Abstract
Major depressive disorder is a global mental illness associated with severe mortality and disability. The dopaminergic system is involved in both the etiology and therapeutics of depression. Distinct functions of dopamine D1 and D2 receptor subtypes have attracted considerable research interest, and their roles in the pathogenesis of depression and interaction with antidepressants need to be comprehensively elucidated. Herein, we investigated the antidepressant effects of a candidate antidepressant from a cinnamamide derivative, M2, and examined underlying neural mechanisms. We observed that a single dose of M2 (30 mg/kg, ip) produced rapid antidepressant-like effects in mice subjected to the forced swim and tail suspension tests. Using whole-cell recordings in mouse coronal brain slices, we found that application of M2 (10–150 μM) concentration-dependently increased the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) of the pyramidal neurons in the medial prefrontal cortex (mPFC). Furthermore, M2-induced enhancement of sEPSC frequency was abolished by sulpiride (10 µM), a dopamine D2 receptor antagonist, but not by the dopamine receptor D1 antagonist, SCH23390 (10 μM). In addition, M2 administration significantly increased expression levels of synaptogenesis-related proteins, including p-mTOR and p-TrkB, in the mPFC at 30 min, and increased postsynaptic protein PSD-95 at 24 h. Our results demonstrated that M2 produces rapid antidepressant actions through a novel mechanism via dopamine D2 receptor-mediated enhancement of mPFC neurotransmission.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a severe mental disorder worldwide, characterized by persistent depressed mood, anhedonia, and intensive suicidal thoughts [1]. Currently available antidepressants afford limited benefits and typically require several days or weeks to exert a significant effect [2,3,4]. Therefore, drugs eliciting rapid and efficient antidepressant action need to be urgently identified.
Dopaminergic systems play a key role in some symptoms commonly observed in patients with depression, such as anhedonia or decreased motivation [5]. The dopaminergic neurons in the ventral tegmental area of the midbrain project to the limbic and cortical areas, constituting the mesolimbic and mesocortical pathways that mediate mood and cognitive function in depression [6]. The medial prefrontal cortex (mPFC), an important component of the mesocortical dopaminergic pathway, plays a crucial role in affective and cognitive deficits in depression. Compared with other monoaminergic systems, the antidepressant function of dopaminergic systems in the mPFC has not been extensively studied. Dopamine exerts its effects via two primary receptor subtypes, D1-like and D2-like receptors, typically displaying bidirectional functional effects in terms of signal transduction and synaptic transmission in the mPFC [7]. Dopamine receptor subtypes in a specific brain region play diverse roles in the pathogenesis and pharmacological treatment of depression. For example, elevated expression of D1 receptors (D1Rs) in the mPFC produces antidepressant-like behaviors [8]. Activation of D1Rs reportedly enhances excitatory synaptic transduction in the PFC [9]. Moreover, it has been shown that D1R agonists modulate antidepressant-like activity in rats [10]. A previous study has revealed that D2 receptor (D2R) agonists reduce spine density [11], and several D2R antagonists have been approved by the Food and Drug Administration (FDA) as atypical antipsychotic sedatives and antidepressants [12]. Early studies found that increased D2R binding can be observed in the striatum and amygdala of patients with depression [13]. Studies have demonstrated that the sensitization of D2-like dopamine receptors in the mesolimbic dopamine system may represent a final common pathway in antidepressant action [14]. However, compared with D1Rs, the role of D2Rs in the mPFC region mediating antidepressant action remains unclear, warranting further investigation.
In recent years, numerous reports have demonstrated that rapid antidepressant effects can be induced by improving structural and functional changes in the mPFC, including promoting excitatory synaptic transmission and neural plasticity [15,16,17,18]. Many studies have supported a major role for dopaminergic pathways in synaptic transmission and plasticity [19,20,21,22,23,24]. Understanding how dopamine receptors in the mPFC participate in antidepressant treatments will be critical for developing more efficacious therapeutic agents. In the present study, a cinnamamide derivative, named M2, as a candidate antidepressant (Fig. 1), displayed rapid and significant antidepressant effects after single-dose administration in wild-type mice. The neural mechanism underlying the sustained antidepressant-like actions produced by M2 remains elusive. Examining the mechanism revealed that M2 enhanced excitatory synaptic transmission and increased the expression of antidepressant-associated synaptic proteins in the mPFC. Further pharmacological research indicated that D2 receptors in the mPFC are related to the antidepressant-like action of M2.
Materials and methods
Animals
All animal procedures were approved by the Animal Care Committees of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, to protect animals used for scientific purposes. All experimental wild-type mice (C57BL/6) were housed in groups of five under a 12/12 h light/dark cycle with ad libitum access to food and water. For electrophysiological experiments, 20–30 day old mice were employed. Male C57BL/6 mice (18–22 g) were used to perform behavioral tests and Western blotting after acute drug administration.
