Abstract
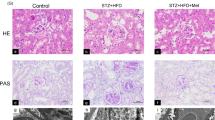
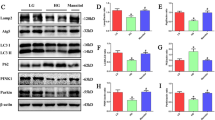
Previous studies have shown mitochondrial dysfunction in various acute kidney injuries and chronic kidney diseases. Lipoic acid exerts potent effects on oxidant stress and modulation of mitochondrial function in damaged organ. In this study we investigated whether alpha lipoamide (ALM), a derivative of lipoic acid, exerted a renal protective effect in a type 2 diabetes mellitus mouse model. 9-week-old db/db mice were treated with ALM (50 mg·kg−1·d−1, i.g) for 8 weeks. We showed that ALM administration did not affect blood glucose levels in db/db mice, but restored renal function and significantly improved fibrosis of kidneys. We demonstrated that ALM administration significantly ameliorated mitochondrial dysfunction and tubulointerstitial fibrotic lesions, along with increased expression of CDX2 and CFTR and decreased expression of β-catenin and Snail in kidneys of db/db mice. Similar protective effects were observed in rat renal tubular epithelial cell line NRK-52E cultured in high-glucose medium following treatment with ALM (200 μM). The protective mechanisms of ALM in diabetic kidney disease (DKD) were further explored: Autodock Vina software predicted that ALM could activate RXRα protein by forming stable hydrogen bonds. PROMO Database predicted that RXRα could bind the promoter sequences of CDX2 gene. Knockdown of RXRα expression in NRK-52E cells under normal glucose condition suppressed CDX2 expression and promoted phenotypic changes in renal tubular epithelial cells. However, RXRα overexpression increased CDX2 expression which in turn inhibited high glucose-mediated renal tubular epithelial cell injury. Therefore, we reveal the protective effect of ALM on DKD and its possible potential targets: ALM ameliorates mitochondrial dysfunction and regulates the CDX2/CFTR/β-catenin signaling axis through upregulation and activation of RXRα.

Schematic figure illustrating that ALM alleviates diabetic kidney disease by improving mitochondrial function and upregulation and activation of RXRα, which in turn upregulated CDX2 to exert an inhibitory effect on β-catenin activation and nuclear translocation. RTEC renal tubular epithelial cell. ROS Reactive oxygen species. RXRα Retinoid X receptor-α. Mfn1 Mitofusin 1. Drp1 dynamic-related protein 1. MDA malondialdehyde. 4-HNE 4-hydroxynonenal. T-SOD Total-superoxide dismutase. CDX2 Caudal-type homeobox transcription factor 2. CFTR Cystic fibrosis transmembrane conductance regulator. EMT epithelial mesenchymal transition. α-SMA Alpha-smooth muscle actin. ECM extracellular matrix. DKD diabetic kidney disease. Schematic figure was drawn by Figdraw (www.figdraw.com).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Panizo S, Martínez-Arias L, Alonso-Montes C, Cannata P, Martín-Carro B, Fernández-Martín JL, et al. Fibrosis in chronic kidney disease: Pathogenesis and consequences. Int J Mol Sci. 2021;22:408.
Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117:662–75.
Ahmad AA, Draves SO, Rosca M. Mitochondria in diabetic kidney disease. Cells. 2021;10:2945.
Boyman L, Karbowski M, Lederer WJ. Regulation of mitochondrial ATP production: Ca2+ signaling and quality control. Trends Mol Med. 2020;26:21–39.
Aranda-Rivera AK, Cruz-Gregorio A, Aparicio-Trejo OE, Pedraza-Chaverri J. Mitochondrial redox signaling and oxidative stress in kidney diseases. Biomolecules. 2021;11:1144.
Duann P, Lin PH. Mitochondria damage and kidney disease. Adv Exp Med Biol. 2017;982:529–51.
Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017;11:637–45.
Jin JY, Wei XX, Zhi XL, Wang XH, Meng D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin. 2021;42:655–64.
Zhang X, Agborbesong E, Li X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int J Mol Sci. 2021;22:11253.
Liu W, Shi LJ, Li SG. The immunomodulatory effect of alpha-lipoic acid in autoimmune diseases. Biomed Res Int. 2019;2019:8086257.
