al. [45] found that the NA inhibitor CS-MC-GIC-4 completely inhibits the expression of NA in Candida albicans, potentially providing a new approach to preventing dental/oral infections. Twelve compounds, such as Isovalerylalkannin, β, β-Dimethylacrylalkannin, and Deoxyshikonin, were isolated and identified from the alcohol extract of Arnebia euchroma (Royle) Johnst. These compounds showed effective inhibitory activity against bacterial NA [46]. Our initial study shows that extracts inhibit both planktonic and biofilm forms of bacteria at different concentrations, ranging from twice the MIC to three lower levels. This inhibition affects the production of acid and water-insoluble polysaccharides [20].
It is well known that biofilms mediated by cariogenic bacteria play a crucial role in the progression of dental caries. In the early 1960s, S. mutans was identified as the primary pathogenic bacteria of dental caries [47]. Among the complex oral microbiome, S. mutans are particularly prominent in the pathophysiology of dental caries, as this bacterium is the main producer of the exopolysaccharide (EPS) matrix [48], an important component of cariogenic biofilms. EPS can promote the adhesion, accumulation, and aggregation of cariogenic bacteria on the tooth surface, further promote the formation of spatially heterogeneous bacterial niches, form complex three-dimensional structures, protect biofilms, and regulate the pathogenesis of dental caries. Therefore, it has been demonstrated that removing or lowering the amount or virulence of S. mutans in the oral microenvironment prevents or slows the development and/or progression of dental caries [49]. Fluoride, CHX, quaternary ammonium salts, and antimicrobial peptides (AMPs) are currently used to control oral S. mutans. Among these, CHX was one of the earliest antibiotics proposed for the prevention and treatment of dental caries and has proven to be the most effective [50]. Due to its strong bacteriostatic capacity, CHX is frequently formulated into mouthwash (0.06–0.2%) and is commonly used in clinics and households to remove dental plaque [8]. In addition, researchers frequently utilize it as the gold standard to evaluate the anti-cariogenic efficacy of the investigated medicines, with 0.12% CHX being the most prevalent [9]. However, excessive CHX usage also results in oral microecological disorder and the overgrowth of drug-resistant bacteria or fungus. In the recent past, the research prospects for the active components of natural medicines have expanded because of their wide variety, amazing effectiveness, and good biological safety [51]. In addition, our previous study showed that the ethanolic extract of AR inhibited the growth, acid production, water-insoluble polysaccharide production and adhesion of the main cariogenic bacteria in the oral cavity [20], which gave us a preliminary understanding of the anti-caries ability of AR extract. Therefore, in this study, we evaluated the effective components of the AR extracts on inhibiting the biofilm activity of S. mutans and their ability to adhere to HAP, as well as its caries prevention effectiveness and biosafety in the rat caries model.
First, in vitro investigations determined the MIC50 (1 mg/mL) and MBC (8 mg/mL) of AR extract against planktonic S. mutans and proved that it inhibits the growth of S. mutans. As the primary pathogenic form of S. mutans, dental plaque is significantly more resistant to external mechanical and pharmacological resistance than the planktonic condition [52]. The impact of AR extract on S. mutans biofilm MBIC50 (4 mg/mL) and MBRC50 (8 mg/mL) was then evaluated. The MBIC50 and MBRC50 of AR extract to the S. mutans biofilm we determined are higher than the MIC50, mainly due to the different culture methods of S. mutans used in the above method, where the former method enables S. mutans to form a stable and dense plaque biofilm structure, thus causing the difference in these results. For the determination of MBRC50, a static biofilm growth assay was used in this experiment, which is particularly effective in examining the early events of biofilm formation, enabling the detection of biofilm formation and changes in a short period of time [53]. In contrast, the bacteria in MIC’s experiments were taken to grow in a continuous flow environment, a process in which the AR extract was able to fully engage with S. mutans cells and exert antimicrobial effects. Plaque biofilm formation mainly involves three stages: acquired film formation on tooth surfaces; bacterial adhesion; and biofilm maturation [54]. Therefore, anti-caries agents should be effective in inhibiting the formation of new biofilms and reducing the viability of existing biofilms, rather than only inhibiting the growth of planktonic bacteria [55]. The AR extract showed a good inhibitory effect on S. mutans biofilm formation. We observed the effect of MBRC50 And the following two concentrations AR extract on established mature S. mutans biofilms under stained fluorescence microscopy. The mature biofilm was encapsulated by the extracellular matrix, which is difficult to remove. Even after treatment with CHX at a clinical concentration of 0.12% for 24 h, the bacterial cells in the biofilm could not be completely removed or killed. It was observed that the structure of the biofilm treated with