Abstract
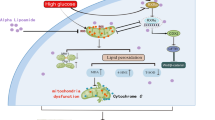
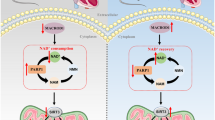
Diabetic nephropathy (DN) is characterized by chronic low-grade renal inflammatory responses, which greatly contribute to disease progression. Abnormal glucose metabolism disrupts renal lipid metabolism, leading to lipid accumulation, nephrotoxicity, and subsequent aseptic renal interstitial inflammation. In this study, we investigated the mechanisms underlying the renal inflammation in diabetes, driven by glucose-lipid metabolic rearrangement with a focus on the role of acetyl-CoA synthetase 2 (ACSS2) in lipid accumulation and renal tubular injury. Diabetic models were established in mice by the injection of streptozotocin and in human renal tubular epithelial HK-2 cells cultured under a high glucose (HG, 30 mmol/L) condition. We showed that the expression levels of ACSS2 were significantly increased in renal tubular epithelial cells (RTECs) from the diabetic mice and human diabetic kidney biopsy samples, and ACSS2 was co-localized with the pro-inflammatory cytokine IL-1β in RTECs. Diabetic ACSS2-deficient mice exhibited reduced renal tubular injury and inflammatory responses. Similarly, ACSS2 knockdown or inhibition of ACSS2 by ACSS2i (10 µmol/L) in HK-2 cells significantly ameliorated HG-induced inflammation, mitochondrial stress, and fatty acid synthesis. Molecular docking revealed that ACSS2 interacted with Sirtuin 1 (SIRT1). In HG-treated HK-2 cells, we demonstrated that ACSS2 suppressed SIRT1 expression and activated fatty acid synthesis by modulating SIRT1-carbohydrate responsive element binding protein (ChREBP) activity, leading to mitochondrial oxidative stress and inflammation. We conclude that ACSS2 promotes mitochondrial oxidative stress and renal tubular inflammation in DN by regulating the SIRT1-ChREBP pathway. This highlights the potential therapeutic value of pharmacological inhibition of ACSS2 for alleviating renal inflammation and dysregulation of fatty acid metabolic homeostasis in DN.

Metabolic inflammation in the renal region, driven by lipid metabolism disorder, is a key factor in renal injury in diabetic nephropathy (DN). Acetyl-CoA synthetase 2 (ACSS2) is abundantly expressed in renal tubular epithelial cells (RTECs) and highly upregulated in diabetic kidneys. Deleting ACSS2 reduces renal fatty acid accumulation and markers of renal tubular injury in diabetic mice. We demonstrate that ACSS2 deletion inhibits ChREBP-mediated fatty acid lipogenesis, mitochondrial oxidative stress, and inflammatory response in RTECs, which play a major role in the progression of diabetic renal tubular injury in the kidney. These findings support the potential use of ACSS2 inhibitors in treating patients with DN.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data supporting the concepts presented in this manuscript can be obtained upon reasonable request from the corresponding author.
References
Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease. J Nephrol. 2017;30:701–17.
Ruiz-Ortega M, Rodrigues-Diez RR, Lavoz C, Rayego-Mateos S. Special Issue “Diabetic Nephropathy: Diagnosis, Prevention and Treatment”. J Clin Med. 2020;9:813.
Nowak N, Skupien J, Niewczas MA, Yamanouchi M, Major M, Croall S, et al. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int. 2016;89:459–67.
Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16:317–36.
Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol Dial Transpl. 2012;27:3049–56.
Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16:206–22.
Veiras LC, Bernstein EA, Cao D, Okwan-Duodu D, Khan Z, Gibb DR, et al. Tubular IL-1beta induces salt sensitivity in diabetes by activating renal macrophages. Circ Res. 2022;131:59–73.
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7.
Ding H, Li J, Li Y, Yang M, Nie S, Zhou M, et al. MicroRNA-10 negatively regulates inflammation in diabetic kidney via targeting activation of the NLRP3 inflammasome. Mol Ther. 2021;29:2308–20.
Gao X, Lin SH, Ren F, Li JT, Chen JJ, Yao CB, et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun. 2016;7:11960.
Huang Z, Zhang M, Plec AA, Estill SJ, Cai L, Repa JJ, et al. ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. Proc Natl Acad Sci USA. 2018;115:E9499–506.
Li X, Qian X, Lu Z. Local histone acetylation by ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Autophagy. 2017;13:1790–1.
Lakhter AJ, Hamilton J, Konger RL, Brustovetsky N, Broxmeyer HE, Naidu SR. Glucose-independent acetate metabolism promotes melanoma cell survival and tumor growth. J Biol Chem. 2016;291:21869–79.
van der Rijt S, Leemans JC, Florquin S, Houtkooper RH, Tammaro A. Immunometabolic rewiring of tubular epithelial cells in kidney disease. Nat Rev Nephrol. 2022;18:588–603.
