Abstract
Our previous studies confirm that exogenous reduced nicotinamide adenine dinucleotide phosphate (NADPH) exerts a neuroprotective effect in animal models of ischemic stroke, and its primary mechanism is related to anti-oxidative stress and improved energy metabolism. However, it is unknown whether nicotinamide adenine dinucleotide (NADH) also plays a neuroprotective role and whether NADPH is superior to NADH against ischemic stroke? In this study we compared the efficacy of NADH, NADPH, and edaravone in ameliorating brain injury and metabolic stress in ischemic stroke. Transient middle cerebral artery occlusion/reperfusion (t-MCAO/R) mouse model and in vitro oxygen glucose deprivation/reoxygenation (OGD/R) model were established. The mice were intravenously administered the optimal dose of NADPH (7.5 mg/kg), NADH (22.5 mg/kg), or edaravone (3 mg/kg) immediately after reperfusion. We showed that the overall efficacy of NADPH in ameliorating ischemic injury was superior to NADH and edaravone. NADPH had a longer therapeutic time window (within 5 h) after reperfusion than NADH and edaravone (within 2 h) for ischemic stroke. In addition, NADPH and edaravone were better in alleviating the brain atrophy, while NADH and NADPH were better in increasing the long-term survival rate. NADPH showed stronger antioxidant effects than NADH and edaravone; but NADH was the best in terms of maintaining energy metabolism. Taken together, this study demonstrates that NADPH exerts better neuroprotective effects against ischemic stroke than NADH and edaravone.
Similar content being viewed by others
Introduction
Stroke is the second most dangerous disease to human health, accounting for 9% of deaths worldwide, after ischemic heart disease [1]. Clinically, ischemic stroke accounts for 75%–80% of the incidence of stroke. Local ischemia following thromboembolism or occlusion of a cerebral artery results in metabolic stress of brain and loss of neurological functions. The pathogenesis of ischemic stroke is complex, among which excessive reactive oxygen species (ROS) produced by mitochondria is one of the most important pathogenic factors [2]. Studies have shown that ROS has multiple biological effects. Under normal physiological conditions, ROS participates in normal physiological processes, including cell signaling, mitotic induction, and immune defense. However, when vascular blood flow to the brain is impeded, the ROS is produced in large quantity, which attacks cellular macromolecules and induces damage to cellular structures, such as lipids, proteins and DNA [3, 4]. The brain accounts for about 2% body weight while consumes 20% of oxygen. Thus, brain neurons are highly dependent on the constant supply of oxygen and glucose for maintaining normal functions and are particularly vulnerable to lack of ATP supply. However, the rapid recovery of blood flow during reperfusion increases the level of tissue oxygenation, and the recovered O2 catalyzes the production of a large amount of ROS, by intracellular ROS-producing enzymes, which directly leads to a second burst of ROS and activates multiple cell death signaling pathways. Therefore, the clearance of ROS is of great significance in the treatment of ischemic stroke.
The current clinical treatments for stroke are thrombolysis and pharmacotherapy. Thrombolytic therapy has a relative narrow time window, largely due to increase in the risk of brain hemorrhage [5]. Drug therapy includes anticoagulants and free radical scavengers. Among them, drugs that antiplatelet aggregation such as aspirin and clopidogrel are very susceptible to cause bleeding while relieving the hypercoagulability of blood, which are only used in clinical as an adjunct therapy. Edaravone, a free radical scavenger, which can reduce infarct volume in stroke patients [6]. However, there is insufficient evidence that edaravone can reduce mortality or improve functional recovery in patients with ischemic stroke [7].