Behavioral tests
Forced swim test (FST)
The FST was performed according to a previously reported method, and the effect of the drugs was evaluated by measuring the period of immobility. Mice were placed in a clear Plexiglas cylinder (30 cm high; 10 cm in diameter) filled with water (23 ± 1 °C) to a depth of 20 cm. The behavior of each mouse was recorded using a camera positioned directly in front of the cylinder for 6 min. The duration of immobility, defined as no movement of the limb or body in the last 4 min, was measured.
Open field test (OFT)
Mice were introduced to an open field apparatus (40 cm × 40 cm × 35 cm), and their behavior was monitored for 10 min using a charge-coupled device camera. The images were captured on a computer with a video tracking system (EthoVision XT 14). The distance moved totally and traveled in the central square (20 cm × 20 cm) was calculated automatically. The test was performed in a quiet environment. Between each mouse test, the apparatus was thoroughly cleaned with 75% alcohol to avoid potential infections.
Tail suspension test (TST)
Mice were suspended 50 cm above the floor using adhesive tape. A camera was used to record the behavior of each mouse for 6 min. Immobility time was recorded for the last 4 min. To avoid potential infection, the apparatus was thoroughly cleaned with 75% alcohol before testing each mouse.
Electrophysiological recording
Mice (20–30 days old) were anesthetized and then transcardially perfused with ice-cold artificial cerebrospinal fluid (aCSF) containing 130 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, 10 mM glucose, and aerated with 95% O2 and 5% CO2. After perfusion, the brain was rapidly removed, coronal brain slices (300-μm thick) containing the mPFC were prepared using a Leica VT1000S vibratome (Leica Microsystems) and allowed to recover at 28 °C for at least 1 h in an oxygenated (95% O2, 5% CO2) aCSF solution.
Subsequently, slices were placed in a recording chamber and continuously perfused (2 mL/min) in aCSF at a bath temperature of 32 °C. All pharmacological agents were dissolved in aCSF and administered through bath perfusion. Patch electrodes with tip resistances between 3 and 9 MΩ were filled with a solution containing 115 mM CsMeSO3, 20 mM CsCl, 2.5 mM MgCl2, 4 mM Na2-ATP, 0.4 mM Na-GTP, 10 mM Na-phosphocreatine, 0.6 mM EGTA, and 10 mM HEPES (pH 7.35). Pyramidal neurons in layer V of the mPFC were imaged and recorded using a ×40 water immersion lens and a Multiclamp 700 B amplifier (Molecular Devices, USA). In the voltage-clamp mode, spontaneous excitatory postsynaptic currents (sEPSCs) were recorded with membrane potentials at −70 mV. Pyramidal cells were identified by their pyramidal shape, large soma, and the presence of apical dendrites. During the recording, bicuculine blocks the GABAA receptor during the entire process.
Western blotting
Western blotting experiments were performed as previously described. For biochemical analyses, an independent group of mice was employed. After administration, the mPFC tissue of the brain was harvested and incubated in RIPA lysis buffer on ice. The tissue was homogenized on ice three times using an ultrasound homogenizer (3 Hz frequency, 10 s). Tissue lysates were centrifuged at 14,000 r/min for 15 min at 4 °C. The supernatant was collected, and the protein concentration was measured by performing the bicinchoninic acid protein assay. Proteins were transferred to polyvinylidene difluoride membranes and blocked with 3% non-fat dry milk in TBST (25 mM Tris–HCl [pH 7.5], 125 mM NaCl, 0.1% Tween 20) for 1 h. The membranes were incubated with specific primary antibodies, including p-mTOR (1:1000, Cst, 2971 s), mTOR (1:1000, Cst, 2983 s), p-TrkB (1:1000, Cst, 4621 s), TrkB (1:1000, Cst, 4603 s), β-tubulin (1:1000, Cst, 2128 s), PSD95 (1:1000, Cst, 3450 s), at 4 °C overnight. Next, the membranes were washed three times with TBST and incubated with secondary antibodies (anti-rabbit IgG, 1:1000) for 1 h. After the final three washes with TBST, bands were detected using enhanced chemiluminescence, followed by exposure to X-ray film (Carestream) for visualization and the Western blotting detection system (GE Healthcare Bioscience).
Drugs
For systemic administration, M2 (Tasly Pharma) was dissolved in saline with dimethyl sulfoxide (DMSO) and Tween-80. S-ketamine (Sigma-Aldrich, USA) and fluoxetine (Sigma-Aldrich, USA) were diluted with saline. Bicuculine (20 mM; Selleckchem) and the D2R antagonist (±)-sulpiride (20 mM; Sigma) were dissolved in DMSO. The D1R antagonist R (+)-SCH23390 (20 mM; Sigma) was dissolved in H2O.
Statistical analysis
All data are expressed as mean ± standard error of the mean. Electrophysiological data were displayed using Clampfit software (pClamp 9.2), and analysis was performed with the Mini Analysis software. We used paired t-tests (for comparison of two groups) and one-way analysis of variance (for multiple groups). GraphPad Prism 9 (GraphPad Software, Inc., La Jolla, CA, USA) was used for all the statistical analyses. Differences were considered statistically significant at P < 0.05.
