Abstract
Mitochondrial dynamics, including mitochondrial fission and fusion, are critical for maintaining mitochondrial functions. Evidence shows that TANK-binding kinase 1 (TBK1) regulates mitochondrial fusion and fission and then mitophagy. Since a previous study demonstrates a strong correlation between mitophagy and osteoarthritis (OA), we herein investigated the potential role of TBK1 in OA process and mitochondrial functions. We demonstrated a strong correlation between TBK1 and OA, evidenced by significantly downregulated expression of TBK1 in cartilage tissue samples of OA patients and in the chondrocytes of aged mice, as well as TNF-α-stimulated phosphorylation of TBK1 in primary mouse chondrocytes. TBK1 overexpression significantly attenuated TNF-α-induced apoptosis and abnormal mitochondrial function in primary mouse chondrocytes. Furthermore, TBK1 overexpression induced remodeling of mitochondrial morphology by directly phosphorylating dynamin-related protein 1 (DRP1) at Ser637, abolishing the fission of DRP1 and preventing its fragmentation function. Moreover, TBK1 recruitment and DRP1 phosphorylation at Ser637 was necessary for engulfing damaged mitochondria by autophagosomal membranes during mitophagy. Moreover, we demonstrated that APMK/ULK1 signaling contributed to TBK1 activation. In OA mouse models established by surgical destabilization of the medial meniscus, intraarticular injection of lentivirus-TBK1 significantly ameliorated cartilage degradation via regulation of autophagy and alleviation of cell apoptosis. In conclusion, our results suggest that the TBK1/DRP1 pathway is involved in OA and pharmacological targeting of the TBK1-DRP1 cascade provides prospective therapeutic benefits for the treatment of OA.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is considered one of the most frequent chronic joint diseases and causes disability and chronic pain, leading to higher socio-economic costs worldwide [1]. Recent evidence reports that the incidence rate and prevalence of OA have been dramatically increased [2]. Previous studies indicate that severe joint pain, swelling, stiffness and limitation of movement are the most prominent clinical features of OA [3]. In addition, irreversible factors including heredity, diet, gender, obesity and diabetes may also facilitate the pathogenesis and advancement of OA [4].
However, TBK1 is a multi-functional serine/threonine-protein kinase that refers to the non-canonical IκB kinase family member and its close homolog IκB kinase ε (IKKε, also named as IKKi) [5,6,7]. During the stimulation from various pathogens or pathogen-related molecules such as interferon regulatory factor 3 (IRF3) in innate immune signaling, TBK1 delivers the integrator of receptor-dependent pathogen identification signals and modulates the function of IFN [8,9,10]. Accumulating evidence suggests that TBK1 plays a regulatory role in several signaling platforms generated by other innate adaptors—MAVS and TRIF [11, 12]. A previous study has uncovered that TBK1 is also involved in selective autophagy including mitophagy and xenophagy [13]. Growing research shows that mitophagy significantly removes many damaged cellular organelles and mitochondrial dysfunctions and xenophagy can regulate the preventive process of invading pathogens [14]. Our research has explored that TBK1 promotes the phosphorylation of autophagy modifiers and receptors that regulate autophagy during autophagosome formation. Mounting evidence reports that targeted autophagy receptors (p62/SQSTM1, OPTN, NDP52/CALCOCO2, TAX1BP1, NBR1, and BNIP3L) with ubiquitin-binding domains and LC3-interacting regions can receive high-binding affinities in the phosphorylation process [15, 16].
A certain mitophagy level regulates mitochondrial quality by removing aging and fragmented mitochondria, which further promotes mitochondrial renewal [17]. Studies have shown that autophagy- and mitophagy-related proteins are overexpressed in cartilage from OA patients and mice induced with monosodium iodoacetate (MIA), which shows a strong correlation between mitophagy and OA [18]. Mitophagy is activated under multiple pathological conditions and with OA development. The significant role and underlying molecular mechanism of mitophagy in the pathogenesis and advancement of OA are mainly unknown. Surprisingly, Haqqi and co-workers found overproduction of ROS, degradation of MMP, accumulation of damaged mitochondria and elevation of cell apoptosis with activated PRKN-mediated mitophagy in inflammatory mediators induced OA chondrocytes [19]. In addition, Shin et al. have revealed that PINK1-mediated mitophagy may promote mitochondrial fragmentation and cell death in human chondrocytes and rats following OA surgery and reduces cartilage damage and alleviate pain behaviors in pink1-knockout mice [18]. In our current research, we aimed to investigate the contributory role of mitophagy in the pathogenesis and progression of OA.
Mitochondria are dynamic organelles characterized by a fission and fusion cycle frequency, which maintains the typical morphology of the mitochondrial network and meets the metabolic demand [20]. Drp1, a cytosolic dynamin GTPase, regulates mitochondrial fission. Drp1 is mainly recruited to the outer mitochondrial membrane (OMM) to prime fission events. Drp1 assembly and participate in the oligomerization process of the OMM, which inhibits mitochondrial fragmentation [21]. The dynamic recruitment of Drp1 at the GTPase activity and its OMM are highly impacted by posttranslational modifications such as phosphorylation and ubiquitination [22, 23]. Research on the phosphorylation of Drp1 in multiple diseases has recently increased massive attention in scientific research fields [24, 25]. However, the precious role of Drp1 phosphorylation in the pathogenesis and development of OA is still largely unknown. Very few studies have been conducted exploring the potential role and underlying pathophysiological mechanism of Drp1 phosphorylation in the advancement of OA.
We speculate that phosphorylation of Drp1 at S637 is required to facilitate the autophagosome engulfment of ubiquitinated mitochondria and mitochondrial quality control. Besides, AMPK pathway activation facilities TBK1 function through ULK1-driven phosphorylation at S172, further stimulating Drp1. Hence, our study unveils a significant relation between S637 phosphorylation of Drp1 and TBK1, which uncovers that manipulating TBK1 and Drp1 phosphorylation may be an ideal therapeutic strategy for the treatment and management of OA.
