Abstract
Objective
Evaluation of the Shear bond strength over zirconia and titanium alloy and degree of conversion of extraoral compared to intraoral self-adhesive resin cements.
Materials and methods
Nine bonding protocols were carried out on zirconia 4Y-TZP and titanium alloy (Ti-6Al-4V). Seven resin cement (one extraoral and six intraoral) were tested in the shear bond strength test and the degree of conversion measurements.
Results
The significantly highest value was obtained for Monobond Plus + Multilink Hybrid Abutment, the extraoral resin cement for both titanium alloy (35.1 MPa) and zirconia (32.9 MPa). For each resin, significantly higher DC values were obtained for the dual-cure mode compared with the self-cure mode. Regardless of the cure mode, Nexus Universal reached the highest DC (78.4%).
Discussion/Conclusions
In this study, the extraoral self-curing resin cement showed the higher bond strength values on zirconia and titanium alloy when associated with a universal primer. Some intraoral dual-cure resin cements showed closed performances when used with universal primers. There is no direct correlation between the degree of conversion of the resin cement and the shear bond strength obtained on the prosthetic materials tested.
Similar content being viewed by others
Introduction
Zirconia and titanium alloys are widely used materials in prosthetic dentistry. In practice, they need to be carefully grit-blasted to achieve good micromechanical retention (50 µm aluminum oxides) [1]. Moreover, the use of functional phosphorylated monomers (10-Methacryloyloxydecyl dihydrogen phosphate (10-MDP), 10-methacryloyloxydecyl dihydrogen thiophosphate (MDTP), glycerol phosphate dimethacrylate (GPDM) and others) in resin cement enhances chemical adhesion [2,3,4], whereas the application of silane has no effect, given the absence of silica in zirconia and titanium alloy [5]. Among the bonding strategies currently available, there are three bonding methods used after grit-blasting. The first is the use of a dedicated prosthetic primer containing functional phosphorylated monomers and silane, called universal primer, then applying a resin cement [6,7,8]. The second method consists of replacing the specific prosthetic primer with a universal adhesive (that also contains functional monomers) and then applying a resin cement [9]. The third is to directly use a resin cement containing functional monomers as part of its formulation [10].
Zirconia can be bonded on dental tissues, such as cementing zirconia crowns on prepared teeth abutments (intraoral bonding) or on titanium base abutment (Ti-base) in prosthetic labs (extraoral bonding) [11]. Some resin cements are dedicated for intraoral use, whereas others are indicated for extraoral use. To follow the trend of simplifying procedures [12, 13], it would be relevant to determine whether intraoral dedicated resin cements could also be used as efficiently as than extraoral ones.
Depending on their chemical composition, these resin cements can be self-cured (chemopolymerized), light-cured (photopolymerized) or dual-cured (association of chemopolymerization and photopolymerization) [14]. There are clinical or laboratory situations in dentistry that do not allow efficient light curing (assembly of inlay cores, thick zirconia crowns, Ti-bases) and under which [15,16,17], resin polymerization relies almost only on the self-curing mode [18]. To our knowledge, there is only one study (in another context) that compares the bond strength of intraoral and extraoral resin cements to zirconia or titanium alloy in the current trend toward simplification [19], and few prior research investigates the role played by light curing in the curing process [20].
The aim of this study was first to evaluate the shear bond strength of one extraoral and various intraoral resin cements to zirconia and titanium alloy according to nine bonding protocols in self-curing mode (in order to assess their bonding strength in the least favorable polymerization mode) and then to measure their degree of conversion in self-curing mode or with additional light curing. The first null hypothesis was that there are no differences in shear bond strengths between the nine protocols tested resin for each substrate. The second null hypothesis was that there were no differences in the degree of conversion between the various resin cements in self-curing mode or with an additional light-cured mode.
Materials and Methods
Materials used
The materials used in this study are summarized in Table 1.