Results
M2 produced antidepressant-like actions
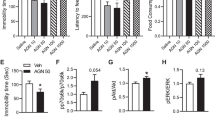
To assess the antidepressant actions of M2, we performed the FST in C57BL/6J mice, and the results are illustrated in Fig. 2. The classic antidepressant fluoxetine and the new antidepressant S-ketamine acted as positive drugs. One-way ANOVA analysis revealed that S-ketamine, fluoxetine, and M2 (30 mg/kg, ip) exhibited a statistically significant effect on immobility time (F (5,53) = 5.122; P < 0.001) in the FST, 30 min after a single administration (Fig. 2a). In the OFT, no significant difference was detected in the total traveled distance [F (5,54) = 2.041; P > 0.05] (Fig. 2b) and the ratio of central traveled distance to total distance [F (5,54) = 1.220; P > 0.05] (Fig. 2c) 30 min after M2 administration (3 mg/kg, 10 mg/kg, and 30 mg/kg, ip), indicating that autonomous activity was unaffected. In addition, compared with the vehicle group, FST and one-way ANOVA data analysis revealed that M2 (30 mg/kg) and S-ketamine significantly reduced swimming immobility time 24 h after a single administration (F (5,54) = 3.021; P < 0.05) (Fig. 2d). Based on the OFT data, no significant difference was observed in the total distance traveled [F (5,54) = 1.141; P > 0.05] (Fig. 2e) and the ratio of central traveled distance to total distance [F (5,54) = 0.9547; P > 0.05] (Fig. 2f) at 24 h after administration. Furthermore, we detected no significant statistical difference in the time spent in the central area in the OFT at 30 min [F (5,54) = 1.137; P > 0.05] (Supplementary Fig. S1c) and 24 h (F (5,54) = 0.6185; P > 0.05) (Supplementary Fig. S1d) after administration. Despite the absence of a statistically significant difference, M2 produced a dose-dependent anxiolytic effect at 30 min and 24 h after administration (Supplementary Fig. S1c, d). Additionally, we observed that the immobility time 30 min after fluoxetine and M2 showed a decreasing trend [F (5,54) = 0.6368; P > 0.05] in the TST (Supplementary Fig. S1a). However, TST and one-way ANOVA data analysis revealed that M2 (3 and 10 mg/kg) significantly reduced the immobility time 24 h after a single administration in a dose-dependent manner (F (5,54) = 4.119; P < 0.05) (Supplementary Fig. S1b). These findings indicated that M2 produced fast and sustained antidepressant-like actions.
a The immobility time in the FST is significantly reduced by S-ketamine (10 mg/kg, ip), fluoxetine (10 mg/kg, ip), and M2 (30 mg/kg, ip) 30 min after administration. b, c All mice exhibit normal locomotor activity, as shown in the open field test 30 min after administration. d The immobility time in the FST is significantly reduced by S-ketamine (10 mg/kg, ip) and M2 (30 mg/kg, ip) 24 h after administration. e, f All mice exhibit normal locomotor activity, as shown in the open field test at 24 h after administration. All values are expressed as mean ± standard error of the mean (SEM). n = 9–10 each group. One-way ANOVA followed by Dunnett’s test. *P < 0.05, ***P < 0.001. FST forced swim test.
M2 increased excitatory synaptic transmission in the prelimbic mPFC
The mPFC plays a critical role in the pathophysiology of depression [25]. Direct optogenetic stimulation of layer V pyramidal neurons reportedly produces a robust antidepressant-like response, suggesting that layer V pyramidal neurons play a critical role in modulating depressive behavior [26, 27]. To determine the impact of M2 on excitatory synaptic transmission in layer V pyramidal neurons, we performed whole-cell patch-clamp recordings of sEPSCs in pyramidal neurons in the prelimbic mPFC.
In the present study, neurons were voltage-clamped at −70 mV, a value approaching the neuronal resting membrane potential, and sEPSCs were evoked in the presence of bicuculine (a GABAA receptor antagonist) to eliminate inhibitory responses. After 6 min of recording to ensure steady baseline responses, brain slices were perfused with an M2-containing aCSF for a 6-min treatment period, followed by a 10 min washout period. We observed that bath application of M2 (150 μM) significantly increased the sEPSC frequency of pyramidal neurons in the mPFC (representative traces are shown in Fig. 3a) and shifted the cumulative distribution of inter-event intervals to the left, consistent with a mean increase in the frequency of sEPSCs (baseline, 1.558 ± 0.3037 Hz, vs. M2 2.529 ± 0.6209 Hz, n = 11, P = 0.0236; Fig. 3c). However, conforming to the representative traces (Fig. 3a), M2 (150 μM) exhibited no obvious effect on the cumulative distribution of the current amplitude and the mean sEPSC amplitude (baseline, 11.93 ± 1.100 pA, vs. M2 12.19 ± 1.129 pA, n = 11, P = 0.4892; Fig. 3b). Furthermore, M2 significantly increased sEPSC normalized frequency in a concentration-dependent manner from 10 to 150 μM (10 μM, 0.9301 ± 0.1117 Hz, vs. 30 μM, 1.041 ± 0.05669 Hz, vs. 50 μM, 1.242 ± 0.1559 Hz, vs. 100 μM, 1.328 ± 0.1257 Hz, vs. 150 μM, 1.538 ± 0.1658 Hz; P = 0.0429; Fig. 3e), with no significant difference observed in the normalized amplitude of sEPSCs (10 μM, 1.004 ± 0.05842 pA, vs. 30 μM, 1.002 ± 0.03972 pA, vs. 50 μM, 0.9489 ± 0.06621 pA, vs. 100 μM, 1.022 ± 0.08641 pA, vs. 150 μM, 1.022 ± 0.09462 pA; P = 0.9443; Fig. 3d). The bath application of 10 and 30 μM M2 had no significant effect on the frequency of sEPSCs in pyramidal neurons, and 50 µM M2 increased sEPSC frequency (Supplementary Fig. S2). With continuous increments in the M2 concentration, the M2-induced frequency was elevated at 100 μM (Supplementary Fig. S2). The sEPSCs of pyramidal neurons were completely inhibited by CNQX (an AMPA receptor antagonist) (Fig. 3a). These results suggested that M2 can enhance the release of excitatory synaptic neurotransmitters from prefrontal pyramidal neuron synapses in the prelimbic mPFC.