AR extract was loose and disordered, and the number of dead bacteria emitting red fluorescence in the biofilm of S. mutans treated with AR extract increased significantly. As the concentration of AR extract increased, the number of dead bacteria increased, and the amount of biofilm decreased. As for the possible mechanism by which AR extract remove mature S. mutans biofilms, based on our results, we hypothesized that 2.0 mg/mL AR extract might be able to enter small channels in biofilms, penetrate the thick extracellular matrix, kill cells, and eventually disrupt and lyse the structure of S. mutans biofilms. However, the higher concentration of AR extract caused the death of bacteria in the biofilm and the structural collapse. Bacterial adhesion is the most important stage and can be disrupted using appropriate methods. The adhesion of S. mutans includes both sucrose-independent and sucrose-dependent modes [56]. Therefore, this study tested the ability of bacteria to adhere to HAP after incubation with AR extract. In this study, AR extract inhibited the adhesion of S. mutans to HAP, among them, 1 mg/mL made 50% of the bacteria lose the ability to adhere. which suggests that AR extract may inhibit bacterial adhesion to the tooth surface. All of the aforementioned data suggested that AR extract not only decreased the area of S. mutans biofilm, but also damaged its biofilm structure, reduced its vitality, and diminished its adhesion capacity.
In vivo, we observed that AR extract has strong antibacterial, anti-adhesion and anti-biofilm formation ability against S. mutans and its biofilm, but it does not mean that it can play the effect role in vivo. The anti-caries drugs actually used in clinic do not completely kill cariogenic bacteria in the mouth, but control the activity and number of cariogenic bacteria at a low level [57]. At the same time, saliva contains some antibacterial substances, and the use of drugs may also have a positive or negative impact on the antibacterial ability of saliva [58]. Therefore, this study further tested the anti-caries activity of AR extract in a rat dental caries model. This study examined the anti-caries efficacy of AR extract in a rat model of dental caries. This study assessed the in vivo anti-caries safety and efficacy of AR extract using a modified Keyes rat dental caries model [59], and the Diet 2000# cariogenic formula with a high sucrose content and high viscosity [60]. To confirm the anti-caries effect of AR extract, we used the Keyes score to evaluate the degree of caries in rats. The Keyes scoring system is a classic caries scoring system that can evaluate the degree of caries in rats [35]. Molars are more sensitive to Diet 2000# and Keyes score, which is strongly supported by the corresponding images (Fig. 6A). The results of the Keyes score confirmed that both AR extract and CHX had better caries-preventing effects than the DDW group on both the smooth surface and groove site. 1 mg/mL AR extract, 2 mg/mL AR extract, and 0.12% CHX can significantly reduce the quantity of superficial and central caries and inhibit the progression of caries into deeper areas. Although the effect of AR extract at a dosage of 0.5 mg/mL was not as pronounced as that of the high-concentration group, it was still able to lower the incidence of superficial caries to some extent. Regarding enamel caries, the 2 mg/mL AR extract group had an anti-caries effect comparable to that of the CHX group. At the same time, X-Ray technology was utilized to assess the severity of carious lesions [61], and CLSM was used to observe the enamel’s potential to demineralize or promote its remineralization, both also showed that the AR extract had a significant anti-caries effect in rat with dental caries. The results of colony counting showed that the AR extract and CHX groups had reduced numbers of S. mutans compared to the DDW group, and both showed inhibition of S. mutans, but were weaker than the CHX group. The data presented above demonstrated that AR extract greatly decreased the cariogenic virulence of S. mutans but did not significantly disturb the microecological balance of oral flora. In addition, in vivo experiments can also help to judge drug safety [62]. Building on previous research, the observation of hepatoprotective activity when oral arginine (AR) extracts were taken, their effects on platelet production and coagulation, as well as their capability to improve and modify liver function damage, has substantiated this claim. In particular, a dosage of 3.5g kg-1day-1 was found to yield the most optimal effects [63]. The main components in AR extracts, including naphthoquinone 6, steroid 18, and triterpenoid 19, demonstrated moderate inhibitory effects on ATP-citrate lyase and protein tyrosine phosphatase 1B, while not exhibiting obvious cytotoxicity [64]. In our experiment, there was no significant difference in body weight between rats fed cariogenic diets, and no pathological alterations (erosion, ulcer, etc.) were observed in the oral mucosa, indicating that AR extract (0.5–2 mg/mL) did not have a significant toxicity effect in vivo. All of the aforementioned data suggested that AR extract might be a source of a novel natural topical anti-caries agent that prevents the incidence and development of dental caries without altering the microflora balance.
