Abstract
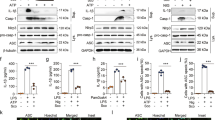
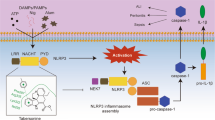
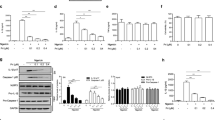
Inhibition of NLRP3 inflammasome activation produces potent therapeutic effects in a wide array of inflammatory diseases. Bergapten (BeG), a furocoumarin phytohormone present in many herbal medicines and fruits, exibits anti-inflammatory activity. In this study we characterized the therapeutic potential of BeG against bacterial infection and inflammation-related disorders, and elucidated the underlying mechanisms. We showed that pre-treatment with BeG (20 μM) effectively inhibited NLRP3 inflammasome activation in both lipopolysaccharides (LPS)-primed J774A.1 cells and bone marrow-derived macrophages (BMDMs), evidenced by attenuated cleaved caspase-1 and mature IL-1β release, as well as reduced ASC speck formation and subsequent gasdermin D (GSDMD)-mediated pyroptosis. Transcriptome analysis revealed that BeG regulated the expression of genes involved in mitochondrial and reactive oxygen species (ROS) metabolism in BMDMs. Moreover, BeG treatment reversed the diminished mitochondrial activity and ROS production after NLRP3 activation, and elevated the expression of LC3-II and enhanced the co-localization of LC3 with mitochondria. Treatment with 3-methyladenine (3-MA, 5 mM) reversed the inhibitory effects of BeG on IL-1β, cleaved caspase-1 and LDH release, GSDMD-N formation as well as ROS production. In mouse model of Escherichia coli-induced sepsis and mouse model of Citrobacter rodentium-induced intestinal inflammation, pre-treatment with BeG (50 mg/kg) significantly ameliorated tissue inflammation and injury. In conclusion, BeG inhibits NLRP3 inflammasome activation and pyroptosis by promoting mitophagy and maintaining mitochondrial homeostasis. These results suggest BeG as a promising drug candidate for the treatment of bacterial infection and inflammation-related disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606.
Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–61.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20.
Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–76.
Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–54.
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109.
Yu JW, Lee MS. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res. 2016;39:1503–18.
Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin X-J, et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560:198–203.
Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA. 2012;109:11282–7.
Mishra SR, Mahapatra KK, Behera BP, Patra S, Bhol CS, Panigrahi DP, et al. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int J Biochem Cell Biol. 2021;136:106013.
Nakahira K, Haspel JA, Rathinam VAK, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–30.
Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14.
Wu KKL, Long K, Lin H, Siu PMF, Hoo RLC, Ye D, et al. The APPL1-Rab5 axis restricts NLRP3 inflammasome activation through early endosomal-dependent mitophagy in macrophages. Nat Commun. 2021;12:6637.
Dagvadorj J, Mikulska-Ruminska K, Tumurkhuu G, Ratsimandresy RA, Carriere J, Andres AM, et al. Recruitment of pro-IL-1α to mitochondrial cardiolipin, via shared LC3 binding domain, inhibits mitophagy and drives maximal NLRP3 activation. Proc Natl Acad Sci USA. 2021;118:e2015632118.
Borgatti M, Mancini I, Bianchi N, Guerrini A, Lampronti I, Rossi D, et al. Bergamot (Citrus bergamia Risso) fruit extracts and identified components alter expression of interleukin 8 gene in cystic fibrosis bronchial epithelial cell lines. BMC Biochem. 2011;12:15.
Chen G, Xu Q, Dai M, Liu X. Bergapten suppresses RANKL-induced osteoclastogenesis and ovariectomy-induced osteoporosis via suppression of NF-κB and JNK signaling pathways. Biochem Biophys Res Commun. 2019;509:329–34.
Liang Y, Xie L, Liu K, Cao Y, Dai X, Wang X, et al. Bergapten: a review of its pharmacology, pharmacokinetics, and toxicity. Phytother Res. 2021;35:6131–47.
Adakudugu EA, Ameyaw EO, Obese E, Biney RP, Henneh IT, Aidoo DB, et al. Protective effect of bergapten in acetic acid-induced colitis in rats. Heliyon. 2020;6:e04710.
Adakudugu EA, Obiri DD, Ameyaw EO, Obese E, Biney RP, Aidoo DB, et al. Bergapten modulates ovalbumin-induced asthma. Sci African. 2020;8:e00457.
Aidoo DB, Obiri DD, Osafo N, Antwi AO, Essel LB, Duduyemi BM, et al. Allergic airway-induced hypersensitivity is attenuated by bergapten in murine models of inflammation. Adv Pharm Sci. 2019;2019:6097349.
Zhou Y, Wang J, Yang W, Qi X, Lan L, Luo L, et al. Bergapten prevents lipopolysaccharide-induced inflammation in RAW264.7 cells through suppressing JAK/STAT activation and ROS production and increases the survival rate of mice after LPS challenge. Int Immunopharmacol. 2017;48:159–68.
Pan H, Lin Y, Dou J, Fu Z, Yao Y, Ye S, et al. Wedelolactone facilitates Ser/Thr phosphorylation of NLRP3 dependent on PKA signalling to block inflammasome activation and pyroptosis. Cell Prolif. 2020;53:e12868.