a A typical trace showing glutamatergic sEPSCs. sPSCs were recorded at a VH of −70 mV in the presence of 10 μM bicuculine. The recorded sEPSCs of pyramidal neurons are completely inhibited by CNQX (an AMPA receptor antagonist). b Cumulative probability distributions of the current amplitude of glutamatergic sEPSCs. Insets show changes in sEPSC amplitude at a concentration of 150 μM M2. Each column shows the mean ± standard error of the mean (SEM) of 11 experiments. c Cumulative probability distributions for the inter-event interval of glutamatergic sEPSCs. Insets show changes in sEPSC amplitude at a concentration of 150 μM M2. Each column shows the mean ± SEM of 11 experiments. *P < 0.05, paired t-test. d The concentration-response relationship shows that the normalized sEPSC amplitude is not altered with the increasing M2 concentration in pyramidal neurons in the prelimbic mPFC. One-way ANOVA followed by Dunnett’s test (P = 0.9443). e The concentration-response relationship shows that the normalized sEPSC frequency is enhanced with the increasing M2 concentration in pyramidal neurons in the prelimbic mPFC. One-way ANOVA followed by Dunnett’s test (P = 0.0429). sEPSCs spontaneous excitatory postsynaptic currents, mPFC medial prefrontal cortex.
M2 increased excitatory synaptic transmission via D2R but not D1R
As a candidate antidepressant drug acting on the monoamine system, M2 is superior to traditional antidepressants. We postulate that the dopamine system plays a key role in the antidepressant mechanism of M2, which regulates information processing in the mPFC through D1R and D2R.
To further explore whether M2 enhanced the excitatory synaptic transmission of pyramidal neurons, we performed recordings in the presence of pharmacological D2R inhibition. D2 receptors are widely expressed in the deep layers of the mPFC [28]. The D2R antagonist sulpiride was pre-perfused to determine whether D2R participated in the antidepressant-like action of M2. We recorded sEPSCs from pyramidal neurons of the prelimbic mPFC in the presence of the GABAA receptor antagonist, bicuculine, until a 5–10 min stable baseline was obtained. Representative traces of sEPSCs are shown in Fig. 4a. We observed that the mean frequency of pyramidal neurons was significantly increased following bath application of sulpiride when compared with the baseline, and additional bath application of M2 did not increase the frequency of sEPSCs based on sulpiride-induced frequency (Basal, 1.670 ± 0.2020 Hz, vs. sulpiride 1.977 ± 0.2516 Hz, vs. sulpiride + M2 2.018 ± 0.2724 Hz; P = 0.0051; n = 14; Fig. 4e). Compared with the baseline, both sulpiride and sulpiride + M2 shifted the cumulative distribution of inter-event intervals to the left (Fig. 4c), consistent with the increase in mean sEPSC frequency (Fig. 4e). The mean sEPSC amplitude was not significantly affected by sulpiride or M2 application (Basal, 10.16 ± 0.7824 pA, vs. sulpiride 9.840 ± 0.4910 pA, vs. sulpiride + M2 9.885 ± 0.4551 pA; P = 0.5308; n = 14; Fig. 4d). Based on cumulative probability plots, no significant difference was detected in the cumulative distribution of sEPSC amplitude following sulpiride or M2 application when compared with the control (Fig. 4b). The results revealed that the M2-enhanced sEPSC frequency was inhibited after pharmacological D2R blockade, suggesting that the D2Rs mediate the M2-induced enhancement of excitatory synaptic transmission in the mPFC.
a Representative traces of sEPSCs, including three stages of Basal, Sul, and Sul+M2. sEPSCs were recorded at −70 mV in the presence of 10 μM bicuculine. b Cumulative probability distributions for the current amplitude of glutamatergic sEPSCs in the three stages. c Cumulative probability distributions for the inter-event interval of glutamatergic sEPSCs. d The mean amplitude of sEPSCs is not significantly altered, showing no effect at 10 μM sulpiride and 150 μM M2-induced changes in sEPSC amplitude. e Compared with the mean frequency of Basal, Sul-induced sEPSCs are significantly increased (P = 0.0086), while Sul+M2-induced sEPSCs are significantly increased (P = 0.0212). No significant change can be observed in the mean frequency of sEPSCs between Sul and Sul+M2 groups (P = 0.8686). Each column represents the mean ± standard error of the mean (SEM). n = 14; paired one-way ANOVA by Tukey’s post hoc test; NS not significant; *P < 0.05. D2R dopamine D2 receptor, sEPSCs spontaneous excitatory postsynaptic currents, Sul sulpiride.