Zhao L, Liu Z, Jia H, Feng Z, Liu J, Li X. Lipoamide acts as an indirect antioxidant by simultaneously stimulating mitochondrial biogenesis and phase ii antioxidant enzyme systems in ARPE-19 Cells. PLoS One. 2015;10:e0128502.
Li X, Liu Z, Luo C, Jia H, Sun L, Hou B, et al. Lipoamide protects retinal pigment epithelial cells from oxidative stress and mitochondrial dysfunction. Free Radic Biol Med. 2008;44:1465–74.
Jeoung NH. Pyruvate dehydrogenase kinases: Therapeutic targets for diabetes and cancers. Diabetes Metab J. 2015;39:188–97.
Hou Y, Li X, Peng S, Yao J, Bai F, Fang J. Lipoamide ameliorates oxidative stress via induction of Nrf2/ARE signaling pathway in PC12 cells. J Agric Food Chem. 2019;67:8227–34.
Zhang Y, Zhou R, Qu Y, Shu M, Guo S, Bai Z. Lipoamide inhibits NF1 deficiency-induced epithelial-mesenchymal transition in murine schwann cells. Arch Med Res. 2017;48:498–505.
Strobbe D, Sharma S, Campanella M. Links between mitochondrial retrograde response and mitophagy in pathogenic cell signalling. Cell Mol Life Sci. 2021;78:3767–75.
Chae S, Ahn BY, Byun K, Cho YM, Yu MH, Lee B, et al. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci Signal. 2013;6:rs4.
Ma X, Warnier M, Raynard C, Ferrand M, Kirsh O, Defossez PA, et al. The nuclear receptor RXRA controls cellular senescence by regulating calcium signaling. Aging Cell. 2018;17:e12831.
Onuki M, Watanabe M, Ishihara N, Suzuki K, Takizawa K, Hirota M, et al. A partial agonist for retinoid X receptor mitigates experimental colitis. Int Immunol. 2019;31:251–62.
Liu H, Yan R, Liang L, Zhang H, Xiang J, Liu L, et al. The role of CDX2 in renal tubular lesions during diabetic kidney disease. Aging. 2021;13:6782–803.
Yu L, Su Y, Paueksakon P, Cheng H, Chen X, Wang H, et al. Integrin α1/Akita double-knockout mice on a Balb/c background develop advanced features of human diabetic nephropathy. Kidney Int. 2012;81:1086–97.
Zhou B, Wen M, Lin X, Chen YH, Gou Y, Li Y, et al. Alpha lipoamide ameliorates motor deficits and mitochondrial dynamics in the parkinson’s disease model induced by 6-hydroxydopamine. Neurotox Res. 2018;33:759–67.
Soulage CO, Pelletier CC, Florens N, Lemoine S, Dubourg L, Juillard L, et al. Two Toxic Lipid Aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4 -hydroxy-nonenal (4-HNE), accumulate in patients with chronic kidney disease. Toxins. 2020;12:567.
Quirós PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol. 2016;17:213–26.
Ouamrane L, Larrieu G, Gauthier B, Pineau T. RXR activators molecular signalling: Involvement of a PPAR alpha-dependent pathway in the liver and kidney, evidence for an alternative pathway in the heart. Br J Pharmacol. 2003;138:845–54.
Annesley SJ, Fisher PR. Mitochondria in health and disease. Cells. 2019;8:680.
Yapa NMB, Lisnyak V, Reljic B, Ryan MT. Mitochondrial dynamics in health and disease. FEBS Lett. 2021;595:1184–204.
Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13:629–46.
Jiménez-Uribe AP, Hernández-Cruz EY, Ramírez-Magaña KJ, Pedraza-Chaverri J. Involvement of tricarboxylic acid cycle metabolites in kidney diseases. Biomolecules. 2021;11:1259.
Wu Y, Chen M, Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion. 2019;49:35–45.
Irazabal MV, Torres VE. Reactive oxygen species and redox signaling in chronic kidney disease. Cells. 2020;9:1342.
Persson HL, Svensson AI, Brunk UT. Alpha-lipoic acid and alpha-lipoamide prevent oxidant-induced lysosomal rupture and apoptosis. Redox Rep. 2001;6:327–34.