NADH is mainly produced by the citric acid cycle in glycolysis and cellular respiration, and it is closely related to mitochondrial function, energy metabolism, oxidative stress, and calcium homeostasis [8, 9]. Exogenous NADH can be transported into the cell through the P2X7 receptor across the membrane [10], and then decomposed into NAD+, hydrogen (H+) [10]. NADH not only increases the level of intracellular NAD+, hydrogen (H+) is also the most ideal substance that can effectively eliminate intracellular free radicals [8, 9, 11], and increase the production of ATP in mitochondria, so as to protect cells and tissues from oxidative damage [11, 12]. NADPH is a co-enzyme of glutathione reductase, which participates in the production of reduced glutathione (rGSH) to eliminate ROS. It also reactivates thioredoxin reductase and catalase as part of the antioxidant system [13, 14]. Our previous studies have shown a therapeutic effect of exogenous NADPH in rodent and primate models of ischemic stroke [15]. However, whether there is a similar therapeutic efficacy among NADH, NADPH, and edaravone in ischemic stroke has not been evaluated. Our aim was to investigate the efficacy and metabolic influence of NADH, NADPH, and edaravone in a rodent model of ischemic stroke.
Materials and methods
Animals and t-MCAO/R model
Male ICR mice (26–30 g, total = 911) were purchased from JOINN Laboratories Inc (Suzhou, China). All animal procedures were approved by the Ethical Committee of Soochow University and followed the NIH Guidelines for the Use and Care of Laboratory Animals.
T-MCAO/R model was described by Longa with minor modification [16]. The surgery was performed using the internal carotid artery line embolization method. Mice were anesthetized with 1.5% isoflurane inhalation and the right common carotid, internal and external arteries of the brain were isolated. A microvascular shear was used to cut an incision at the distal end of the external carotid artery and a silicone-coated nylon (6-0) monofilament (6023 Doccol Corporation, Redlands, CA, USA) was inserted to block the blood supply. When the blood flow was blocked for 2 h, the wire plug was removed and the blood supply was restored. Mice in the sham-operated group had the same procedure as those in the ischemia group, except that they were not given insertion of a wire bolt. During the whole operation, the temperature of the mouse anus was controlled at 37 °C by the automatic temperature-controlled heating pad.
Mice were randomly assigned to each group, and the exclusion criteria and mortality rates for each group are shown in Supplementary Table S1. All behavioral evaluations of the experimental animals were conducted by researchers who did not know the treatment of each animal.
Cell culture, oxygen and glucose deprivation/reoxygenation (OGD/R), and cell viability assay
Primary cultures of cortical neurons were prepared from ICR mouse embryos at 17 days of gestation as previously described [17]. All experiments were performed using cortical neurons which were cultured for 8 days in vitro. In order to model ischemia-like condition in vitro, oxygen and glucose deprivation/reoxygenation (OGD/R) was performed as described before [18]. The cell viability was evaluated with a Cell Counting Kit-8 (CCK-8; CK04, Dojindo, Japan). The primary cortical neurons were inoculated in 96-well plates with a density of ~1 × 105 cells/well. After 24 h of reoxygenation, 100 μL neuron medium containing 10 μL CCK-8 solution was added into each well, and the plates were incubated in a humid incubator (5% CO2, 37 °C) for 2 h. The absorbance of each well was detected at wavelengths of 450 nm with a scanning microplate reader (M1000 PRO, TECAN, Switzerland) [19].
Drug administration in vivo and in vitro
NADPH was purchased from Suzhou Ren Ben Pharmaceutical Co. (11185140, Basel, Switzerland, Suzhou, China). NADH was provided by Suzhou Ren Ben Pharmaceutical Co. (N05-180301, Suzhou, China). Edaravone was purchased from Simcere (80-180908, Nanjing, China). In vivo studies, NADH (2.5, 7.5, 22.5, and 67.5 mg/kg) and edaravone (1, 3, 10 mg/kg) were dissolved in 0.9% normal saline [20], and NADPH was dissolved in NaHCO3 buffer at pH = 9. The mice were given by tail vein injection at different time after reperfusion. Mice in the control group were given a corresponding dose of normal saline. In vitro studies, NADH (2.5, 5, 10, 20, 40 μM), NADPH (2.5, 5, 10, 20 μM), or edaravone (5, 10, 20, 40, 100, 200, 400 μM), dissolved in neuron medium, were given to cultured primary cortical neurons at the onset of reoxygenation. The control group did not perform the experiment of oxygen glucose deprivation and was cultured normally.