To further explore whether M2 enhanced the excitatory synaptic transmission of pyramidal neurons, we performed the recordings in the presence of pharmacological D1R inhibition. Representative traces of sEPSCs are shown in Fig. 5a. The application of the D1R receptor antagonist SCH23390 displayed a weak effect on the mean frequency of sEPSCs in the pyramidal neurons of the prelimbic mPFC. Additional M2 bath application significantly increased the mean frequency of sEPSCs (Basal, 1.167 ± 0.2189 Hz, vs. SCH 1.028 ± 0.1747 Hz, vs. SCH+M2 1.348 ± 0.2202 Hz; P = 0.0588; n = 9; Fig. 5e). SCH+M2 significantly shifted the cumulative distribution of inter-event intervals to the left, which is consistent with the elevated M2-induced sEPSC frequency (Fig. 5c). The mean amplitude of sEPSCs did not differ between the baseline, SCH23390, and SCH23390+M2 stages (basal, 11.36 ± 1.090 pA vs. SCH, 10.63 ± 0.7449 pA, vs. SCH+M2 10.23 ± 0.6384 pA; P = 0.3909; n = 9; Fig. 5d). In addition, no significant difference was detected in the cumulative distribution of mEPSC amplitude after SCH23390 or M2 application when compared with the control (Fig. 5b). The results showed that the M2-induced enhancement of sEPSC frequency persisted after the pharmacological blockade of D1Rs, suggesting that D1Rs are not involved in the M2-mediated enhancement of excitatory synaptic transmission in the mPFC.
a Representative traces of sEPSC including three stages of Basal, SCH, and SCH+M2. sEPSCs were recorded at −70 mV in the presence of 10 μM bicuculine. b Cumulative probability distributions for the current amplitude of glutamatergic sEPSCs under the three stages. c Cumulative probability distributions for the inter-event interval of glutamatergic sEPSCs. d The mean amplitude of sEPSC shows no significant change at 10 μM SCH23390 and 150 μM M2 induced changes in sEPSC amplitude. e Compared with the mean frequency of SCH23390-induced sEPSC, SCH+M2 shows a significant increase (P = 0.0078). Each column displays the mean ± standard error of the mean (SEM). n = 9; paired one-way ANOVA by Tukey’s post hoc test; NS no significance; **P < 0.01.
The pharmacological characterization of M2 supported the involvement of D2R in regulating excitatory synaptic transmission in the mPFC. Although blockade of D1R (with SCH23390) did not significantly affect the M2-mediated effect, the inhibition of D2R (with sulpiride) blunted the M2-induced increase in synaptic transmission in the mPFC.
M2 activated mTOR signaling and increased postsynaptic-related proteins
Previous studies have demonstrated that ketamine rapidly activates the mTOR pathway, leading to increased function of new synapses in the mPFC of mice [29, 30]. In contrast, the clinical antidepressant fluoxetine did not significantly influence mTOR signaling. To further clarify the possible M2-induced intracellular signaling pathways underlying the rapid antidepressant effects, we examined the expression of mTOR signals. M2 significantly altered the expression levels of Ser 2448 phosphorylation of mTOR (p-mTOR) 30 min after the injection without modifying total mTOR (Fig. 6a). Likewise, S-ketamine increased the expression of p-mTOR instead of mTOR. However, fluoxetine had no obvious effect on the phosphorylation or non-phosphorylation of mTOR. As shown in Fig. 6c, the expression levels of p-mTOR/mTOR were significantly increased in the M2 (P < 0.05) and S-ketamine groups (P < 0.05) when compared with the vehicle group.
a Western blotting images of p-TrkB, TrkB, p-mTOR, mTOR, and β-tubulin at 30 min after administration. b Relative protein levels of p-TrkB/TrkB in the mPFC. c Relative protein levels of p-mTOR and mTOR in the mPFC. d Western blotting images of p-TrkB, TrkB, p-mTOR, mTOR, and β-tubulin at 24 h after administration. e Relative protein levels of p-TrkB/TrkB in the mPFC. f Relative protein levels of p-mTOR and mTOR in the mPFC. g Western blot images of PSD-95 at 24 h after administration. h Relative protein expression levels of PSD-95 in the mPFC. Data are expressed as mean ± standard error of the mean (SEM). *P < 0.05; **P < 0.01; ****P < 0.0001. mPFC medial prefrontal cortex.