Shear bond strength (SBS) test and failure mode
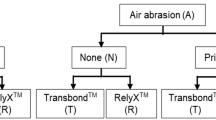
Nine bonding protocols (Table 2) were carried out on zirconia 4Y-TZP (Katana Zirconia STML, Kuraray-Noritake) and titanium alloy (Ti-6Al-4V) (NeoNickel, Chassieu, France) for a total of 18 variable groups. Ten samples were produced per variable groups.
Blocks of zirconia and titanium alloy were included in self-curing acrylic resin and then abraded with water-cooled sandpaper (800 grits) to expose a flat surface (>7 mm2). All these samples were grit-blasted with Al2O3 (50 µm) for 10 s at 2 bars, rinsed with water/air spray, then with a 99% ethanol solution and dried.
On each sample (n = 180), a cylindrical Teflon mold was placed to build a 3 mm-high cylinder of resin cement with a flat base of 7 mm² (diameter = 3 mm). The tested bonding protocols were performed following the manufacturer’s instructions (which can lead to various protocols for a specific resin cement). The resin cement was set in self-curing mode (in the dark) under 50 g pressure for 60 min. Then, the mold was removed, and the excess, if present, was gently removed with a scalpel. All the samples were stored in demineralized water at 37 °C for 24 h. Detailed bonding protocols are summarized in Table 2.
For each group (n = 10), SBS values were determined in a universal testing machine (LRX, Lloyd Instruments, Fareham, UK). The shear force was applied at the resin cement/prosthetic material interface, with a chisel-shaped blade parallel to the prosthetic material surface. A cross-head speed of 0.5 mm/min was chosen.
The debonded specimens were observed under a binocular microscope (BZH10 Olympus, Hamburg, Germany) at 30× magnification, and the failure modes were classified according to the following four types: cohesive failure within the prosthetic material, adhesive failure at the interface between the resin cement and the prosthetic material, mixed failure (adhesive and cohesive failure within the prosthetic material), cohesive failure within the resin cement.
Degree of conversion measurements
For each resin cement tested (n = 7), 6 cylindrical specimens (diameter 6 mm x height 3 mm) were made with Teflon molds: 3 were performed in with light-activation for 60 s at 1200 mW per square cm (Valo Grande lamp, Ultradent Products Inc, South Jordan, UT, USA) immediately after mixing, and 3 were performed only in self-curing mode (mixed in the dark). All samples were then stored in the dark in distilled water at 37 °C for one week before the degree of conversion (DC) measurement.
DC was determined using Fourier transformed infrared spectroscopy (FTIR) with a NicoletTM iS10 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) in attenuated total reflectance (ATR) mode following proposed protocol from previous publications [21]. These spectra were recorded with OMNIC software (Thermo Electron Corporation, Waltham, MA, USA). After measuring the background, all measurements were obtained in the spectral region of 500 to 4000 cm−1 under the following conditions: a resolution of 4 cm−1 and 32 internal scans per reading. For all resin cements analyzed, spectra in the unpolymerized state were also collected to serve as a reference. The spectra of each specimen were recorded 3 times.
For each spectrum, the height of the absorption band of the aliphatic C=C bond (1638 cm−1) and that of the aromatic C=C bond (1608 cm−1) were measured, first in the monomer state and then after the polymerization process. DC was determined by evaluating the change in the height ratio of the aliphatic C=C peak and the aromatic C=C peak during the polymerization process [22], according to the following formula:
Abs: Absorbance, P: Polymerized, NP: Nonpolymerized
Statistical analysis
Normal distribution of SBS and DC values was confirmed by the Shapiro‒Wilk test, and the equality of variances was assessed using the Levene test before the other tests were performed. SBS and DC data were expressed as the mean values and standard deviations.
For SBS results, two one-way ANOVAs followed by Tukey’s post hoc test for each bonding substrate (zirconia or Ti-6Al-4V) were used to investigate the difference in SBS between the different groups. The failure mode was analyzed by Fisher’s exact test for single comparisons between groups and pairwise analysis.
For DC results, a 2-way ANOVA followed by Tukey’s post hoc test was performed for the factors “resin” and “mode of polymerization”. In all tests, the significance level was p < 0.05. Statistical calculations were performed using R software (R version 3.6.1, R Foundation for statistical computing, Vienna, Austria).