Materials and methods
Study design
In vitro and in vivo experiments were conducted using clinical samples from patients with end-stage OA and C57BL/6 mice. The in vitro experiment was mostly done with primary chondrocytes from patients and mice. Western blot technique and immunohistochemistry staining were performed to measure targeted protein expression and related signaling pathways in OA. To further investigate the underlying molecular mechanisms of TBK1 in OA, Lentivirus and siRNA were used to regulate specific gene expression and fluorescence staining, mitochondrial morphology staining and protein detection were performed. Mice were subjected to a surgical model of OA. The researchers who administered the intraarticular injections and measured capacitance were completely unaware of the treatment. The investigators were blinded to therapy when cutting, staining, scoring, and doing histomorphometric assessments. The severity of OA was assessed by two independent investigators.
Human articular cartilage collection
Normal human articular cartilage tissues were received from femoral condyles and tibial plateaus from 4 patients who underwent amputation and there were no significant clinical and imaging features. OA human articular cartilage tissues were attained from four patients having total knee arthroplasty. Then, the articular cartilages were picked up for conducting the Western blot experiment. The information of donors is listed in Table 1.
Antibody and reagents
Malvidin-3-O-arabinoside chloride (M3A) was acquired from Med Chem Express (Monmouth Junction, NJ, USA). H&E staining kit and Safranine O-Fast Green FCF Cartilage Stain Kit were purchased from Solarbio Science & Technology (Beijing, China). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was acquired from the Beyotime Institute of Biotechnology (Shanghai, China). Primary antibody against TBK1, P-TBK1 (Ser172), Phospho-DRP1 (Ser616), Beclin1, ATG5, LC3A/B, COX-IV, AMPKα, p-AMPKα, ULK1, p-ULK1, Cleaved Caspase-3 and GAPDH were purchased from Cell Signaling Technology (Beverly, MA, USA). Hsp60, DRP1, DRP1 (Ser637), goat anti-rabbit IgG H&L (Alexa Fluor® 488) and goat anti-mouse IgG H&L (Alexa Fluor® 647) were purchased from Abcam (Cambridge, UK). Goat anti-mouse IgG (H + L)-HRP and goat anti-rabbit IgG (H + L)-HRP were acquired from Bioworld Technology (Minneapolis, MN, USA). The immunoprecipitation kit and mitotrcker fluorescent probe were purchased from Thermo Scientific (Madison, WI, USA). Fetal bovine serum was purchased from Merck (Darmstadt, Germany). TUNEL apoptosis detection kit was received from Yeasen Biochemical (Shanghai, China).
Chondrocyte isolation and culture
The C57BL/6 mice (6 males and 6 females, aged: 5 days) were euthanized with sodium pentobarbital overdose. Articular cartilage was dissected into small sections and was then digested with 0.2% type II collagenase (Sigma-Aldrich, St. Louis, MO, USA) in Dulbecco’s modified Eagle’s medium/nutrient mixture F12 (DMEM/F12) (Gibco, Carlsbad, CA, USA) at 37 °C for 16 h. The chondrocytes were then filtered through a 70 μm cell strainer and maintained in DMEM/F12 containing 10% fetal bovine serum (Gibco) in an incubator at 37 °C with 5% CO2. Primary chondrocytes were used for subsequent experiments
TUNEL staining
TUNEL staining was performed to detect DNA fragmentation. The method and process of TUNEL staining were followed according to the manufacturer’s instructions. The cells were sufficiently stained with the TUNEL assay kit and DAPI reagent. The results were finally observed and images were captured under a confocal microscope (Nikon, Japan).
Western blotting
The Western blotting assay was carried out by following the previous study. Briefly, the cells and tissues were adequately washed with PBS and lysed in 1× RIPA buffer solution with protease and phosphatase inhibitors. The protein samples were thoroughly detached via SDS gel electrophoresis and then moved to the PVDF membrane (Millipore, USA). Next, the membrane was adequately blocked by 5% non-fat dry skim-milk solution(Beyotime Biotechnology, China) for 2 h at room temperature. After adequate blocking, the membrane was washed with PBS solution 3 times at room temperature and kept with specific primary antibodies for overnight incubation at 4 °C. The primary antibodies used in the Western blot assay were diluted at 1:1000. The specific secondary antibody (Abcam, 1:5000) was then incubated on the membrane for 2 h at room temperature. Immunoblotting was performed by a commercial chemiluminescence kit (Sigma-Aldrich, St. Louis, MO, USA). The ImageJ software version 4.1 was finally performed to measure the relative protein expression levels.
Immunohistochemistry staining
The 5 μm thickness of tissue sections was sufficiently deparaffinized, rehydrated and incubated in 3% H2O2 solution for 30 min at room temperature. Antigen retrieval was performed in EDTA antigen retrieval solution (Beyotime) for 20 min at 95 °C followed by cooling naturally at room temperature for 1 h. Then, the slides were adequately blocked with 5% BSA for 45 min. Next, the sections slides were immunized with specific primary antibodies (Dilution 1:100) at 4 °C for overnight. After adequately washing with PBST solution, the tissue slides were incubated with HRP-conjugated secondary antibodies (dilution 1:200; Beyotime, A0216 or A0208) for 2 h at 37 °C. The tissue slides were finally stained with DAB reagent and attentively observed under an electronic microscope (Nikon, Japan).
Immunofluorescence staining
Chondrocytes were adequately washed in PBS solution and fixed with 4% paraformaldehyde solution. Then, 0.1% TritonX-100 was applied to permeabilize the cells for 5 min at room temperature. PBS solution (2 mL) was used to wash glass coverslips covered with chondrocytes. The chondrocyte cells were adequately blocked by 5% BSA solution at 37 °C for 45 min. After sufficient incubation, the cell sample slides were cleansed with PBS solution. Subsequently, the specific primary antibodies were applied to inoculate the cells at 4 °C for overnight. Primary antibodies against LC3, P-TBK1, and Drp1 were diluted at 1:200, 1:100, and 1:200, respectively. After overnight incubation, the cells were adequately washed with PBS solution for 3 times. The cell sample slides were then incubated with Alexa Fluor 488 or Alexa Fluor 594-conjugated secondary antibodies for 2 h and stained with DAPI reagent for 5 min. A confocal fluorescence microscope (Nikon, Japan) was finally performed to capture images of the sample slides.