Neurotrophins are potential mediators of neuroplastic changes induced by antidepressants [31]. To examine the activation of neurotrophin signaling in the brain, tyrosine kinase receptor B (TrkB) phosphorylation was used as an assay. M2 significantly altered the expression levels of Tyr 705/706 phosphorylation (p-TrkB) 30 min after the injection (Fig. 6a), as determined by the higher expression level of p-TrkB/TrkB when compared with the vehicle group (P < 0.05, Fig. 6b); S-ketamine and fluoxetine induced no significant difference when compared with the vehicle. Antidepressants did not affect total TrkB protein levels, implying that these drugs increased TrkB phosphorylation but not TrkB protein synthesis. These findings suggested that the rapid antidepressant-like effect of M2 could be related to the activation of mTOR and TrkB proteins.
Activation of mTOR has been functionally linked with local protein synthesis in synapses, resulting in the production of proteins involved in the formation, maturation, and function of new synapses [29]. PSD-95 is a postsynaptic density protein associated with neuroplasticity. We found that M2 administration increased the levels of the postsynaptic protein PSD-95 after 24 h (P < 0.0001, Fig. 6g, h). Under the same conditions, S-ketamine, but not fluoxetine, increased the expression of PSD-95 when compared with vehicle (P < 0.01, Fig. 6h). Twenty-four hours after administration, we noted that M2, S-ketamine, and fluoxetine did not significantly alter the expression levels of p-mTOR/mTOR and p-TrkB/TrkB when compared with the vehicle (Fig. 6d–f). It has been proposed that mTOR signaling not only regulates the number and structure of synapses but also controls synaptic transmission by modifying synaptic functions. This result is consistent with the electrophysiological experiment, which found that M2 enhanced the synaptic transmission function of the prefrontal cortex. The antidepressant actions of M2 may be mediated via the rapid activation of mTOR signaling, which results in rapid and sustained elevation of synapse-associated proteins in the PFC.
Discussion
In the present study, our results revealed that M2 produced antidepressant-like effects as evaluated by FST, TST, and OFT. Furthermore, we found that M2 enhanced excitatory synaptic transmission in the mPFC; this effect was blocked by the D2R antagonist, not the D1R antagonist. On examining the M2-mediated intracellular signaling, our results showed that the activation of mTOR signaling and synaptogenesis-related proteins might mediate the antidepressant actions of M2.
The classical selective serotonin reuptake inhibitor (SSRI), fluoxetine, affects monoamine neurotransmission immediately [32] but typically requires several weeks to exert an antidepressant effect during clinical therapy. Herein, compared with fluoxetine, a single-dose intraperitoneal administration of M2 produced a sustained antidepressant-like effect, displaying a significantly reduced swimming immobility time 24 h after administration in the FST and TST. M2 exhibited greater antidepressant efficacy and more rapid onset of action than traditional antidepressants. Furthermore, we observed that M2 increased excitatory synaptic transmission via D2Rs in the prelimbic mPFC.
The PFC plays an important role in the pathogenesis of MDD [33]. The rodent mPFC is speculated to be analogous to the primate mPFC [34]. Post-mortem studies of suicides with MDD have reported that the PFC is reduced in volume [33], along with decreased excitatory transmitter transmission. Additionally, studies have shown the presence of dysfunction in the cortical-limbic circuitry during depression, as supported by elevated glucose metabolism in the prefrontal cortex and reduced metabolism in limbic regions after treatment with antidepressants [35]. Additional findings suggest that antidepressants enhance the activity of D2-like receptors in the nucleus accumbens and increase neurotransmission in the mesolimbic dopamine system to mediate antidepressant effects [36]. Several studies have compared D2Rs in the midbrain; however, the role of D2Rs in the mPFC in terms of cognitive enhancement remains under investigation. Pyramidal output neurons in the mPFC integrate and transfer information from extra-cortical inputs and local circuits to other cortical areas and subcortical structures [37]. The proportion of pyramidal neurons expressing D1R is similar in layers II–III and VI (19%–21%). Pyramidal cells expressing D2 receptors were more abundant in layer V (25%) than in layers II–III (5%) or VI (13%) [38]. D1Rs are expressed at the postsynaptic membrane, and D2Rs localize at both presynaptic and postsynaptic membranes, with the presynaptic receptors functioning as inhibitory autoreceptors [39]. The differential expression pattern on layer neurons indicates that D2R can differentially regulate these pyramidal cells. Our data indicated that M2 acutely potentiates excitatory neurotransmission in brain slices, and the M2-mediated enhancement was blocked by applying a D2R antagonist rather than a D1R antagonist, suggesting that D2R activity mediates the antidepressant effects via the mPFC. Several D2R antagonists have been approved by the FDA as atypical antipsychotic sedatives and antidepressants [11], and most studies on D2Rs have primarily examined the midbrain [40]; therefore, the role of D2R in the mPFC warrants further investigation.