However, it is well known that a single carious bacterium does not cause dental caries, nor is it a static process. There are various interactions between the complex plaque biofilm and planktonic bacteria in the oral cavity. Whether AR extract can reduce the number or toxicity of cariogenic bacteria to maintain the stability of oral microecology needs further research and verification. The results of the medium and high concentration AR extract intervention groups were similar to those of the CHX group, according to the Keyes scoring results. In the meantime, the low-concentration AR extract intervention group can also reduce the incidence of superficial caries to a certain extent, indicating that the low-concentration AR extract intervention also has the effect of preventing caries; however, it is unclear whether it reduces the incidence of caries or slows the progression of caries, and longer experiments are required to confirm.
The widely accepted criteria for validating animal models include predictive validity, face validity, and construct validity. However, a single model is unlikely to fully replicate a human disease and may not satisfy all three criteria [65]. S. mutans can cause dental caries in rats, which is used to evaluate the in vivo anti-caries efficacy of AR extract, but human dental caries is caused by a combination of various cariogenic bacteria, complex diet, host factors and time of action, but also a dynamic development process. The caries model used in this study simulates the phenotypic manifestation of human dental caries, including key traits like cavitations, thereby giving it “face validity” characteristics. It replicates the disease’s etiological mechanisms such as Streptococcus mutans’ ability to cause dental caries in the presence of sugar in both animals and humans, demonstrating “construct validity.” In addition, various in vivo studies on traditional Chinese herbal medicines have demonstrated their efficacy in preventing caries. These studies have shown that when the experimental group is subjected to consistent conditions of age, bacterial infection, and cariogenic diet, it consistently exhibits outcomes similar to the model group [32, 66, 67], thus validating its predictive validity. This aligns with the aim of “achieving a blend of diverse yet complementary models that can offer validity across the predictive, face, and construct validity criteria.” However, the dental caries model caused by S. mutans still has some limitations, and it is still necessary to look forward to animal model research that is more in line with the occurrence and development of human dental caries, to provide high-level evidence for the clinical application of AR extract. In addition, in future research, we also need to overcome the limitations of natural medicine, identify the natural medicine monomers with anti-caries activity in AR extract, and produce one or more mouthwashes or toothpastes containing AR for preventing dental caries.
Conclusions
Taken as a whole, this study provided a deeper understanding of the anti-caries properties of AR and confirmed that AR extract is a highly effective caries prevention and treatment drug, which can significantly inhibit the planktonic state and biofilm activity of S. mutans, inhibit the formation of biofilm, and destroy its structure in vitro. Moreover, in a rat model of dental caries, AR extract strongly prevented the growth and adherence of S. mutans in the oral cavity of rats, as well as decreased the incidence and development of superficial and deep caries lesions while maintaining high biosafety. The above results open up new ways to make new dental materials and products for oral health that contain AR.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Prim. 2017;3:1–16. https://doi.org/10.1038/nrdp.2017.30.
Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. “Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. https://doi.org/10.1016/s0140-6736(17)32154-2.
Lu H, Tao D, Lo E, Li R, Wang X, Tai B, et al. The 4th national oral health survey in the mainland of China: background and methodology. Chin J Dent Res. 2018;21:161–5. https://doi.org/10.3290/j.cjdr.a41079.
Priya A, Selvaraj A, Divya D, Karthik Raja R, Pandian SK. In vitro and in vivo anti-infective potential of thymol against early childhood caries causing dual species candida albicans and Streptococcus Mutans. Front Pharmacol. 2021;12:3067 https://doi.org/10.3389/fphar.2021.760768.
Cui T, Luo W, Xu L, Yang B, Zhao W, Cang H. Progress of antimicrobial discovery against the major cariogenic pathogen Streptococcus Mutans. Curr Issues Mol Biol. 2019;32:601–44. https://doi.org/10.21775/cimb.032.601.
Ben-Zaken H, Kraitman R, Coppenhagen-Glazer S, Khalifa L, Alkalay-Oren S, Gelman D, et al. Isolation and characterization of Streptococcus Mutans phage as a possible treatment agent for caries. Viruses. 2021;13:825 https://doi.org/10.3390/v13050825.
Pekbelgin Karaoğlu H, Sağlam Ö, Özdemir S, Gonca S, Koçak MB. Novel symmetrical and unsymmetrical fluorine-containing metallophthalocyanines: synthesis, characterization and investigation of their biological properties. Dalton Trans. 2021;50:9700–8. https://doi.org/10.1039/d1dt00991e.
Brookes ZLS, Bescos R, Belfield LA, Ali K, Roberts A. Current uses of chlorhexidine for management of oral disease: a narrative review. J Dent. 2020;103:103497 https://doi.org/10.1016/j.jdent.2020.103497.
Wyganowska-Swiatkowska M, Kotwicka M, Urbaniak P, Nowak A, Skrzypczak-Jankun E, Jankun J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int J Mol Med. 2016;37:1594–1600. https://doi.org/10.3892/ijmm.2016.2550.
Qiu W, Zhou Y, Li Z, Huang T, Xiao Y, Cheng L, et al. Application of antibiotics/antimicrobial agents on dental caries. BioMed Res Int. 2020;2020:5658212 https://doi.org/10.1155/2020/5658212.
Zhan L. Rebalancing the caries microbiome dysbiosis: targeted treatment and sugar alcohols. Adv Dent Res. 2018;29:110–6. https://doi.org/10.1177/0022034517736498.
Philip, NI Ecological Approaches to Dental Caries Prevention: Alteration of Biofilms by Natural Products and Casein-Phosphopeptide-Amorphous Calcium Phosphate. Ph.D thesis, The University of Queensland, 288 Herston Road Corner Bramston Tce and Herston Rd Herston QLD 4006, 2019.
Moghadam ET, Yazdanian M, Tahmasebi E, Tebyanian H, Ranjbar R, Yazdanian A, et al. Current herbal medicine as an alternative treatment in dentistry: in vitro, in vivo and clinical studies. Eur J Pharmacol. 2020;889:173665 https://doi.org/10.1016/j.ejphar.2020.173665.
Yang, S The divine farmer’s mate medica: a translation of the Shen Nong Ben Cao Jing, 1st ed BLUE POPPY PRESS A Division of Blue Poppy Enterprises. Inc.: 5441 Western Ave., Suite 2 BOULDER, CO 80301, America, 1998; pp. 58.
Kumar A, Shashni S, Kumar P, Pant D, Singh A, Verma RK. Phytochemical constituents, distributions and traditional usages of Arnebia Euchroma: a review. J Ethnopharmacol. 2021;271:113896 https://doi.org/10.1016/j.jep.2021.113896.
Chinese Commission Pharmacopoeia. Pharmacopoeia of the People’s Republic of China. 1st ed.; China Medical Science Press: No. 22A, Wenhuiyuan North Road, Haidian District, Beijing, China, 2020; pp. 355-69.