Results
SBS values and failure mode analysis
The mean SBS values and standard deviations are reported in Table 3.
The significantly highest SBS value was obtained for MP + MHA, the extraoral resin cement for both Ti-6Al-4V (35.1 MPa) and zirconia (32.9 MPa). In contrast, TTC had the lowest SBS values regardless of the prosthetic material (15.9 MPa for Ti-6Al-4V and 12.7 MPa for zirconia).
The use of universal primer or universal adhesive did not significantly improve the bond strength on zirconia (29.1 MPa for GC-O vs. 29.4 MPa for GMP + GC-O; 24.7 MPa for PSA-U vs. 24.3 MPa for CUB + PSA-U), whereas it was significantly increased on titanium alloy for GC-O (25.9 MPa for GC-O vs. 32.0 MPa for GMP + GC).
Adhesive failures occurred in every group. No significant differences in the failure occurrences were shown between groups.
Degree of conversion
The degree of conversion values obtained for each resin cement are reported in Table 4.
For each resin, significantly higher DC values were obtained for the dual-cure mode compared with the self-cure mode. Regardless of the cure mode, N-U reached the highest DC (78.4%), whereas TTC had the lowest DC (60.3%).
Discussion
Shear bond strength values and failure mode analysis
It is difficult to establish an immediate in vitro threshold bond strength, despite that such a measurement could ensure long-term clinical adhesion. However, long-term clinical studies show good bonding performance on zirconia and Ti-6Al-4V when the standard protocols are followed correctly [23,24,25,26].
In this study, macro-shear bond strength test was used to assess SBS values of the various adhesive protocols to Zirconia and Ti-6Al-4V. The macro-shear bond strength test is one of the two most frequently used tests, along with the micro-tensile bond strength test, to assess the strength of an adhesive procedure [27,28,29]. Some papers have compared the relevance of micro-tensile and macro-shear bond strength tests, showing that the resultant forces at the interface are neither pure shear for the macro-shear test [28], nor pure traction for the micro-traction test [28]. Although the micro-traction test is considered more representative than the macro-shear test, the latter offers a number of technical advantages in terms of sample preparation in the laboratory and was selected for this study.
Bonding strength values on Ti-6Al-4V and zirconia were measured concomitantly, due to the dual indication of these two materials in certain prosthetic indications such as bonding crowns on abutments in implantology. It is therefore interesting to know the performance of the same protocol on these two substrates. However, in mouth, the two materials have different clinical indications. Zirconia can be bonded in a large number of different fixed prosthetic indications [30, 31], while titanium is less frequently used in bonded indications, except in certain cases of intra-radicular posts or periodontal splints [32, 33].
The extraoral resin cement bonding protocol MP + MHA was chosen for study here because of its widespread use in the literature with good clinical retention results [34,35,36]. As a result, it is considered a gold standard when a prosthetic assembly is performed outside the mouth. In our study, the MP + MHA extraoral protocol led to the highest bond strength values over both zirconia and Ti-6Al-4V. The first null hypothesis is therefore rejected.