Previous reports have indicated that dopamine regulates glutamatergic synaptic transmission in the mPFC through a presynaptic mechanism [41], mediated via presynaptic D2R [42]. The D2R antagonist (sulpiride) increased the sEPSC frequency, supporting the critical role of D2R in glutamatergic synaptic transmission in the mPFC. M2, a triple reuptake inhibitor, increases the dopamine concentration in synaptic clefts and functionally increases glutamatergic transmission in a concentration-dependent manner in the mPFC. The pharmacological inhibition of D2R (sulpiride), rather than D1R (with SCH23390), blunted the M2-induced increase in synaptic transmission. Accordingly, we inferred that M2 might act at presynaptic D2R, resulting in a mechanism of disinhibition that exerts an antidepressant effect. The involvement of D2R in M2-mediated actions increases synaptic transmission, as well as triggers the activation of intracellular signaling pathways.
In 2010, Li et al. achieved a breakthrough and found that ketamine elicits rapid efficacy against depressive symptoms by increasing the number and function of neural spines in the prefrontal cortex via activation of the mTOR pathway, which is well-known to mediate cell proliferation, survival, and metabolism [29]. Classical antidepressants, such as fluoxetine, did not significantly impact mTOR signaling. Our results revealed that the rapid activation of mTOR signaling in the mPFC after a single M2 administration was similar to that induced by ketamine. The mTOR signaling pathway could be activated by both glutamatergic receptors [43] and D2Rs after M2 treatment [44]. Rapid activation of mTOR signaling, resulting in prompt and sustained elevation of synapse-associated proteins in the PFC, may represent a potential antidepressant mechanism. Twenty-four hours after M2 administration, the expression of p-mTOR (Ser 2448) returned to control levels, whereas the expression of the synaptic protein (PSD-95) was significantly increased, suggesting that the antidepressant-like effect of M2 may be related to the signaling and function of synaptic proteins. It has been proposed that the TrkB receptors, the binding target of brain-derived neurotrophic factor, might mediate the upstream intracellular signaling of the mTOR signaling pathway to transmit the neurogenesis effect. Moreover, studies have implicated antidepressant effects associated with increased levels of phosphorylated and activated forms of TrkB [45]. At 30 min after the single M2 administration, the expression levels of p-TrkB rapidly increased. Our results suggested that the antidepressant-like action of M2 was associated with the mTOR signaling pathway, leading to increased synaptic signaling proteins and enhanced functions of spine synapses in the mPFC.
The mechanism of activation of mTOR and TrkB in the mPFC is critical for mediating the antidepressant action of highly effective antidepressants. M2 enhanced excitatory synaptic transmission and increased the synthesis of synaptic plasticity-related proteins via the mTOR pathway in the mPFC. Unlike classical antidepressants, e.g., SSRIs, M2 produced a rapid and sustained antidepressant-like action even after single-dose administration. Notably, regulation of dopaminergic systems appears important in this process. Our results indicate that M2 produces antidepressant-like actions via a new mechanism, which involves the enhancement of D2R-mediated neurotransmission in the mPFC.
References
Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiat. 2015;72:334–41.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4.
Sinyor M, Schaffer A, Levitt A. The sequenced treatment alternatives to relieve depression (STAR*D) trial: a review. Can J Psychiatry. 2010;55:126–35.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Dailly E, Chenu F, Renard CE, Bourin M. Dopamine, depression and antidepressants. Fundam Clin Pharmacol. 2004;18:601–7.
Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol Psychiatry. 1992;32:1–17.
Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb Cortex. 2007;17:2820–7.
Freund N, Thompson BS, Sonntag K, Meda S, Andersen SL. When the party is over: depressive-like states in rats following termination of cortical D1 receptor overexpression. Psychopharmacology. 2016;233:1191–201.
Zhang B, Guo F, Ma Y, Song Y, Lin R, Shen FY, et al. Activation of D1R/PKA/mTOR signaling cascade in medial prefrontal cortex underlying the antidepressant effects of l-SPD. Sci Rep. 2017;7:3809.
Desormeaux C, Demars F, Davenas E, Jay TM, Lavergne F. Selective activation of D1 dopamine receptors exerts antidepressant-like activity in rats. J Psychopharmacol. 2020;34:1443–8.
Castillo-Gomez E, Varea E, Blasco-Ibanez JM, Crespo C, Nacher J. Effects of chronic dopamine D2R agonist treatment and polysialic acid depletion on dendritic spine density and excitatory neurotransmission in the mPFC of adult rats. Neural Plast. 2016;2016:1615363.
Ruther E, Degner D, Munzel U, Brunner E, Lenhard G, Biehl J, et al. Antidepressant action of sulpiride. Results of a placebo-controlled double-blind trial. Pharmacopsychiatry. 1999;32:127–35.
D’Haenen HA, Bossuyt A. Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol Psychiatry. 1994;35:128–32.
Willner P, Hale AS, Argyropoulos S. Dopaminergic mechanism of antidepressant action in depressed patients. J Affect Disord. 2005;86:37–45.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72.
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41.
Li Q, Zhang B, Cao H, Liu W, Guo F, Shen F, et al. Oxytocin exerts antidepressant-like effect by potentiating dopaminergic synaptic transmission in the mPFC. Neuropharmacology. 2020;162:107836.