Dulta K, Kaur G, Kumar K, Mazhar M, Bhardwaj G, Sharma, A. Species of arnebia genus found in the western himalayas: Arnebia euchroma (Royle ex Benth.), Arnebia benthamii (Wall. Ex G Don) Johnston, Arnebia guttata Bunge. Immunity Boosting Medicinal Plants of the Western Himalayas 2023; pp. 77-105, https://doi.org/10.1007/978-981-19-9501-9_4.
Zhang ZP, Liu Y, Zou HD, Pan J, Hao ZC, Guan W, et al. Euchronin A–F isolated from the Arnebia euchroma (Royle) Johnst. and their anti-proliferative activities in vitro. J Natl Med. 2023; 1-9, https://doi.org/10.1007/s11418-023-01738-2.
Kretschmer N, Durchschein C, Heubl G, Pferschy-Wenzig EM, Kunert O, Bauer R. Discrimination of Zicao Samples Based on DNA Barcoding and HPTLC Fingerprints, and Identification of (22E)-Ergosta-4, 6, 8 (14), 22-tetraen-3-one As a Marker Compound. Planta Med. 2023;89:824–32. https://doi.org/10.1055/a-1855-1778.
Yuan X, Cong Z, Wu Z, Zhao J. In vitro effect of Arnebia euchroma (Royle) Johnst. root extract on the main cariogenic bacteria in oral cavity. Lishizhen Med Mater Med Res. 2020;31:552–5. https://doi.org/10.3969/j.issn.1008-0805.2020.03.013.
Huang XY, Fu HL, Tang HQ, Yin ZQ, Zhang W, Shu G, et al. Optimization extraction of shikonin using ultrasound-assisted response surface methodology and antibacterial studies. Evid-Based Comp Alternative Med. 2020;2020:1–10. https://doi.org/10.1155/2020/1208617.
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, CLSI M100-ED33. 33rd ed. Clinical and Laboratory Standards Institute: 1055 Westlakes Drive, Suite 300 Berwyn, PA 19312, America, 2023; pp. 272-3.
Liu Y, Xu Y, Song Q, Wang F, Sun L, Liu L, et al. Anti-biofilm activities from bergenia crassifolia leaves against Streptococcus Mutans. Front Microbiol. 2017;8:1738 https://doi.org/10.3389/fmicb.2017.01738.
Niu Y, Wang K, Zheng S, Wang Y, Ren Q, Li H, et al. Antibacterial effect of caffeic acid phenethyl ester on cariogenic bacteria and Streptococcus Mutans biofilms. Antimicrobial Agents Chemother. 2020;64:e00251–20. https://doi.org/10.1128/aac.00251-20.
Xiao J, Zuo Y, Liu Y, Li J, Hao Y, Zhou X. Effects of Nidus Vespae extract and chemical fractions on glucosyltransferases, adherence and biofilm formation of Streptococcus Mutans. Arch Oral Biol. 2007;52:869–75. https://doi.org/10.1016/j.archoralbio.2007.02.009.
Vijayakumar A, Sarveswari HB, Vasudevan S, Shanmugam K, Solomon AP, Neelakantan P. Baicalein inhibits Streptococcus Mutans biofilms and dental caries-related virulence phenotypes. Antibiotics. 2021;10:215 https://doi.org/10.3390/antibiotics10020215.
Rahmati Z, Abdi J, Vossoughi M, Alemzadeh I. Ag-doped magnetic metal organic framework as a novel nanostructured material for highly efficient antibacterial activity. Environ Res. 2020;188:109555 https://doi.org/10.1016/j.envres.2020.109555.
Vaillancourt K, LeBel G, Pellerin G, Ben Lagha A, Grenier D. Effects of the licorice isoflavans licoricidin and glabridin on the growth, adherence properties, and acid production of Streptococcus Mutans, and assessment of their biocompatibility. Antibiotics. 2021;10:163 https://doi.org/10.3390/antibiotics10020163.
Shahzad M, Millhouse E, Culshaw S, Edwards CA, Ramage G, Combet E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2014;6:719–29. https://doi.org/10.1039/c4fo01087f.