Intracellular ROS detection
Intracellular ROS level was detected by the commercial ROS kit (Beyotime Biotechnology, China), according to the manufacturer’s instructions. ROS can oxidize the substrate DCFH-DA to the DCF fluorescent. Briefly, the cells were incubated with DCFH-DA and the fluorescent intensities were finally measured.
Cell viability assay
CCK-8 assay was applied to assess the cell viability of chondrocytes. 96-well plates with a density of 1 × 104 cells were used to co-incubate the chondrocytes for 24 h. Then, PBS solution was used to wash the cells and 10 μL of the CCK-8 reagent was applied to incubate in each well. After that, the cells were substantially cultured for 4–6 h at 37 °C. A microplate reader (Leica Microsystems, Germany) was employed to quantify the absorbance level at a wavelength of 450 nm. All experiments were significantly repeated three times.
Molecular modeling analysis
The protein-protein complex containing AMPK was employed to execute molecular-docking studies obtained from the PDB database (https://www.rcsb.org/). After minimizing with PyMoL (version 1.7.6), the minimal energy configuration for molecular-docking analysis was quantified through defecting parameters. A protein-ligand docking analysis was performed using the Auto Dock Tools (version 1.5.6). The images were formed through the UCSFPyMoL and 2D interactions were finally plotted by Ligplus v1.4.
Lentivirus and siRNAs transfection
Lenti-TBK1 transfection (GeneChem, Shanghai, China) was followed to analyze the overexpression of TBK1. The cells were transfected with Lentivirus at 40%; >95% confluences of the cells were feasible 12 h later. After the transformation of the medium, the cells were incubated for additional 3 days. Finally, Western blot assay was carried out to determine the efficacy of transfection levels. The sequence of siRNA used for this study is shown in Table 2.
Mitochondrial membrane potential (MMP) assay
MMP was detected by Mito-Tracker Red CMXRos (Molecular ProbesTM, Thermo Fisher Scientific Inc.), which stained mitochondria and promoted mitochondrial function in an MMP-mediated way. Then, the nuclei were sufficiently treated with DAPI reagent for 5 min at 37 °C. Finally, experimental slides were assessed under a confocal microscope (Nikon, Japan) and the fluorescence intensity was measured by ImageJ software version 4.1 (Bethesda, MD, USA).
Co-immunoprecipitation (CO-IP)
CO-IP was conducted by applying a commercial kit, according to the manufacturer’s instructions. After sufficient treatments, the cells were instantly placed on ice and washed with ice-cold PBS solution for 3 times. Total proteins were lysated as described above. To determine the binding capacity of p-TBK1-p-Drp1, the protein lysates were immunoprecipitated by incubating with Dynabeads consisting of p-TBK1 primary antibody for overnight at 4 °C. After overnight incubation, the immunocomplexes were aggregated according to the manufacturer’s instructions. Immunolabeling was finally determined by Western blotting.
Animal models
DMM experimental model was constructed on 8–10 weeks old C57BL/6 male mice bought from the Animal Center of Wenzhou Medical University, Wenzhou, Zhejiang province, China. All experimental mice were allocated into: Sham; DMM + LV-NC and DMM + LV-TBK1. Intra-articular injection of lentivirus was administered to mice at 0-, 15-, 30- and 45-days post-OA surgery. OA mouse models were constructed by surgical destabilization of the medial meniscus as formerly represented [26]. Experimental mice were sacrificed by the overdose administration of pentobarbital sodium at the end of 8 weeks. All study protocols and experimental procedures were approved by The Animal Care and Research Ethics Committee of Wenzhou Medical University, Wenzhou, Zhejiang province, China.
Real-time polymerase chain reaction (RT-PCR)
Total RNAs were extracted from the mouse knee joint tissues using TRIzol reagent (Invitrogen), according to the supplier’s instructions. RNA purity was measured by assaying OD at 260 and 280 nm and A260/A280 ≥ 1.8 was the standard. Its integrity was measured by agarose gel electrophoresis. cDNA synthesis was completed by using the First-Strand Synthesis for RT-PCR kit (TaKaRa). RT-PCR was performed with SYBR Premix Ex Taq kits (Takara) and the Bio-Rad CFX96 Real-Time System (Bio-Rad). Relative gene expression levels were calculated using the 2−∆∆Ct method, where β-actin was considered as the internal control. The sequences of the primers used in the RT-PCR technique are shown in Table 3.
Histopathological staining
Mouse knee joint tissues were fixed in 10% formaldehyde solution for 24 h, permeated in EDTA decalcified solution for nearly 1 month and adequately washed with running water. After sufficient dehydration by different gradient ethanol and transparent with xylene, the tissues were incorporated in paraffin and sliced into 5 μm thickness. Finally, the tissue sample slides were stained with H&E and SO kits to observe the morphological features of the knee joint tissue area by two experienced pathologists under an electronic microscope (Nikon, Japan).
Statistical analysis
All experimental data were evaluated by SPSS statistics software (Version 20.0, USA), containing the one-way analysis of Duncan’s test variance, indicated as the mean ± standard deviation (SD). The variation between the groups was evaluated at the statistically significant level of P < 0.05 value.
Results
Downregulated expression and activation of TBK1 are associated with the pathogenesis and advancement of OA
To investigate the significant relationship between TBK1 and OA, we compared the expression levels of TBK1 in cartilage tissue samples of healthy and OA patients. Our Western blot assay revealed that the expression levels of TBK1 protein were significantly downregulated in the degenerated cartilages than in the healthy group (Fig. 1a, b). Mounting evidence indicates that natural aging is one of the most significant risk factors for primary OA. Thus, we measured the change of TBK1 expression under the natural senescence condition and established a 13-month-old mouse model. The knee joint tissues were isolated from young and old groups.
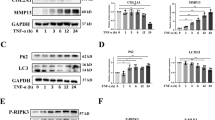
a, b Representative Western blots and quantification data of TBK1 in human knee cartilage tissue as measured by Western blotting analysis. c The Safranin O staining and immunohistochemical staining of mice knee joint tissue. d Relative positive cells per section of TBK1 in immunohistochemical staining of mice knee joint tissue sections. e, g Representative Western blots and quantification data of p-TBK1 and TBK1 of chondrocytes treated with TNF-α in concentration gradient. f, h Representative Western blots and quantification data of p-TBK1 and TBK1 in chondrocytes treated with TNF-α in time gradient. All experiments were performed as means ± SD of 3 times in duplicates. *P < 0.05; **P < 0.01; nsP > 0.05.