Socodato R. Dopamine D1 receptor signaling and endocannabinoid cooperate to fuel striatal plasticity: an editorial highlight for “Cyclic AMP-dependent protein kinase and D1 dopamine receptors regulate diacylglycerol lipase-alpha and synaptic 2-arachidonoyl glycerol signaling” on page 334. J Neurochem. 2020;153:297–9.
Hagena H, Manahan-Vaughan D. Dopamine D1/D5, but not D2/D3, receptor dependency of synaptic plasticity at hippocampal mossy fiber synapses that is enabled by patterned afferent stimulation, or spatial learning. Front Synaptic Neurosci. 2016;8:31.
Urakubo H, Yagishita S, Kasai H, Ishii S. Signaling models for dopamine-dependent temporal contiguity in striatal synaptic plasticity. PLoS Comput Biol. 2020;16:e1008078.
Anderson EM, Gomez D, Caccamise A, McPhail D, Hearing M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol Stress. 2019;10:100152.
Wang S, Liu S, Wang Q, Sun Y, Yao L, Li D, et al. Dopamine modulates excitatory synaptic transmission by activating presynaptic D1-like dopamine receptors in the RA projection neurons of zebra finches. Front Cell Neurosci. 2020;14:126.
Kawamoto K, Otsuguro K, Ishizuka M, Ito S. Inhibitory effects of dopamine on spinal synaptic transmission via dopamine D1-like receptors in neonatal rats. Br J Pharmacol. 2012;166:788–800.
Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–43.
Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res. 2010;44:683–7.
Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci. 2014;8:230.
Santana N, Artigas F. Laminar and cellular distribution of monoamine receptors in rat medial prefrontal cortex. Front Neuroanat. 2017;11:87.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Guo F, Zhang B, Fu Z, Ma Y, Gao Y, Shen F, et al. The rapid antidepressant and anxiolytic-like effects of YY-21 involve enhancement of excitatory synaptic transmission via activation of mTOR signaling in the mPFC. Eur Neuropsychopharmacol. 2016;26:1087–98.
Neto FL, Borges G, Torres-Sanchez S, Mico JA, Berrocoso E. Neurotrophins role in depression neurobiology: a review of basic and clinical evidence. Curr Neuropharmacol. 2011;9:530–52.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66.
McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17180–5.
Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19.
Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82.
Gershon AA, Vishne T, Grunhaus L. Dopamine D2-like receptors and the antidepressant response. Biol Psychiatry. 2007;61:145–53.
Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–31.
Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–60.
Andersen PH, Gingrich JA, Bates MD, Dearry A, Falardeau P, Senogles SE, et al. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends Pharmacol Sci. 1990;11:231–6.
Lavin A, Grace AA. Stimulation of D1-type dopamine receptors enhances excitability in prefrontal cortical pyramidal neurons in a state-dependent manner. Neuroscience. 2001;104:335–46.
Wallace J, Jackson RK, Shotton TL, Munjal I, McQuade R, Gartside SE. Characterization of electrically evoked field potentials in the medial prefrontal cortex and orbitofrontal cortex of the rat: modulation by monoamines. Eur Neuropsychopharmacol. 2014;24:321–32.
Real JI, Simoes AP, Cunha RA, Ferreira SG, Rial D. Adenosine A2A receptors modulate the dopamine D2 receptor-mediated inhibition of synaptic transmission in the mouse prefrontal cortex. Eur J Neurosci. 2018;47:1127–34.
Xia B, Huang X, Sun G, Tao W. Iridoids from Gardeniae fructus ameliorates depression by enhancing synaptic plasticity via AMPA receptor-mTOR signaling. J Ethnopharmacol. 2021;268:113665.
Dadalko OI, Siuta M, Poe A, Erreger K, Matthies HJ, Niswender K, et al. mTORC2/rictor signaling disrupts dopamine-dependent behaviors via defects in striatal dopamine neurotransmission. J Neurosci. 2015;35:8843–54.
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–313 e19.
Acknowledgements
This work was supported by funds from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12040220), the National Natural Science Foundation of China (31671049, 31371066 to YL), the National Key New Drug Creation Program of China (No. 2018ZX09711002-002-012), and the Shanghai Municipal Science and Technology Major Project and the Science and Technology Commission of Shanghai Municipality (Nos. 184319071000 and 19140903102 to YL and FG). The work in this manuscript was funded in part by a research grant from Tasly Pharma, Inc.
Author information
Authors and Affiliations
Contributions
YXC and XYJ performed the experiments and analyzed the data. RHX and MZ assisted with behavioral experiments. XHM, FG, and YL designed the study and supervised the project; YXC, FG, and YL wrote and edited the manuscript. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Che, Yx., Jin, Xy., Xiao, Rh. et al. Antidepressant-like effects of cinnamamide derivative M2 via D2 receptors in the mouse medial prefrontal cortex. Acta Pharmacol Sin 43, 2267–2275 (2022). https://doi.org/10.1038/s41401-021-00854-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00854-7