Measurement of infarct volume
The mice were anesthetized 24 h after t-MCAO/R and brains were divided into 5 coronal slices, then stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC, Sinopharm Chemical Reagent, Shanghai, China) for 15–20 min at 42 °C [21]. The infarct volume measurement method is shown in Fig. 1. The infarct volume was expressed as a percentage of total hemisphere.
The mice were anesthetized 24 h after t-MCAO/R and brains were sliced into 5 coronal sections, then stained with 1% TTC. After the TTC staining, the brain slices were fixed in 4% paraformaldehyde for 24 h, and then arranged in sequence on the glass slide and scanned with a scanner. The infarct area is white while the normal tissue is red. The volume of the infarcted brain tissue (white part) is taken as the percentage of the area of the normal hemi-brain tissue (red half-brain) as the index of cerebral infarction volume. As shown in the figure, infarct volume = \(\frac{{({\mathrm{S1 + S2 + S3 + S4 + S5}}) - ({\mathrm{s}}1 + {\mathrm{s}}2 + {\mathrm{s}}3 + {\mathrm{s}}4 + {\mathrm{s}}5)}}{{{\mathrm{S1 + S2 + S3 + S4 + S5}}}} \times 100\%\).
Assessment of neurological score
Neurological score was assessed 24 h after reperfusion by a researcher who was unaware of the experimental grouping and drug administration. After 24 h of reperfusion, neurological deficits were evaluated using a 5-point scoring standard as described before [22].
Measurement of brain water content
Mice were sacrificed 24 h after reperfusion. Their brains were rapidly dissected and immediately weighed to obtain wet weight, and then the dry weight was weighed after baking at 107 °C for 48 h. Brain water content was measured as follows: Percentage of brain water content = (wet weight − dry weight)/wet weight × 100% [23].
Behavioral tests
A battery of behavioral tests (Beam Walk Test, Rotarod test and Y maze test) was performed 28 days after post-t-MCAO/R as described before [24,25,26]. These tests were conducted by two investigators who were unaware of the experimental conditions.
Measurement of NADPH, NADP+, NADH, and NAD+ levels
Mice were intravenously administered NADPH (7.5 mg/kg), NADH (22.5 mg/kg), and edaravone (3 mg/kg). Mice (6 per group) were anesthetized with 1% pentobarbital sodium and then perfused with saline to remove blood in brains. The cerebral cortex is separated and homogenized for subsequent assays. Brain NADPH, NADP+ concentrations were measured with the enzychrom NADPH/NADP+ assay kit (BioAssay Systems, Hayward, CA, USA) following manufacturer’s instructions. Brain NADH, NAD+ concentrations were measured with the enzychrom NADH/NAD+ assay kit (BioAssay Systems).
Measurement of Ca2+, ROS, and MMP
The primary cortical neurons were deprived of oxygen and glucose for 4 h, and were reoxygenated with neuron medium containing 20 μM NADH, 10 μM NADPH, or 100 μM edaravone for 3 h. The fluorescent probe Fluo-3 AM (Beyotime, Shanghai, China) was used to detect the intracellular Ca2+ concentration. DCFH-DA (Beyotime) was used to assess the ROS level. The level of mitochondrial membrane potential (MMP) in cells was studied using the JC-1 assay kit and Mito Tracker Red CMXRos (Beyotime).
Measurement of LDH release and apoptosis
The primary cortical neurons were deprived of oxygen and glucose for 4 h, and were reoxygenated with neuron medium containing 20 μM NADH, 10 μM NADPH, or 100 μM edaravone for 24 h. LDH cytotoxicity assay kit (C0016, Beyotime) was used to detect extracellular LDH release. One step TUNEL apoptosis assay kit (C1086, Beyotime) was used to detect apoptosis.