Similarly, the expression levels of TBK1 were significantly downregulated in the chondrocytes of aged mice (Fig. 1c, d). In addition, the expression level of p-TBK1 S172 and TBK1 proteins was considerably augmented in a TNF-α concentration-dependent way. Under the concentration of 15 ng/mL, the ratio of P-TBK1/TBK was increased and peaked at 24 h in a time-dependent manner (Fig. 1e–h). To further investigate the actual function of TBK1 in phosphorylation, we induced TNF-α for 24 h in our study. Collectively, these findings suggest a strong correlation between TBK1 and OA.
TBK1 overexpression attenuates TNF-α-mediated apoptosis and abnormal mitochondrial function
To further explore the function of TBK1, we investigated the promising role of TBK1 in apoptosis and mitochondrial fission and fusion under TNF-α stimulation. The chondrocytes were transfected with lentivirus to upregulate the basal expression of TBK1 (Fig. 2a, b). More importantly, our Western blot analysis revealed that TBK1 overexpression could significantly downregulate the ratio level of TNF-α-induced Cleaved-caspase3/Caspase3 and upregulate the ratio of Bcl-2/Bax (Supplementary Fig. S1). Furthermore, HSP60 and COX-IV were applied as mitochondrial markers. Our results exhibited that TBK1 upregulation could reduce HSP60 levels while enhancing COX-IV expression in chondrocytes (Fig. 2c, d). We next investigated whether TBK1 affects mitochondrial fusion/fission by analyzing mitochondrial structure. The images obtained by confocal microscopy showed filamentous sausage-like mitochondria in the cells. After TNF-α treatment, the fragmented mitochondria were minimized, punctuated and enhanced spherical shapes in a time-dependent manner.
a, b Representative Western blots and quantification data of TBK1 in chondrocytes transfected with lentivirus. c, d Representative Western blots and quantification data of mitochondrial marker protein and apoptosis marker protein in chondrocytes. e Mito-tracker staining in chondrocytes treated with LV-NC or LV-TBK1 under TNF-α stimulation. f, g Representative images and quantification data of ROS level in chondrocytes. h quantification data of ATP level in chondrocytes. i Representative images and quantification data of TUNEL staining in chondrocytes. All experiments were performed as means ± SD of 3 times in duplicates. **P < 0.01; nsP > 0.05.
Interestingly, this trend was reversed by TBK1 overexpression (Fig. 2e). We found that the formation of ROS was increased during mitochondrial respiration. We performed the ROS and TUNEL staining. The findings of TUNEL were persistent with Western blot outcomes and TBK1 could markedly obstruct the activation of oxidative stress.
TBK1 normalizes mitochondrial dynamics through directly phosphorylating DRP1 at Ser637
Emerging evidence indicates that p-Drp1 S616 phosphorylation or p-Drp1S637 dephosphorylation facilitates Drp1 oligomerization and then induces mitochondria constriction and cleavage [27]. In our research, the levels of p-Drp1 S616 under TNF-α concentration were increased and peaked at 24 h. By contrast, an interesting trend was found in the p-Drp1S637 panel in both concentration and time-dependent manner. Our results revealed that the p-Drp1S637 dephosphorylation was gradually aggravated in TNF-α stimulation (Fig. 3a–f). The direct interaction between p-TBK1 and Drp1 was further verified by double-immunofluorescence staining. Consequently, we found a clear definition in the intracellular granules between red and green fluorescence with more co-localization in the TNF-α treatment group compared with the control group (Fig. 3g). Co-immunoprecipitation analysis showed a strong interaction between p-TBK1 with p-Drp1 S637 (Fig. 3h). In addition, TBK1 overexpression inhibited cytochrome c release and further contributed to the loss of Drp1 induced by TNF-α in the cytoplasm. On the other hand, TBK1 regulated the Drp1 phosphorylation process and promoted p-Drp1S637 translocation to mitochondria without impacting p-Drp1 S616 and induced mitochondrial fusion (Fig. 3i–l).
a–c The expression of Drp1 and its phosphorylation at site S616, S637 after treatment with TNF-α in the concentration of 0, 5, 10, 15 μM. d–f The expression of Drp1 and its phosphorylation at site S616, S637 after treatment with TNF-α in a timely manner. g Immunofluorescence double-labeled staining for co-localization of DRP1 and P-TBK1 in chondrocytes (Green: p-TBK1, Red: DRP1). h Representative Western blots data of TBK1, P-DRP1 S616, and S637 in chondrocytes. i, j The expression of Drp1 and Cyto c in the cytoplasm, measured by Western blotting. **P < 0.01. k, l The expression of p-Drp1 S616, p-Drp1 S637, and Cyto c in mitochondria, measured by Western blotting. All experiments were performed as means ± SD of 3 times in duplicates. *P < 0.05; **P < 0.01; nsP > 0.05.
TBK1 regulates mitophagy in Drp1
To verify whether TBK1 is necessary and sufficient for rescuing blocked autophagy, we applied the siRNA technique against ATG5 to restrict the autophagic process (Fig. 4a, b). lentivirus-shRNA was used to analyze whether TBK1 regulates TNF-α-induced mitophagy. The immunofluorescence findings showed that Lv-shTBK1 decreased the formation of LC3 puncta and diminished co-localization between LC3 and mitochondria (Supplementary Fig. S2). In parallel, our research group performed the Western blot assay to determine the anti-apoptosis effect of TBK1. As shown in Fig. 4c, d, the overexpression of TBK1 could inhibit the Cleaved Caspase-3 pathway and robust Cyto c release. These effects were reversed by the administration of Si-Atg5, thereby indicating the role of promising autophagy in the TBK1-mediated anti-apoptotic effect. We also measured multiple autophagy-associated proteins such as LC3-I, II, Beclin and p62. Our results revealed that the expression of Beclin and the ratio of LC3-II/I were remarkably lifted.
a, b Representative Western blots and quantification data of ATG5 in chondrocytes transfected with siRNA. c, d Representative Western blots and quantification data of Cleaved-caspase-3 and Cyto c in chondrocytes. e, f Representative Western blots and quantification data of LC3-I, II, Beclin, and p62 in chondrocytes. g Fluorescence double-labeled staining for co-localization of LC3 and mitochondrial chondrocytes (Green: p-TBK1, Red: DRP1). h Representative images data of TUNEL staining in chondrocytes. All experiments were performed as means ± SD of 3 times in duplicates. **P < 0.01; nsP > 0.05.