The two highest values found for each prosthetic material were MHA and GC-O using their dedicated universal primers (MP and GMP, respectively). There is a general trend of specific primers such as MP or GMP to be more effective than universal adhesives used as primers on titanium alloy and zirconia [37]. MP is a diluted ethanolic solution that consists of three mutually stable bonding monomers, silane, phosphoric acid agent and disulfide [38]. GMP has a slightly different composition with the presence of γ-MPTMS, 10-MDP, MDTP, bis-GMA and TEGDMA [39]. As zirconia and titanium alloys possess a high affinity to phosphoric acid in forming poorly soluble phosphates [40], these specific primers containing methacrylate monomers with a functional phosphoric-acid group result in a strong bond and hydrolysis resistance with them. Although the detailed adhesion mechanism remains largely unexplored, the bond between metal oxides and acid monomers is produced by intermolecular forces such as hydrogen bonds [41]. The most common acid monomer used to achieve this chemical bonding is 10-MDP, which is thus present in the two universal primers tested. This monomer is considered one of the most effective materials (if not the most effective) for creating chemical bonds with the two substrates studied [10, 42,43,44]. It is often combined with other molecules in universal primers for synergistic action and to obtain higher bond strength values on zirconia and nonprecious metals but also to make them effective on precious metals (sulfur compounds) or vitreous ceramics (presence of silane) [42]. These various mixtures within universal primers are suspected of being detrimental to achieving the higher interaction with our two substrates tested but are nevertheless commonly accepted as methods at least as reliable for bonding these substrates as tribochemical grit-blasting which consists in applying a thin layer of silica to the surface of the prosthetic element followed by the use of a primer containing silane [45, 46]. Other zirconia bonding processes have also been proposed in the literature and are currently being evaluated [47].
Here, all dental universal adhesives tested as an alternative to universal primers also contained 10-MDP or glyceryl dimetacrylates as functional monomers but led to lower bond strength values than when a universal primer was used. All curing procedures were carried out in a dark room with a self-curing mode to avoid activating light-curing polymerization of resin cements. Under these conditions, universal adhesives used as nonspecific primers were not light-cured either. This could also contribute to the lower bond strength values, even though manufacturers report self-cure contact with their associated resin cements.
Intraoral self-adhesive resin cements showed different behaviors when used directly on prosthetic materials without their specific primers or associated universal adhesives. GC-O had a completely different behavior between bonding on zirconia or titanium alloy. On zirconia, GC-O with or without its universal silane GMP exhibited almost the same SBS value, whereas significantly low values were observed on Ti6-Al-4V. This could be linked to the absence of MDTP in its composition, whereas this molecule is present on GMP.
For the other self-adhesive resin cements tested without their associated universal primer or universal adhesive on zirconia, we note that there were a number of factors that may explain the lower performance compared to GC-O. Kerr, who produces the OBU and the N-U, is the only brand that uses GPDM instead of 10-MDP in self-adhesive resin cements or dental adhesives. Prior research has shown that 10-MDP has a higher affinity for zirconia than GPDM [44]. Moreover, no synergy seems possible between GPDM and 10-MDP to achieve higher bond strength [48]. Regarding PSA-U and its universal adhesive CUB, the use of the adhesive did not influence the SBS. Bonding on zirconia has lower SBS values despite the presence of MDP in the resin cement and in the adhesive. One explanation could be the higher conversion rate observed for GC-O compared to PSA-U in self-curing mode. Prior research has shown many times that the higher the value, the greater the bond strength values developed for the same product, thanks to improved mechanical properties [49, 50]. TTC has the lowest SBS values on zirconia and Ti-6Al-4V. This result can be explained by the fact that there is no 10-MDP or GPDM in its formula. Rather, its composition includes 4-META, a functional monomer with a high affinity for metal [3], but a lower affinity for zirconia [51], which may explain its better adhesion performance on Ti-6Al-4V than on zirconia. Second, TTC had the lowest conversion rate in dual-cured or self-cured mode of all the other resin cements tested.
Degree of conversion
All the material in dual cure mode yielded a higher DC compared to the self-cured mode. This outcome has been reported in many studies [52, 53]. Light not only activates the photoinitiators present in the resin but also allows the heat generated by light irradiation to improve the mobility of the resin molecules, thereby increasing the cross-linking rate of the polymerized resin [54].
GPDM is a monomer found only in N-U, which has two methacrylate groups and one phosphate group. These two methacrylate groups can promote better cross-linking in polymerized networks despite other functional monomers, such as 10-MDP [48]. This could explain the observation of this material showing the higher overall DC of N-U in dual-cure.
Dual curing is therefore concluded to optimize the degree of conversion in any clinical or laboratory situation, which can have a major impact on the long-term adhesion stability, particularly in terms of water absorption and resin solubility in the bonding interface [55], which can become infiltrated if light-cured to a limited extent [56, 57]. A high DC results in better mechanical properties and biocompatibility [17, 49, 50, 58].