Western blot analysis
Brain tissues from the ischemic cortex were dissected into RIPA lysis buffer. Total protein was extracted by ultrasound cell crusher cryogenic homogenator and the BCA kit (T9300A, Takara) was used to detect the protein concentration in each sample. The specific steps for Western blot analysis were described previously [27]. The following primary antibodies were used in the present study: mouse anti-nitrotyrosine (#05233, Millipore, Darmstadt, Germany), mouse anti-γH2AX (#ab2893, Abcam, Cambridge, UK), rabbit anti-4-hydroxynonenal (#ab46545, Abcam, Cambridge, UK) and mouse anti-GAPDH (#ab9484, Millipore, Darmstadt, Germany). Secondary antibodies used in Western blotting including anti-mouse IgG and anti-rabbit IgG (Li-Cor Bioscience, Lincoln, NE, USA). The protein bands were observed using the Odyssey Infrared Imaging System (Li-Cor Bioscience). After the images were collected, the protein expression was finally determined using the ImageJ Launcher software and normalized to a loading control GAPDH (National Institutes of Health, Bethesda, MD, USA).
Measurement of SOD, rGSH, MDA, H2O2, and ATP levels
SOD vitality, GSH/GSSG ratio and intracellular concentration of MDA, H2O2 and ATP were measured 3 h after reperfusion in ischemic brain tissue in mice. The vitality of SOD, the ratio of GSH/GSSG, and the levels of MDA, H2O2, and ATP were determined using a SOD assay kit (S0101, Beyotime), a GSH/GSSG assay kit (S0053, Beyotime), a MDA assay kit (S0131, Beyotime), a H2O2 assay kit (S0038, Beyotime) and a ATP assay kit (S0026, Beyotime) following the manufacturer’s instructions.
Statistical analysis
Data were expressed as means ± SD (standard deviation of the mean). One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to compare more than two groups; two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to compare two factors; paired or unpaired t-test was used to compare two groups. A value of P < 0.05 was regarded as statistically significant.
Results
NADPH shows better dose-response relationship than that of NADH and edaravone in ischemic stroke model
In order to verify the consistency of the model, we used a laser Doppler Monitor to detect the cerebral blood flow in mice during cerebral ischemia and at the early stage of reperfusion. We clearly observed the decrease in blood flow during cerebral ischemia in mice and the increase in cerebral blood flow during reperfusion (Supplementary Fig. S1), thus proving the reliability of our cerebral ischemia-reperfusion model.
To determine the dose-response relationships of NADPH, NADH, and edaravone in the treatment of mouse model of ischemic stroke, NADPH (2.5–22.5 mg/kg), NADH (2.5–67.5 mg/kg), or edaravone (1–10 mg/kg) was administered at the onset of reperfusion. It was found that infarct volume was 64.33% ± 8.445% in vehicle-treated group, 52.70% ± 8.668% (P < 0.05) in 2.5 mg/kg NADPH-treated group and 34.54% ± 11.65% (P < 0.001) in 7.5 mg/kg NADPH-treated group (Fig. 2a), but NADPH 22.5 mg/kg had no significant effect. NADPH (7.5 mg/kg) significantly alleviated neurological deficits and reduced brain water content in mice. Similarly, compared with the vehicle-treated group (62.56% ± 8.283%), 22.5 mg/kg NADH markedly reduced infarct volume (44.75% ± 9.634%, P < 0.001), alleviated neurological deficits and decreased brain water content in mice (Fig. 2b). Studies have shown that 3 or 10 mg/kg are the commonly used doses of edaravone in stroke treatment [7, 28,29,30]. Our present results found that compared with the vehicle-treated group (59.10% ± 7.580%), 3 mg/kg edaravone could significantly reduce the infarct volume (38.57% ± 7.601%, P < 0.001), alleviate neurological deficits and decrease brain water content in mice (Fig. 2c). These results confirmed that NADPH (7.5 mg/kg), NADH (22.5 mg/kg), and edaravone (3 mg/kg) were the optimal doses for the treatment of ischemic stroke in mice when given immediately after reperfusion.