In contrast, these markers were significantly reduced after the Drp1 knockdown (Fig. 4e, f). As demonstrated in Fig. 4g by confocal microscopy, the cells displayed smaller green staining of LC3B puncta and red staining of mitochondria fluorescent probe, indicating the lower levels of autophagy and a few numbers of mitochondria fused with lysosomes. We ascertained a significant augmentation in large red/yellow puncta in cells treated with lentivirus to upregulate TBK1 expression, consistent with activated mitophagy. However, transfection with Drp1 by siRNA attenuated TBK1-mediated autophagosome formation. Then, we performed the TUNEL assay to measure apoptosis-mediated cell death. After transfection of Drp1 or Atg5 by siRNA, the ratio of cells with TUNEL+ (apoptotic marker) positive cell showed augmented expression levels (Fig. 4h). Collectively, these findings indicate that TBK1 may suppress apoptosis-mediated cell death by promoting mitophagy and regulating Drp1.
AMPK activation by M3A induced DRP1 phosphorylation in a TBK1-dependent manner in chondrocytes
Previous studies have indicated that the AMPK signaling pathway can activate TBK1 through direct phosphorylation [28,29,30]. Specifically, phosphorylation at Ser555 regulates ULK1 activation and translocation to mitochondria [31] and then connects energy sensing to mitophagy [32]. Malvidin-3-O-arabinoside (M3A) has been exhibited to trigger the AMPK signaling pathway, contributing to the subsequent enhancement of autophagy [33]. To further explore the interaction between M3A and AMPK signaling pathways (Fig. 5a, b), we performed molecular-docking analysis including blind and focused docking. M3A is prone to link the pivotal regulatory domain with a highly stipulated binding energy of −4.08 kcal/mol, indicating a stationary conformation between M3A and AMPK (Fig. 5c). The two-dimensional interaction graph demonstrated that M3A generated strong hydrogen bonding with the amino acid residues of Leu212, Asp215, Pro213 and Tyr232 (Fig. 5d). These significant interactions contributed to stabilizing the binding location of M3A in the projected ligand-binding sites. According to the results of the CCK-8 assay, application of 40 and 60 μM M3A could induce cellular damage (Fig. 5e). We further performed experiment using 20 μM concentration. To probe the mechanistic basis by which APMK activates Drp1 phosphorylation, we pretreated chondrocytes with different concentrations of M3A and then transfected with lentivirus-TBK1 or si-TBK1 under TNF-α stimulation. We clearly found that M3A could increase the ratio of p-TBK1/TBK1, p-Drp1 Ser637/Drp1, p-AMPK/AMPK1 and p-ULK1/ULK1 in a concentration-dependent manner, whereas LV-shTBK1 could repress the proportion of p-TBK1/TBK1 and p-Drp1 Ser616/Drp1 without influencing on AMPK/ULK1 signaling pathway (Fig. 5f). Collectively, these findings implay that APMK regulates the phosphorylation of Drp1 via promoting the expression of TBK1.
a The model of M3A. b The ribbon model of AMPK. c The 3D docking analysis between M3A and AMPK complex. d The 2D binding model between M3A and AMPK complex. e The CCK-9 analysis result of chondrocytes treated with different concentrations of M3A. f Representative Western blots data of P-TBK1, TBK1, P-DRP1 Ser637, DRP1, P-AMPK1, AMPK, P-ULK1, and ULK1 in chondrocytes. All experiments were performed as means ± SD of 3 times in duplicates. **P < 0.01; nsP > 0.05.
Protective effects of intraarticular injection of lentivirus-TBK1 in DMM-induced OA mice model
To clarify the protective role of TBK1 in OA cartilage degeneration, we established a DMM-induced mouse model. After 2 months of surgery, the treated knee cartilage was collected. The results of PCR showed that the expression levels of TBK1 mRNA were considerably lower in the DMM + Lv-NC group compared to the LV-NC + sham group. Furthermore, the expression level of TBK1 was dramatically elevated after intraarticular injection of the lentivirus-TBK1 (Fig. 6a). We performed H&E and S&O staining to evaluate cartilage tissue in OA. The DMM + LV-NC group demonstrated severe cartilage erosion, massive proteoglycan loss and obvious cytopenia compared to the LV-NC + sham group. Nevertheless, the LV-TBK1 + DMM group exhibited a complete cartilage surface and higher collagen content than the DMM + LV-NC group (Fig. 6b). Consistent with the findings of histopathological staining, the OARSI score of the DMM + LV-NC group was markedly higher than other groups. Intriguringly, the LV-TBK1 + DMM group showed lower scores than the DMM + LV-NC group.
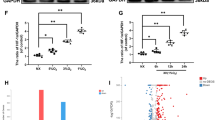
a The mRNA quantification data of mice knee joint tissue. b Representative H&E, S&O staining of cartilage in each group at 8-week after surgery. c OARIS scores of cartilages in each group. d–f The expression of LC3-II and Cleaved-caspase-3 were evaluated by immunohistochemistry staining. All experiments were performed as means ± SD of 3 times in duplicates. **P < 0.01; nsP > 0.05.
From the biomolecular perspective, the DMM + LV-NC group released abundant Cleaved Caspase-3, leading to the loss of chondrocytes and proteoglycans, while administration of LV-TBK1 rescued this significant effect. We further found that TBK1 promoted the expression of LC3-II in vivo (Fig. 6d–f). We also testified the signaling pathway mechanism in vivo. The immunohistochemistry results revealed that the expression levels of p-AMPK, P-TBK1 S172 and P-DRP1 S637 were decreased in OA development. After overexpression of TBK1, the expression level of P-TBK1 S172 and P-DRP1 S637 proteins was increased. Nevertheless, a similar trend did not indicate that TBK1 may influence AMPK phosphorylation. Analysis of DRP1 and P-DRP1 expression in tissue slides demonstrates an inverse trend (Supplementary Fig. S3).Taken together, these experimental data suggest that TBK1 alleviates OA development by activating autophagy and suppressing apoptosis.