Surprisingly, MHA, the only extraoral and self-cured resin cement, had higher DC with light curing than self-cure mode. This resin cement is recommended for Ti-base abutment sealing in laboratory and no light-curing is advised. This recommendation is explained by the astonishing presence of camphorquinone, a light curing initiatior in its composition. Therefore, although not recommended by the manufacturer, additional light-curing of this material in the laboratory could result in beneficial properties, especially as the high temperatures caused by possible heating do not appear to alter its mechanical and adhesive properties. This procedure would also be a useful technique for improving its biological properties in the oral environment [19].
This study being in vitro, it includes a certain number of limitations due to this fact, but also to its construction scheme:
-
It would be interesting to carry out adhesion tests with ageing, in particular to evaluate the evolution over time of the simplified intra-oral adhesives studied in this study, which contain a certain hydrophilicity due to their self-adhesive chemistry.
-
Because of their in vitro nature, these results cannot be directly transposed to the clinic. Long-term studies are needed to draw clinical conclusions.
Finally, as a follow-up to this study, the behavior of the simplified intra-oral adhesives from this study on dental tissues will be investigated in addition to the current investigation on prosthetic tissues.
Conclusions
In this study, the extraoral self-curing resin cement showed the higher bond strength values on zirconia and Ti-6Al-4V alloy when associated with a universal primer. Some intraoral dual-cure resin cements showed closed performances when used with universal primers.
The extreme simplification of bonding procedures based on the use of self-adhesive resin cements containing functional monomers alone or monomers associated with a universal adhesive led to variable results depending on brands and formulations. These results may nevertheless be sufficient for these applications given that they yielded bond strength values close to those of conventional procedures.
There is no direct correlation between the degree of conversion of the resin cement and the shear bond strength obtained on the prosthetic materials tested. Additional light curing is advised in every case of bonding to enhance the degree of conversion.
However, further clinical studies validating these results are required before generalizing these conclusions.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Chatterjee N, Ghosh A. Current scenario on adhesion to zirconia; surface pretreatments and resin cements: A systematic review. J Indian Prosthodont Soc. 2022;22:13–20.
Koizumi H, Nakayama D, Komine F, Blatz MB, Matsumura H. Bonding of resin-based luting cements to zirconia with and without the use of ceramic priming agents. J Adhes Dent. 2012;14:385–92.
Tsuchimoto Y, Yoshida Y, Mine A, Nakamura M, Nishiyama N, Van Meerbeek B, et al. Effect of 4-MET- and 10-MDP-based primers on resin bonding to titanium. Dent Mater J. 2006;25:120–4.
Chen Y, Lu Z, Qian M, Zhang H, Xie H, Chen C. Effect of 10-Methacryloxydecyl Dihydrogen Phosphate concentration on chemical coupling of Methacrylate Resin to Yttria-stabilized Zirconia. J Adhes Dent. 2017;19:349–55.
Matinlinna JP, Lung CYK, Tsoi JKH. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent Mater. 2018;34:13–28.
Özcan M, Volpato CÂM. Adhesion protocol for bonding abutments or fixed dental prostheses on titanium bases in implant-borne reconstructions: how and why? J Adhes Dent. 2016;18:268–9.
Taira Y, Matsumura H, Yoshida K, Tanaka T, Atsuta M. Adhesive bonding of titanium with a methacrylate-phosphate primer and self-curing adhesive resins. J Oral Rehabil. 1995;22:409–12.
Quigley NP, Loo DSS, Choy C, Ha WN. Clinical efficacy of methods for bonding to zirconia: A systematic review. J Prosthet Dent. 2021;125:231–40.
Dos Santos RA, de Lima EA, Mendonça LS, de Oliveira JE, Rizuto AV, de Araújo Silva Tavares ÁF, et al. Can universal adhesive systems bond to zirconia? J Esthet Restor Dent Publ Am Acad Esthet Dent Al. 2019;31:589–94.