Lu J, Chen PP, Zhang JX, Li XQ, Wang GH, Yuan BY, et al. GPR43 activation-mediated lipotoxicity contributes to podocyte injury in diabetic nephropathy by modulating the ERK/EGR1 pathway. Int J Biol Sci. 2022;18:96–111.
Guo Y, Ni J, Chen S, Bai M, Lin J, Ding G, et al. MicroRNA-709 mediates acute tubular injury through effects on mitochondrial function. J Am Soc Nephrol. 2018;29:449–61.
Wu L, Liu C, Chang DY, Zhan R, Zhao M, Man Lam S, et al. The attenuation of diabetic nephropathy by Annexin A1 via regulation of lipid metabolism through the AMPK/PPARalpha/CPT1b pathway. Diabetes. 2021;70:2192–203.
Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 2018;23:869–81.e868.
Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55:561–72.
Opazo-Rios L, Mas S, Marin-Royo G, Mezzano S, Gomez-Guerrero C, Moreno JA, et al. Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int J Mol Sci. 2020;21:2632.
Qi W, Hu C, Zhao D, Li X. SIRT1-SIRT7 in diabetic kidney disease: biological functions and molecular mechanisms. Front Endocrinol (Lausanne). 2022;13:801303.
Zhang N, Hu Y, Ding C, Zeng W, Shan W, Fan H, et al. Salvianolic acid B protects against chronic alcoholic liver injury via SIRT1-mediated inhibition of CRP and ChREBP in rats. Toxicol Lett. 2017;267:1–10.
Nicholas DA, Proctor EA, Agrawal M, Belkina AC, Van Nostrand SC, Panneerseelan-Bharath L, et al. Fatty acid metabolites combine with reduced beta oxidation to activate Th17 inflammation in human type 2 diabetes. Cell Metab. 2019;30:447–61.e445.
Lee HK. Fatty acid overload to compromised oxidative phosphorylation activates inflammation in type 2 diabetes: hidden beasts and how to find them. J Diabetes Investig. 2020;11:290–3.
Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–53.
Hou Y, Wang Q, Han B, Chen Y, Qiao X, Wang L. CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis. 2021;12:523.
Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713–21.
Finucane OM, Lyons CL, Murphy AM, Reynolds CM, Klinger R, Healy NP, et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1beta secretion and insulin resistance despite obesity. Diabetes. 2015;64:2116–28.
Ralston JC, Lyons CL, Kennedy EB, Kirwan AM, Roche HM. Fatty acids and NLRP3 inflammasome-mediated inflammation in metabolic tissues. Annu Rev Nutr. 2017;37:77–102.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Ruysschaert JM, Lonez C. Role of lipid microdomains in TLR-mediated signalling. Biochim Biophys Acta. 2015;1848:1860–7.
O’Farrell M, Duke G, Crowley R, Buckley D, Martins EB, Bhattacharya D, et al. FASN inhibition targets multiple drivers of NASH by reducing steatosis, inflammation and fibrosis in preclinical models. Sci Rep. 2022;12:15661.
Liu X, Cooper DE, Cluntun AA, Warmoes MO, Zhao S, Reid MA, et al. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell. 2018;175:502–13.
Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586–91.
Lu J, Chen PP, Zhang JX, Li XQ, Wang GH, Yuan BY, et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKalpha activity. Theranostics. 2021;11:4728–42.
Acknowledgements
This research was supported by the Jiangsu Innovative and Entrepreneurial Talent Programme (JSSCBS20211515), the Nanjing Postdoctoral Science Foundation, the Medical Science and Technology Development Foundation’s Key Project, Nanjing Department of Health (YKK21094), and the National Natural Science Foundation of China (82170736, 81970629). This research did not use artificial intelligence, language models, machine learning, or similar technologies to create content or assist with the writing or editing of manuscripts.
Author information
Authors and Affiliations
Contributions
The study was designed by JL and KLM. The experiments were conducted and diabetic mouse models established by JL, XQL, PPC, JXZ, and LL. Data analysis was performed by GHW and XQL. The manuscript was initially drafted by JL, while revisions were made by KLM and CMJ. All authors have approved the final version of the manuscript. Guarantor statement: KLM and CMJ have taken responsibility for the contents of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, J., Li, Xq., Chen, Pp. et al. Acetyl-CoA synthetase 2 promotes diabetic renal tubular injury in mice by rewiring fatty acid metabolism through SIRT1/ChREBP pathway. Acta Pharmacol Sin 45, 366–377 (2024). https://doi.org/10.1038/s41401-023-01160-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01160-0