Garcia SS, Blackledge MS, Michalek S, Su L, Ptacek T, Eipers P, et al. Targeting of Streptococcus Mutans biofilms by a novel small molecule prevents dental caries and preserves the oral microbiome. J Dent Res. 2017;96:807–14. https://doi.org/10.1177/0022034517698096.
Zhang Q, Qin S, Xu X, Zhao J, Zhang H, Liu Z, et al. Inhibitory effect of Lactobacillus Plantarum CCFM8724 towards Streptococcus Mutans- and Candida Albicans-induced caries in rats. Oxid Med Cell Longev. 2020;2020:1–10. https://doi.org/10.1155/2020/4345804.
Zhou Y, Zhang B, Wang Y, Hu R. Effects of sulforaphene on the cariogenic properties of Streptococcus Mutans in vitro and dental caries development in vivo. Antibiotics. 2023;12:1359 https://doi.org/10.3390/antibiotics12091359.
Shirato M, Nakamura K, Tenkumo T, Niwano Y, Kanno T, Sasaki K, et al. Inhibition of tooth demineralization caused by Streptococcus mutans biofilm via antimicrobial treatment using hydrogen peroxide photolysis. Clin Oral Investig. 2023;27:739–50. https://doi.org/10.1007/s00784-022-04821-2.
Keyes PH. Dental caries in the molar teeth of rats II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958;37:1088–99. https://doi.org/10.1177/00220345580370060901.
Zhou Y, Shimada Y, Matin K, Sadr A, Yoshiyama M, Sumi Y, et al. Assessment of root caries under wet and dry conditions using swept-source optical coherence tomography (SS-OCT). Dent Mater J. 2018;37:880–8. https://doi.org/10.4012/dmj.2017-273.
Sheng Z, Jiang Y, Liu J, Yang B. UHPLC-MS/MS analysis on flavonoids composition in astragalus membranaceus and their antioxidant activity. Antioxidants. 2021;10:1852 https://doi.org/10.3390/antiox10111852.
Jain R, Bhardwaj P, Pandey SS, Kumar S. Arnebia Euchroma, a plant species of cold desert in the himalayas, harbors beneficial cultivable endophytes in roots and leaves. Front Microbiol. 2021;12:696667 https://doi.org/10.3389/fmicb.2021.696667.
Meng QJ, Yi H, Yang H, Zhu LW, Feng J, Liu XQ. Studies on flash extraction methods of Arnebia Euchroma. China J Chin Mater Med. 2013;38:2302–5. https://doi.org/10.4268/cjcmm20131416.
Ullah B, Hassan S, Khan MN, Razzaq A, Al-Sadoon MK, Wahab S, et al. Phytochemical screening, antimicrobial, antipellicle and antibiofilm activities of the root of alpine medicinal plant (Arnebia euchroma (Royle) IM Johnst.). Pol J Environ Stud. 2023;33:1–18. https://doi.org/10.15244/pjoes/171298.
He J-M, Sun S-C, Sun Z-L, Chen J-T, Mu Q. Isovalerylshikonin, a new resistance-modifying agent from Arnebia Euchroma, supresses antimicrobial resistance of drug-resistant Staphylococcus Aureus. Int J Antimicrobial Agents. 2019;53:70–73. https://doi.org/10.1016/j.ijantimicag.2018.08.021.
Asghari F, Rabiei Faradonbeh D, Malekshahi ZV, Nekounam H, Ghaemi B, Yousefpoor Y, et al. Hybrid PCL/Chitosan-PEO nanofibrous scaffolds incorporated with A. Euchroma extract for skin tissue engineering application. Carbohydr. Polym. 2022;278:118926 https://doi.org/10.1016/j.carbpol.2021.118926.
Fazal A, Yang M, Wang M, Ali F, Wen Z, Yin T, et al. Assessment of shikonin and acetyl-shikonin for mitigating quorum sensing potential of C. violaceum. Plant Growth Regul. 2021;94:233–43. https://doi.org/10.1007/s10725-021-00712-w.