Comino-Garayoa R, Peláez J, Tobar C, Rodríguez V, Suárez MJ. Adhesion to Zirconia: A systematic review of surface pretreatments and resin cements. Mater Basel Switz. 2021;14:2751.
Guncu MB, Aktas G, Guncu GN, Anıl D, Turkyilmaz I, Antonoff LR. Clinical, technical, and radiologic outcomes of 182 implant-supported Zirconia single crowns using Titanium-base abutments: a retrospective study. Int J Prosthodont. 2022;35:553–9.
Cadenaro M, Josic U, Maravić T, Mazzitelli C, Marchesi G, Mancuso E, et al. Progress in dental adhesive materials. J Dent Res. 2023;102:254–62.
Perdigão J, Araujo E, Ramos RQ, Gomes G, Pizzolotto L. Adhesive dentistry: Current concepts and clinical considerations. J Esthet Restor Dent Publ Am Acad Esthet Dent Al. 2021;33:51–68.
Heboyan A, Vardanyan A, Karobari MI, Marya A, Avagyan T, Tebyaniyan H, et al. Dental luting cements: an updated comprehensive review. Mol Basel Switz. 2023;28:1619.
Di Francescantonio M, Aguiar TR, Arrais CAG, Cavalcanti AN, Davanzo CU, Giannini M. Influence of viscosity and curing mode on degree of conversion of dual-cured resin cements. Eur J Dent. 2013;7:81–5.
Archegas LRP, de Menezes Caldas DB, Rached rN, Soares P, Souza EM. Effect of ceramic veneer opacity and exposure time on the polymerization efficiency of resin cements. Oper Dent. 2012;37:281–9.
Meng X, Yoshida K, Atsuta M. Influence of ceramic thickness on mechanical properties and polymer structure of dual-cured resin luting agents. Dent Mater. 2008;24:594–9.
Inokoshi M, Nozaki K, Takagaki T, Okazaki Y, Yoshihara K, Minakuchi S, et al. Initial curing characteristics of composite cements under ceramic restorations. J Prosthodont Res. 2021;65:39–45.
Lang R, Hiller K-A, Kienböck L, Friedl K, Friedl KH. Influence of autoclave sterilization on bond strength between zirconia frameworks and Ti-base abutments using different resin cements. J Prosthet Dent. 2022;127:617.e1–617.e6.
Aldhafyan M, Silikas N, Watts DC. Influence of curing modes on conversion and shrinkage of dual-cure resin-cements. Dent Mater Publ Acad Dent Mater. 2022;38:194–203.
Dantagnan C-A, François P, Le Goff S, Attal JP, Dursun E. Degree of conversion of 3D printing resins used for splints and orthodontic appliances under different postpolymerization conditions. Clin Oral Investig. 2023;27:2935–42.
Collares FM, Portella FF, Leitune VCB, Samuel SM. Discrepancies in degree of conversion measurements by FTIR. Braz Oral Res. 2013;27:453–4.
Kern M. Bonding to oxide ceramics—laboratory testing versus clinical outcome. Dent Mater Publ Acad Dent Mater. 2015;31:8–14.
Blatz MB, Vonderheide M, Conejo J. The effect of resin bonding on long-term success of high-strength ceramics. J Dent Res. 2018;97:132–9.
Attia A, Kern M. Long-term resin bonding to zirconia ceramic with a new universal primer. J Prosthet Dent. 2011;106:319–27.
Alammar A, Blatz MB. The resin bond to high-translucent zirconia-A systematic review. J Esthet Restor Dent Publ Am Acad Esthet Dent Al. 2022;34:117–35.
Braga RR, Meira JB, Boaro LC, Xavier TA. Adhesion to tooth structure: A critical review of “macro” test methods. Dent Mater. 2010;26:e38–e49.
El Mourad AM. Assessment of bonding effectiveness of adhesive materials to tooth structure using bond strength test methods: a review of literature. Open Dent J. 2018;12:664–78.