Discussion
Previous studies have indicated that impaired mitochondrial homeostasis is implicated in the pathogenesis and advancement of orthopedics degenerative conditions such as IVDD and OA [34,35,36]. Mitochondria, a highly dynamic organelle, frequently alter their morphological structures in continuous cycles of fission and fusion [37]. Giacomello and co-workers have recently explained that mitochondrial elongation can be induced by over-activated mitochondrial fusion or inactivated mitochondrial fission [38]. Appropriate regulation of the mitochondrial fusion and fission increases metabolic demands and eliminates many damaged organelles, further preventing lipogenesis, cancer, neurodegenerative disease and skeletal muscle tissue injury [39,40,41]. Nevertheless, the molecular events shaped by mitochondrial dynamics are still poorly understood. Hyperfused mitochondrial networks are often associated with efficient OXPHOS to produce ATP. In contrast, uncontrolled fission in response to the cellular nutrient state generates fragmented and round mitochondria associated with metabolic dysfunction, enhanced ROS production and improved mitophagy. Recent researches have revealed that mitochondria can further induce the most joint degenerative disease of bone and soft tissue. Mitochondria-derived ROS formation further facilitates the pathogenesis and advancement of OA and the process of senescence aging [42]. However, the cellular processes and biological significance of these regulations in OA remain largely unexplored.
Mitochondrial dynamics may be the key strategy to manipulate the production processes of peroxides in OA. It has been suggested that TBK1 regulates mitochondrial homeostasis in a posttranslational modification way. The previous research conducted by Sun et al. [43] indicates that TBK1 leads to the phosphorylation of RAB7, promoting autophagic flux and damaged mitochondria to restore mitochondrial dynamics. A pioneering research conducted by Andrew and co-workers [44] has revealed that TBK1 is mainly recruited with OPTN to depolarized mitochondria and directly phosphorylates OPTN to efficiently sequestrate damaged mitochondria and induce oxidative stress and inflammatory response. TBK1 is a node molecule that participates in many signaling pathways. Thus, we hypothesized whether TBK1 is involved in regulating mitochondrial dynamics during OA.
Our current research suggests that TBK1 participates in developing trauma-induced OA and old-associated OA. We found that the overexpression of TBK1 can promote mitochondrial dynamics changes, regulate inflammatory response and attenuate apoptosis. Mitochondrial regulatory factors consist of mitochondrial proteins, mitochondrial dynamics (biogenesis, cleavage and fusion) and mitochondrial autophagy. Significantly, our study reports that inflammation and TBK1-driven phosphorylation of DRP1 provides a novel mechanism to adjust DRP1 activity and mitochondrial dynamics via directly phosphorylating at Ser637. The four recognized DRP1 receptors recruit DPR1 to the outer mitochondrial membrane. Emerging evidence indicates that this process is promoted through the phosphorylation of the C-terminal of DRP1 by kinases (such as PKA, CDK1 and ERK2) and is currently known as the primary regulatory mechanism of DRP1 [45,46,47]. A previous work published by Fröhlich et al. indicates that the mitochondrial DRP1 receptor promotes the formation of a putative cleavage site on the surface of the mitochondria in the circular polymer structure of DRP1, which is stabilized by the complex interaction between its MD and the GTPase response domain (GED) [48].
Further research uncovers that the subsequent binding and hydrolysis of GTP lead to the conformational changes of DRP1, which further induces membrane shrinkage and displacement. Phosphorylation of DRP1 or dephosphorylation at Ser637 regulates its translocation to the mitochondrial membrane and mitochondrial cleavage [49]. Specifically, our study shows that p-TBK1 directly interacts and phosphorylates Drp1 at Ser637 to abrogate the mitochondrial fission of DRP1.
The interaction between phosphorylated DRP1 and mitochondrial receptors is also severely downregulated. TKB1-mediated phosphorylation results in a drastic drop in higher-order DRP1 oligomers, which are necessary for DRP1 ring-like structure assembly [50, 51] and might eliminate the assembly-dependent GTPase activity of DRP1 [52]. TBK1-mediated phosphorylation contributes to the cellular mechanism of DRP1 inactivation. Our findings demonstrate a previously unknown mechanism for signaling-induced inactivation of DRP1 and its contribution to the pathogenesis of multiple diseases.
In addition to its potential effects on mitochondrial dynamics, we found that the TBK1/DRP1 axis is also a key regulator of mitophagy, a specific and selective form of autophagy. Previous research indicated the TBK1-mediated regulation of autophagy and mitochondrial function through p62 phosphorylation in IVDD [16]. TBK1-induced mitophagy could exist in chondrocytes. Our data show that a basal level of mitophagy occurs in chondrocytes via promoting mitophagy and TBK1 expression. More importantly, siRNA-mediated knockdown of Drp1 could suppress TBK1-induced mitophagy and its potential anti-apoptotic effect. Next, we sought to find the upstream molecules to identify the activation of TBK1. To evaluate its impact on mitophagy, we analyzed mitochondrial autophagy-related pathways such as PRKN (parkin RBR E3 ubiquitin-protein ligase)-dependent, PRKN-independent and AMPK-ULK1 signaling pathways.
Furthermore, we applied specific pathway agonists to verify their upstream and downstream relationship with the TBK1/DRP1 signaling axis. In our experiment, we included the AMPK pathway and selected M3A as the signaling pathway agonist to verify its correlation with TBK1. Mechanistically, our results display that TBK1 activation depends on the AMPK/ULK1 pathway, which then promotes DRP1 phosphorylation and mitophagy (Fig. 7).