Gao Z, Chen X, Wang C, Song J, Xu J, Liu X, et al. New strategies and mechanisms for targeting Streptococcus mutans biofilm formation to prevent dental caries: a review. Microbiol Res. 2023, 127526, https://doi.org/10.1016/j.micres.2023.127526.
Li J, Li S, Li H, Guo X, Guo DU, Yang Y, et al. Antibiofilm activity of shikonin against Listeria monocytogenes and inhibition of key virulence factors. Food Control. 2021;120:107558 https://doi.org/10.1016/j.foodcont.2020.107558.
El Zawawy NA, El-Safty S, Kenawy ER, Ibrahim Salem S, Ali SS, Mahmoud YA. Exploring the biomedical potential of a novel modified glass ionomer cement against the pandrug-resistant oral pathogen Candida albicans SYN-01. J Oral Microbiol. 2023;15:2195741 https://doi.org/10.1080/20002297.2023.2195741.
Huang Y, Guo X, Wang Z, Yin C, Chen M, Xie J, et al. High-resolution neuraminidase inhibition profiling of Arnebia euchroma (Royle) IM Johnst. based on HR-MS and target isolation: An example study of anti-infectious constituents in traditional Chinese medicine. J. Ethnopharmacol. 2024;319:117074 https://doi.org/10.1016/j.jep.2023.117074.
Lemos JA, Palmer SR, Zeng L, Wen ZT, Kajfasz JK, Freires IA, et al. The biology of Streptococcus Mutans. Microbiol Spectr. 2019;7:435–48. https://doi.org/10.1128/microbiolspec.gpp3-0051-2018.
Astasov-Frauenhoffer M, Varenganayil MM, Decho AW, Waltimo T, Braissant O. Exopolysaccharides regulate calcium flow in cariogenic biofilms. PLOS ONE. 2017;12:e0186256 https://doi.org/10.1371/journal.pone.0186256.
Alam MK, Zheng L, Liu R, Papagerakis S, Papagerakis P, Geyer CR. Synthetic antigen-binding fragments (Fabs) against S. Mutans and S. Sobrinus inhibit caries formation. Sci Rep. 2018;8:10173 https://doi.org/10.1038/s41598-018-28240-0.
Poppolo Deus F, Ouanounou A. Chlorhexidine in dentistry: pharmacology, uses, and adverse effects. Int Dent J. 2022;72:269–77. https://doi.org/10.1016/j.identj.2022.01.005.
Guglielmi P, Pontecorvi V, Rotondi G. Natural compounds and extracts as novel antimicrobial agents. Expert Opin Ther Pat. 2020;30:949–62. https://doi.org/10.1080/13543776.2020.1853101.
Liu D, Ma X, Ji Y, Chen R, Zhou S, Yao H, et al. Bioresponsive nanotherapy for preventing dental caries by inhibiting multispecies cariogenic biofilms. Bioact Mater. 2022;14:1–14. https://doi.org/10.1016/j.bioactmat.2021.12.016.
Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2006;1:Unit-1B.1 https://doi.org/10.1002/9780471729259.mc01b01s00.
Struzycka I. The oral microbiome in dental caries. Pol J Microbiol. 2014;63:127–35. https://doi.org/10.33073/pjm-2014-018.
Tu H, Fan Y, Lv X, Han S, Zhou X, Zhang L. Activity of synthetic antimicrobial peptide GH12 against Oral Streptococci. Caries Res. 2016;50:48–61. https://doi.org/10.1159/000442898.
Wen ZT, Yates D, Ahn S-J, Burne RA. Biofilm formation and virulence expression by Streptococcus Mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10:111 https://doi.org/10.1186/1471-2180-10-111.
Mu R, Zhang H, Zhang Z, Li X, Ji J, Wang X, et al. Trans-cinnamaldehyde loaded chitosan based nanocapsules display antibacterial and antibiofilm effects against cavity-causing Streptococcus mutans. J Oral Microbiol. 2023;15:2243067 https://doi.org/10.1080/20002297.2023.2243067.