Zhang XK, Su Y, Chen L, Chen F, Liu J, Zhou H. Regulation of the nongenomic actions of retinoid X receptor-αby targeting the coregulator-binding sites. Acta Pharmacol Sin. 2015;36:102–12.
De Bosscher K, Desmet SJ, Clarisse D, Estébanez-Perpiña E, Brunsveld L. Nuclear receptor crosstalk - defining the mechanisms for therapeutic innovation. Nat Rev Endocrinol. 2020;16:363–77.
Lu Z, Liu H, Fu W, Wang Y, Geng J, Wang Y, et al. 20(S)-Protopanaxadiol inhibits epithelial-mesenchymal transition by promoting retinoid X receptor alpha in human colorectal carcinoma cells. J Cell Mol Med. 2020;24:14349–65.
Tunctan B, Kucukkavruk SP, Temiz-Resitoglu M, Guden DS, Sari AN, Sahan-Firat S, et al. Bexarotene, a selective RXRα agonist, reverses hypotension associated with inflammation and tissue injury in a rat model of septic shock. Inflammation. 2018;41:337–55.
Li JE, Futawaka K, Yamamoto H, Kasahara M, Tagami T, Liu TH, et al. Cinnamaldehyde contributes to insulin sensitivity by activating PPARδ, PPARγ, and RXR. Am J Chin Med. 2015;43:879–92.
Chai D, Lin X, Zheng Q, Xu C, Xie H, Ruan Q, et al. Retinoid X receptor agonists attenuates cardiomyopathy in streptozotocin-induced type 1 diabetes through LKB1-dependent anti-fibrosis effects. Clin Sci. 2020;134:609–28.
Ayala-Peña VB, Pilotti F, Volonté Y, Rotstein NP, Politi LE, German OL. Protective effects of retinoid x receptors on retina pigment epithelium cells. Biochim Biophys Acta. 2016;1863:1134–45.
Wang HB, Wei H, Wang JS, Li L, Chen AY, Li ZG. Down-regulated expression of LINC00518 prevents epithelial cell growth and metastasis in breast cancer through the inhibition of CDX2 methylation and the Wnt signaling pathway. Biochim Biophys Acta Mol Basis Dis. 2019;1865:708–23.
Schunk SJ, Floege J, Fliser D, Speer T. WNT-β-catenin signalling—a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17:172–84.
Simon-Tillaux N, Hertig A. Snail and kidney fibrosis. Nephrol Dial Transpl. 2017;32:224–33.
Gnemmi V, Bouillez A, Gaudelot K, Hémon B, Ringot B, Pottier N, et al. MUC1 drives epithelial-mesenchymal transition in renal carcinoma through Wnt/β-catenin pathway and interaction with SNAIL promoter. Cancer Lett. 2014;346:225–36.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82060141 and 81960141), Merit-based Funding for High-level Talent Innovation and Entrepreneurship in Guizhou Province [(2021)02], Science and Technology Top Talent Project of General Higher Education Institutions in Guizhou Province (Qianjiaohe KY [2021] 032).Schematic figure was drawn by Figdraw (www.figdraw.com).
Author information
Authors and Affiliations
Contributions
HFZ, HML, LT, BG, and YYW designed the experiments, interpreted the data, and wrote the manuscript; HFZ, HML, JYX, XCZ, WLT, LQL conducted the experiments; YXZ, DW, RYC, LLL, MJS, FZ, TZ, YX analyzed the data; and all authors contributed helpful suggestions for this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Hf., Liu, Hm., Xiang, Jy. et al. Alpha lipoamide inhibits diabetic kidney fibrosis via improving mitochondrial function and regulating RXRα expression and activation. Acta Pharmacol Sin 44, 1051–1065 (2023). https://doi.org/10.1038/s41401-022-00997-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00997-1
Keywords
This article is cited by
-
Association between glycemic variability and short-term mortality in patients with acute kidney injury: a retrospective cohort study of the MIMIC-IV database
Scientific Reports (2024)
-
Targeted inhibition of the HNF1A/SHH axis by triptolide overcomes paclitaxel resistance in non-small cell lung cancer
Acta Pharmacologica Sinica (2024)
-
Antioxidant supplementation may effect DNA methylation patterns, apoptosis, and ROS levels in developing mouse embryos
Histochemistry and Cell Biology (2024)