Though there are some differences with earlier works [53], our results are consistent with previous studies linking TBK1 to mitophagy in multiple diseases such as amyotrophic lateral sclerosis, Parkinson and other neurodegenerative diseases [54,55,56]. In our previous study, we also showed the positive role of TBK1 in IVDD via the regulation of mitophagy [16]. IVDD and OA are similar joint diseases involving the degradation of cartilaginous tissues mainly induced by oxidative stress, cell apoptosis and extracellular matrix (ECM) degradation. In addition, the previous study focused on exploring the significant relationship between TBK1 and ECM degradation and signaling pathways associated with inflammation in OA. However, our study reveals underlying molecular mechanisms of mitophagy and mitochondrial dynamic change under TBK1 regulation in OA. Besides, the cell and animal models used in these studies were also totally different. In other research, Peng et al. used IL-1β to induce matrix degradation and inflammation condition and performed anterior cruciate ligament transection (ACLT) surgery to establish an OA animal model. Animal models of different types and cell culture conditions can be used for other research purposes, which may deliver different conclusions in the future. Further experiments are highly required to explore the promising role of TBK1 in inducing inflammation and ECM for the advancement of OA.
Our findings imply that the TBK1-DRP1 axis is an essential factor for mitochondrial fusion. In addition, the TBK1-DRP1 signaling axis facilitates mitochondrial autophagy and resistance of chondrocytes to apoptosis. We also uncovered the phosphorylation site of DRP1, which could be the downstream molecules of TBK1. Collectively, the above findings suggest another phosphorylation site of DRP1 may explore the fundamental changes in cell biology research.
Conclusion
Our proposed research model uncovers that TBK1 activation results from inflammatory stress and further promotes mitochondrial dynamics, mitophagy and anti-apoptotic effect. Our current study suggests that pharmacological targeting of the TBK1-DRP1 cascade provides prospective therapeutic benefits for the treatment and management of OA.
References
Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–26.
Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386:376–87.
Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthr Cartil. 2018;26:319–25.
Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–64.
Helgason E, Phung QT, Dueber EC. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: the emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett. 2013;587:1230–7.
Balka KR, Louis C, Saunders TL, Smith AM, Calleja DJ, D’Silva DB, et al. TBK1 and IKKε act redundantly to mediate STING-induced NF-κB responses in myeloid cells. Cell Rep. 2020;31:107492.
Ma X, Helgason E, Phung QT, Quan CL, Iyer RS, Lee MW, et al. Molecular basis of Tank-binding kinase 1 activation by transautophosphorylation. Proc Natl Acad Sci USA. 2012;109:9378–83.
Zhao B, Du F, Xu P, Shu C, Sankaran B, Bell SL, et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature. 2019;569:718–22.
Aziz N, Son Y-J, Cho JY. Thymoquinone suppresses IRF-3-mediated expression of type I interferons via suppression of TBK1. Int J Mol Sci. 2018;19:1355.
Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–6.
Zhou R, Zhang Q, Xu P. TBK1, a central kinase in innate immune sensing of nucleic acids and beyond. Acta Biochim Biophys Sin. 2020;52:757–67.
Li J, Li J, Miyahira A, Sun J, Liu Y, Cheng G, et al. Crystal structure of the ubiquitin-like domain of human TBK1. Protein Cell. 2012;3:383–91.
Randow F, Youle RJ. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe. 2014;15:403–11.
Singh A, Kendall SL, Campanella M. Common traits spark the mitophagy/xenophagy interplay. Front Physiol. 2018;9:1172.
Herhaus L, Bhaskara RM, Lystad AH, Gestal-Mato U, Covarrubias-Pinto A, Bonn F, et al. TBK1-mediated phosphorylation of LC3C and GABARAP-L2 controls autophagosome shedding by ATG4 protease. EMBO Rep. 2020;21:e48317.
Hu S, Chen L, Al Mamun A, Ni L, Gao W, Lin Y, et al. The therapeutic effect of TBK1 in intervertebral disc degeneration via coordinating selective autophagy and autophagic functions. J Adv Res. 2021;30:1–13.
Xian H, Liou YC. Functions of outer mitochondrial membrane proteins: mediating the crosstalk between mitochondrial dynamics and mitophagy. Cell Death Differ. 2021;28:827–42.
Shin HJ, Park H, Shin N, Kwon HH, Yin Y, Hwang JA, et al. Pink1-mediated chondrocytic mitophagy contributes to cartilage degeneration in osteoarthritis. J Clin Med. 2019;8:1849.
Ansari MY, Khan NM, Ahmad I, Haqqi TM. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr Cartil. 2018;26:1087–97.
Suárez-Rivero JM, Villanueva-Paz M, De la Cruz-Ojeda P, De la Mata M, Cotán D, Oropesa-Ávila M, et al. Mitochondrial dynamics in mitochondrial. Dis Dis. 2017;5:1.
Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845.
Oshima Y, Cartier E, Boyman L, Verhoeven N, Polster BM, Huang W, et al. Parkin-independent mitophagy via Drp1-mediated outer membrane severing and inner membrane ubiquitination. J Cell Biol. 2021;220:e202006043.
Huang J, Xie P, Dong Y, An W. Inhibition of Drp1 SUMOylation by ALR protects the liver from ischemia-reperfusion injury. Cell Death Differ. 2021;28:1174–92.
Simula L, Antonucci Y, Scarpelli G, Cancila V, Colamatteo A, Manni S, et al. PD-1-induced T cell exhaustion is controlled by a Drp1-dependent mechanism. Mol Oncol. 2022;16:188–205.
Makani VKK, Mendonza JJ, Edathara PM, Yerramsetty S, Pal Bhadra M. BORIS/CTCFL expression activates the TGFβ signaling cascade and induces Drp1 mediated mitochondrial fission in neuroblastoma. Free Radic Biol Med. 2021;176:62–72.
Hu S, Zhang C, Ni L, Huang C, Chen D, Shi K, et al. Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020;11:481.
Feng ST, Wang ZZ, Yuan YH, Wang XL, Sun HM, Chen NH, et al. Dynamin-related protein 1: a protein critical for mitochondrial fission, mitophagy, and neuronal death in Parkinson’s disease. Pharmacol Res. 2020;151:104553.
Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, et al. Global Phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 2015;22:922–35.
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41.
Shang L, Chen S, Du F, Li S, Zhao L, Wang X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci USA. 2011;108:4788–93.
Bach M, Larance M, James DE, Ramm G. The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem J. 2011;440:283–91.
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–61.