De Munck J, Mine A, Poitevin A, Van Ende A, Cardoso MV, Van Landuyt KL, et al. Meta-analytical review of parameters involved in dentin bonding. J Dent Res. 2012;91:351–7.
Hammoudi W, Trulsson M, Svensson P, Smedberg JI. Long-term results of a randomized clinical trial of 2 types of ceramic crowns in participants with extensive tooth wear. J Prosthet Dent. 2022;127:248–57.
Alghauli MA, Wille S, Lehmann F, Kern M. Survival and debonding resistance of posterior cantilever resin-bonded fixed dental prostheses for moderately and severely worn dentition during thermomechanical loading. Dent Mater Publ Acad Dent Mater. 2023;39:634–9.
Liu Y, Fang M, Zhao R, Liu H, Tian M, Zhong S, et al. Effects of periodontal splints on biomechanical behaviors in compromised periodontal tissues and cement layer: 3D finite element analysis. Polymers. 2022;14:2835.
Soares AP, Bitter K, Lagrange A, Rack A, Shemesh H, Zaslansky P. Gaps at the interface between dentine and self-adhesive resin cement in post-endodontic restorations quantified in 3D by phase contrast-enhanced micro-CT. Int Endod J. 2020;53:392–402.
Erhan Çömlekoğlu M, Nizam N, Çömlekoğlu MD. Immediate definitive individualized abutments reduce peri-implant bone loss: a randomized controlled split-mouth study on 16 patients. Clin Oral Investig. 2018;22:475–86.
Elter B, Tak Ö. Influence of cement shade, ceramic thickness, and airborne-particle abrasion of titanium surface on the final color of monolithic lithium disilicate glass-ceramic hybrid-abutment systems in vitro. Quintessence Int Berl Ger 1985. 2022;53:678–88.
Dhesi GS, Sidhu S, Al-Haj Husain N, Özcan M. Evaluation of adhesion protocol for titanium base abutments to different ceramic and hybrid materials. Eur J Prosthodont Restor Dent. 2021;29:22–34.
Amaral M, Belli R, Cesar PF, Valandro LF, Petschelt A, Lohbauer U. The potential of novel primers and universal adhesives to bond to zirconia. J Dent. 2014;42:90–98.
Ebert T, Elsner L, Hirschfelder U, Hanke S. Shear bond strength of brackets on restorative materials: Comparison on various dental restorative materials using the universal primer Monobond® Plus. J Orofac Orthop. 2016;77:73–84.
Wille S, Lehmann F, Kern M. Durability of resin bonding to lithium disilicate using different self-etching and conventional ceramic primers after long-term aging. Dent Mater Publ Acad Dent Mater. 2022;38:444–50.
Völkel T: Wissenschaftliche Dokumentation Monobond Plus. In. Wissenschaflicher Dienst 2011.
Kern M, Thompson VP. Durability of resin bonds to a cobalt-chromium alloy. J Dent. 1995;23:47–54.
Kodaira A, Koizumi H, Hiraba H, Takeuchi Y, Koike M, Shimoe S. Bonding of resin luting materials to titanium and titanium alloy. J Oral Sci. 2022;64:181–4.
Salem RST, Ozkurt-Kayahan Z, Kazazoglu E. In vitro evaluation of shear bond strength of three primer/resin cement systems to monolithic Zirconia. Int J Prosthodont. 2019;32:519–25.
Arai M, Takagaki T, Takahashi A, Tagami J. The role of functional phosphoric acid ester monomers in the surface treatment of yttria-stabilized tetragonal zirconia polycrystals. Dent Mater J. 2017;36:190–4.
Ye S, Lin J-C, Kang L-L, Li CL, Hou SS, Lee TL, et al. Investigations of silane-MDP interaction in universal adhesives: A ToF-SIMS analysis. Dent Mater Publ Acad Dent Mater. 2022;38:183–93.
Dimitriadi M, Panagiotopoulou A, Pelecanou M, Yannakopoulou K, Eliades G. Stability and reactivity of γ-ΜPTMS silane in some commercial primer and adhesive formulations. Dent Mater Publ Acad Dent Mater. 2018;34:1089–101.