Cheng X, He F, Si M, Sun P, Chen Q. Effects of antibiotic use on saliva antibody content and oral microbiota in sprague dawley rats. Front. Cell. Infect. Microbiol. 2022;12:721691 https://doi.org/10.3389/fcimb.2022.721691.
Orland FJ, Blayney JR, Harrison RW, Reyniers JA, Trexler PC, Wagner M, et al. Use of the germfree animal technic in the study of experimental dental caries: I. Basic observations on rats reared free of all microorganisms. J Dent Res. 1954;33:147–74. https://doi.org/10.1177/00220345540330020201.
Huang Y, Wang T, Chen Y, Lin H, Chen, D Amyloid hexapeptide prevent dental caries by antibiofilm formation. J Dent. 2023, 104596, https://doi.org/10.1016/j.jdent.2023.104596.
Fukuzato Y, Matsuura T, Ozaki K, Matsuura M, Sano T, Nakahara Y, et al. Morphological study on dental caries induced in WBN/KobSlc Rats (Rattus Norvegicus) fed a standard laboratory diet. Lab Anim. 2009;43:376–81. https://doi.org/10.1258/la.2008.0080064.
Zhang Z, Ji Y, Liu D, Zhou S, Wang Z, Chen R, et al. Heat shock protein inhibitors show synergistic antibacterial effects with photodynamic therapy on caries-related Streptococci in vitro and in vivo. Msphere. 2023;8:e00679–22. https://doi.org/10.1128/msphere.00679-22.
Ablat N, Ablimit M, Abudoukadier A, Kadeer B, Maihemuti A, Bakewaiyi A, et al. Liver protection and hemostatic effects of medicinal plant Arnebia euchroma (Royle) IM Johnst extract in a rat model. J Ethnopharmacol. 2023;300:115739 https://doi.org/10.1016/j.jep.2022.115739.
Tan Y, Tian D, Li C, Chen Y, Shen Y, Li J, et al. Naphthoquinones and triterpenoids from Arnebia euchroma (Royle) Johnst and their hypoglycemic and lipid-lowering effects. Fitoterapia. 2022;162:105288 https://doi.org/10.1016/j.fitote.2022.105288.
Hajishengallis G. Illuminating the oral microbiome and its host interactions: animal models of disease. FEMS Microbiol Rev. 2023;47:fuad018 https://doi.org/10.1093/femsre/fuad018.
Zhang P, Wu S, Li J, Bu X, Dong X, Chen N, et al. Dual-sensitive antibacterial peptide nanoparticles prevent dental caries. Theranostics. 2022;12:4818 https://doi.org/10.7150/thno.73181.
Yi Y, Wang L, Chen L, Lin Y, Luo Z, Chen Z, et al. Farnesal-loaded pH-sensitive polymeric micelles provided effective prevention and treatment on dental caries. J. Nanobiotechnol. 2020;18:1–16. https://doi.org/10.1186/s12951-020-00633-2.
Funding
This research was funded by the National Natural Science Foundation of China (81760194); Xinjiang Medical University Postgraduate Research and Innovation Project (CXCY2022034).
Author information
Authors and Affiliations
Contributions
Zeyu Wu and Jie Song have contributed equally to this work. Jin Zhao and Zeyu Wu: designed the experiments and revised the draft. Jie Song and Zeyu Wu: performed the experiments and wrote the paper draft. Yangyang Zhang and Xiyu Yuan: performed the experiments and statistical analysis of data. Jin Zhao: guided the experiments. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declaration
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The “Ethics Review Committee of Xinjiang Medical University” gave its clearance for this research’s conduct (IACUC-JIPD-2019030). The China Ministry of Science and Technology’s Guide for the Care and Use of Laboratory Animals was followed in all experiments. Volunteers were asked to read the completed consent form and sign it if they agreed with its contents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Z., Song, J., Zhang, Y. et al. Inhibitory and preventive effects of Arnebia euchroma (Royle) Johnst. root extract on Streptococcus mutans and dental caries in rats. BDJ Open 10, 15 (2024). https://doi.org/10.1038/s41405-024-00196-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41405-024-00196-6