Li Y, Xu Y, Xie J, Chen W. Malvidin-3-O-arabinoside ameliorates ethyl carbamate-induced oxidative damage by stimulating AMPK-mediated autophagy. Food Funct. 2020;11:10317–28.
Jiang W, Liu H, Wan R, Wu Y, Shi Z, Huang W. Mechanisms linking mitochondrial mechanotransduction and chondrocyte biology in the pathogenesis of osteoarthritis. Ageing Res Rev. 2021;67:101315.
Sun K, Jing X, Guo J, Yao X, Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17:2082–92.
Hartman R, Patil P, Tisherman R, St Croix C, Niedernhofer LJ, Robbins PD, et al. Age-dependent changes in intervertebral disc cell mitochondria and bioenergetics. Eur Cell Mater. 2018;36:171–83.
Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–59.
Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21:204–24.
Labbé K, Mookerjee S, Le Vasseur M, Gibbs E, Lerner C, Nunnari J. The modified mitochondrial outer membrane carrier MTCH2 links mitochondrial fusion to lipogenesis. J Cell Biol. 2021;220:e202103122.
Mohamed Asik R, Suganthy N, Aarifa MA, Kumar A, Szigeti K, Mathe D, et al. Alzheimer’s disease: a molecular view of β-amyloid induced morbific events. Biomedicines. 2021;9:1126.
Hoene M, Kappler L, Kollipara L, Hu C, Irmler M, Bleher D, et al. Exercise prevents fatty liver by modifying the compensatory response of mitochondrial metabolism to excess substrate availability. Mol Metab. 2021;54:101359.
Rimessi A, Previati M, Nigro F, Wieckowski MR, Pinton P. Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. Int J Biochem Cell Biol. 2016;81:281–93.
Sun M, Zhang W, Bi Y, Xu H, Abudureyimu M, Peng H, et al. NDP52 protects against myocardial infarction-provoked cardiac anomalies through promoting autophagosome-lysosome fusion via recruiting TBK1 and RAB7. Antioxid Redox Signal. 2022;36:1119–35.
Moore AS, Holzbaur EL. Dynamic recruitment and activation of ALS-associated TBK1 with its target optineurin are required for efficient mitophagy. Proc Natl Acad Sci USA. 2016;113:E3349–58.
Ko HJ, Tsai CY, Chiou SJ, Lai YL, Wang CH, Cheng JT, et al. The phosphorylation status of Drp1-Ser637 by PKA in mitochondrial fission modulates mitophagy via PINK1/Parkin to exert multipolar spindles assembly during mitosis. Biomolecules. 2021;11:424.
Giovarelli M, Zecchini S, Martini E, Garrè M, Barozzi S, Ripolone M, et al. Drp1 overexpression induces desmin disassembling and drives kinesin-1 activation promoting mitochondrial trafficking in skeletal muscle. Cell Death Differ. 2020;27:2383–401.
Kashatus JA, Nascimento A, Myers LJ, Sher A, Byrne FL, Hoehn KL, et al. Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol Cell. 2015;57:537–51.
Fröhlich C, Grabiger S, Schwefel D, Faelber K, Rosenbaum E, Mears J, et al. Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein. Embo J. 2013;32:1280–92.
Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–44.
Montecinos-Franjola F, Bauer B, Mears J, Ramachandran R. GFP fluorescence tagging alters dynamin-related protein 1 oligomerization dynamics and creates disassembly-refractory puncta to mediate mitochondrial fission. Sci Rep. 2020;10:14777.
Große L, Wurm C, Brüser C, Neumann D, Jans D, Jakobs S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016;35:402–13.
Chang CR, Manlandro CM, Arnoult D, Stadler J, Posey AE, Hill RB, et al. A lethal de novo mutation in the middle domain of the dynamin-related GTPase Drp1 impairs higher order assembly and mitochondrial division. J Biol Chem. 2010;285:32494–503.
Sun P, Xue Y. Silence of TANK-binding kinase 1 (TBK1) regulates extracellular matrix degradation of chondrocyte in osteoarthritis by janus kinase (JAK)-signal transducer of activators of transcription (STAT) signaling. Bioengineered. 2022;13:1872–9.
Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW. The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol Cell. 2015;60:7–20.
Harding O, Evans CS, Ye J, Cheung J, Maniatis T, Holzbaur ELF. ALS- and FTD-associated missense mutations in TBK1 differentially disrupt mitophagy. Proc Natl Acad Sci USA. 2021;118:e2025053118.
Herhaus L. TBK1 (TANK-binding kinase 1)-mediated regulation of autophagy in health and disease. Matrix Biol. 2021;100-101:84–98.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (81972150, 82172428), Wenzhou Inovation Team (Growth Factor Drug Development, No. 201801), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-028), Zhejiang Province Science and Technology Plan Research and Xinmiao Talent Program (Grant/Award Numbers: 2021R413082).
Author information
Authors and Affiliations
Contributions
SLH, JS designed the study and supervised the entire process. SLH and AM wrote the paper. YFS, XMJ, YXY, CZP performed the animal experiments. SLH, YFS, XMJ, YXY, CZP, YPC, SLL, and JJL performed the in vitro experiments. CZP, ZYL participated in data analysis.JX,XYW,SLH supervised and conceptualized the study. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
According to the Animal Care and Use Committee of Wenzhou Medical University, Wenzhou, Zhejiang Province, China, all surgical interventions, treatments and postoperative animal care protocols and procedures were entirely followed for conducting the research (ethical committee number: wydw2021-0583). The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of The Second Affiliated Hospital of Wenzhou Medical University. All animals used in the study were cared for in accordance with the ethical guidelines on animal experimentation of Laboratory Animals of China National Institutes of Health. Healthy C57BL/6 mice were obtained from the Experimental Animal Center of Wenzhou Medical University, Zhejiang Province, China.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Sl., Mamun, A.A., Shaw, J. et al. TBK1-medicated DRP1 phosphorylation orchestrates mitochondrial dynamics and autophagy activation in osteoarthritis. Acta Pharmacol Sin 44, 610–621 (2023). https://doi.org/10.1038/s41401-022-00967-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00967-7
Keywords
This article is cited by
-
The role and intervention of mitochondrial metabolism in osteoarthritis
Molecular and Cellular Biochemistry (2023)