Kakkad N, Yadav NS, Hazari P, Narwani S, Somkuwar K, Basha S, et al. Comparative evaluation of tensile bond strength of Poly Ether Ether Ketone (PEEK) and Zirconia copings using resin cement with or without adhesive: an in vitro study. Mater Basel Switz. 2022;15:4167.
Calamita RS, Oliveira AAD, Pizzanelli GG, Salvador MVO, Mesquita AMM, Pecorari VGA et al. Interaction of different concentrations of 10-MDP and GPDM on the zirconia bonding Dent Mater Off Publ Acad Dent Mater. 2023:S0109-5641(23)00113-6
Hoorizad Ganjkar M, Heshmat H, Hassan, Ahangari R. Evaluation of the effect of porcelain laminate thickness on degree of conversion of light cure and dual cure resin cements using FTIR. J Dent Shiraz Iran. 2017;18:30–36.
Alkhudhairy F, AlKheraif A, Naseem M, Khan R, Vohra F. Degree of conversion and depth of cure of Ivocerin containing photo-polymerized resin luting cement in comparison to conventional luting agents. Pak J Med Sci. 2018;34:253–9.
Shimizu H, Inokoshi M, Takagaki T, Uo M, Minakuchi S. Bonding Efficacy of 4-META/MMA-TBB resin to surface-treated highly translucent dental Zirconia. J Adhes Dent. 2018;20:453–9.
Chen L, Suh BI, Gleave C, Choi WJ, Hyun J, Nam J. Effects of light-, self-, and tack-curing on degree of conversion and physical strength of dual-cure resin cements. Am J Dent. 2016;29:67–70.
David-Pérez M, Ramírez-Suárez JP, Latorre-Correa F, Agudelo-Suárez AA. Degree of conversion of resin-cements (light-cured/dual-cured) under different thicknesses of vitreous ceramics: systematic review. J Prosthodont Res. 2022;66:385–94.
Theobaldo JD, Aguiar FHB, Pini NIP, Lima DANL, Liporoni PCS, Catelan A. Effect of preheating and light-curing unit on physicochemical properties of a bulk fill composite. Clin Cosmet Investig Dent. 2017;9:39–43.
Hofmann N, Renner J, Hugo B, Klaiber B. Elution of leachable components from resin composites after plasma arc vs. standard or soft-start halogen light irradiation. J Dent. 2002;30:223–32.
Kuguimiya RN, Rode KM, Carneiro PMA, Aranha AC, Turbino ML. Influence of curing units and indirect restorative materials on the hardness of two dual-curing resin cements evaluated by the nanoindentation Test. J Adhes Dent. 2015;17:243–8.
da Silva EM, Noronha-Filho JD, Amaral CM, Poskus LT, Guimarães JG. Long-term degradation of resin-based cements in substances present in the oral environment: influence of activation mode. J Appl Oral Sci. 2013;21:271–7.
Novais VR, Raposo LHA, de Miranda RR, Lopes CC, Simamoto PC Jr, Soares CJ. Degree of conversion and bond strength of resin-cements to feldspathic ceramic using different curing modes. J Appl Oral Sci Rev FOB. 2017;25:61–8.
Author information
Authors and Affiliations
Contributions
VF contributed to the research concept, study design, data collection, statistical analysis, writing the original draft, and reviewing and editing the final manuscript. CAD contributed to data collection, statistical analysis, writing the original draft. SAG AD contributed to data collection. ED contributed to reviewing and editing the final manuscript. JPA contributed to the research concept, study design, reviewing and editing the final manuscript. PF contributed to the research concept, study design, statistical analysis, writing the original draft, and reviewing and editing the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fouquet, V., Dantagnan, CA., Abdel-Gawad, S. et al. In vitro shear bond strength over zirconia and titanium alloy and degree of conversion of extraoral compared to intraoral self-adhesive resin cements. BDJ Open 9, 54 (2023). https://doi.org/10.1038/s41405-023-00178-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41405-023-00178-0