Zhu X, Zhang HW, Chen HN, Deng XJ, Tu YX, Jackson AO, et al. Perivascular adipose tissue dysfunction aggravates adventitial remodeling in obese mini pigs via NLRP3 inflammasome/IL-1 signaling pathway. Acta Pharmacol Sin. 2019;40:46–54.
Feng WD, Wang Y, Luo T, Jia X, Cheng CQ, Wang HJ, et al. Scoparone suppresses mitophagy-mediated NLRP3 inflammasome activation in inflammatory diseases. Acta Pharmacol Sin. 2022. https://doi.org/10.1038/s41401-022-01028-9. Online ahead of print.
Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–206.
Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99–114.
Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nuñez G, He Y, et al. Endoplasmic reticulum stress activates the inflammasome via NLRP3- and Caspase-2-driven mitochondrial damage. Immunity. 2015;43:451–62.
Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13:148–59.
Quiros PM, Goyal A, Jha P, Auwerx J. Analysis of mtDNA/nDNA ratio in mice. Curr Protoc Mouse Biol. 2017;7:47–54.
Kampira E, Dzobo K, Kumwenda J, van Oosterhout JJ, Parker MI, Dandara C. Peripheral blood mitochondrial DNA/nuclear DNA (mtDNA/nDNA) ratio as a marker of mitochondrial toxicities of stavudine containing antiretroviral therapy in HIV-infected Malawian patients. OMICS. 2014;18:438–45.
Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–85.
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833.
Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42.
Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–18.
von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013;31:73–106.
Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18:2114–27.
Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–55.e20.
Shen C, Zhang Z, Xie T, Xu J, Yan J, Kang A, et al. Jinxin oral liquid inhibits human respiratory syncytial virus-induced excessive inflammation associated with blockade of the NLRP3/ASC/Caspase-1 pathway. Biomed Pharmacother. 2018;103:1376–83.
Seoane PI, Lee B, Hoyle C, Yu S, Lopez-Castejon G, Lowe M, et al. The NLRP3-inflammasome as a sensor of organelle dysfunction. J Cell Biol. 2020;219:e202006194.
Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111–29.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5.
Próchnicki T, Latz E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab. 2017;26:71–93.
Evavold CL, Hafner-Bratkovič I, Devant P, D’Andrea JM, Ngwa EM, Boršić E, et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell. 2021;184: 4495–511.e19.
Kleele T, Rey T, Winter J, Zaganelli S, Mahecic D, Perreten Lambert H, et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature. 2021;593:435–9.
Dufies O, Doye A, Courjon J, Torre C, Michel G, Loubatier C, et al. Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice. Nat Microbiol. 2021;6:401–12.
Liu Y, Jing YY, Zeng CY, Li CG, Xu LH, Yan L, et al. Scutellarin suppresses NLRP3 inflammasome activation in macrophages and protects mice against bacterial sepsis. Front Pharmacol. 2017;8:975.
Bull DM, Bookman MA. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977;59:966–74.
Delfini M, Stakenborg N, Viola MF, Boeckxstaens G. Macrophages in the gut: masters in multitasking. Immunity. 2022;55:1530–48.
Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16:531–43.
Zhang Q, Chen LH, Yang H, Fang YC, Wang SW, Wang M, et al. GPR84 signaling promotes intestinal mucosal inflammation via enhancing NLRP3 inflammasome activation in macrophages. Acta Pharmacol Sin. 2022;43:2042–54.
Liu Z, Zaki MH, Vogel P, Gurung P, Finlay BB, Deng W, et al. Role of inflammasomes in host defense against Citrobacter rodentium infection. J Biol Chem. 2012;287:16955–64.
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21.
Aldulaimi O. Screening of fruits of seven plants indicated for medicinal use in Iraq. Pharmacogn Mag. 2017;13:S189–S195.
Acknowledgements
This work was supported by the National Natural Science Foundation (NNSF) of China (Nos. 82001663), Young Elite Scientists Sponsorship Program by China Association for Science and Technology (No. 2020-QNRC1-03), and Joint Fund of Beijing University of Traditional Chinese Medicine and USANA. We are grateful to Prof. Feng Shao (National Institute of Biological Sciences, Beijing, China) for providing iBMDM, Prof. Chen Dong (Tsinghua University, Beijing, China) for providing C. rodentium.
Author information
Authors and Affiliations
Contributions
YW, XJ and ALX conceived the study; TL, WDF, JYW and FX performed the experiments. LDK, XJW, XL and RL contributed to the interpretation of results. TL and XJ wrote the manuscript and YW and ALX made pivotal revisions; YJC provided computational analysis; YW and ALX supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, T., Jia, X., Feng, Wd. et al. Bergapten inhibits NLRP3 inflammasome activation and pyroptosis via promoting mitophagy. Acta Pharmacol Sin 44, 1867–1878 (2023). https://doi.org/10.1038/s41401-023-01094-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01094-7
Keywords
This article is cited by
-
Bergapten enhances mitophagy to regulate intestinal barrier and Th17/Treg balance in mice with Crohn’s disease-like colitis via PPARγ/NF-κB signaling pathway
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)