Mice were subjected to the t-MCAO for 2 h and NADPH (2.5, 7.5, 22.5 mg/kg), NADH (2.5, 7.5, 22.5, 67.5 mg/kg), or edaravone (1, 3, 10 mg/kg) were administered to mice by tail vein injection immediately after reperfusion. a Representative images of TTC staining and quantitative analysis of cerebral infarction volume, neurological score, and brain water content in mice treated with NADPH 24 h after t-MCAO/R. *P < 0.05, ***P < 0.001 vs Vehicle (n = 8–17). ###P < 0.001 vs 7.5 mg/kg. b Representative images of TTC staining and quantitative analysis of cerebral infarction volume, neurological score, and brain water content in mice treated with NADH (n = 7–14) 24 h after t-MCAO/R. *P < 0.05, ***P < 0.001 vs Vehicle (n = 11). #P < 0.05, ##P < 0.01 vs 22.5 mg/kg. c Representative images of TTC staining and quantitative analysis of cerebral infarction volume, neurological score, and brain water content in mice treated with edaravone 24 h after t-MCAO/R. ***P < 0.001 vs Vehicle (n = 12–18). ##P < 0.01, ###P < 0.001 vs 3 mg/kg. Data were expressed as the Mean ± SD. Statistical comparisons were carried out with a one-way ANOVA followed by Tukey’s multiple comparisons test.
NADPH shows longer therapeutic window than that of NADH and edaravone in ischemic stroke model
To investigate their therapeutic time windows for treatment of ischemic stroke, NADPH (7.5 mg/kg), NADH (22.5 mg/kg), and edaravone (3 mg/kg) were administered to mice at different time points after reperfusion. TTC staining showed that NADPH (7.5 mg/kg) significantly reduced infarct volume when administered within 5 h after reperfusion (Fig. 3a). Similar results were obtained with brain water content and neurological score (Fig. 3a). In comparison, NADH (22.5 mg/kg) significantly reduced infarct volume, decreased brain water content and alleviated neurological deficits only when it was given within 2 h after reperfusion (Fig. 3b). edaravone (3 mg/kg) could only maintain efficacy within 2 h after reperfusion (Fig. 3c). These results suggested that NADPH, NADH, edaravone demonstrate the best protective effects when administered immediately after reperfusion. NADPH has a longer therapeutic time window than that of NADH and Edaravone.
Mice were subjected to the t-MCAO for 2 h and NADPH (7.5 mg/kg), NADH (22.5 mg/kg), or edaravone (3 mg/kg) was injected into the tail vein at indicated time after reperfusion. a Quantitative analysis of the effects of NADPH on infarction volume, neurological deficit score and brain water content 24 h after reperfusion. **P < 0.01, ***P < 0.001 vs Vehicle (n = 9–15). ###P < 0.001 vs 0 h. b Quantitative analysis of the effects of NADH on infarction volume, neurological deficit score, and brain water content 24 h after reperfusion. ***P < 0.001 vs Vehicle (n = 9–17). #P < 0.05, ###P < 0.001 vs 0 h. c Quantitative analysis of the effects of edaravone on infarction volume, neurological deficit score, and brain water content 24 h after reperfusion. ***P < 0.001 vs Vehicle (n = 15). ###P < 0.001 vs 0 h. Data were expressed as the Mean ± SD. Statistical comparisons were carried out with a one-way ANOVA followed by Tukey’s multiple comparisons test.
NADPH shows better neuroprotective effects than NADH or edaravone in ischemic stroke model
Based on the results in Figs. 2 and 3, we compared the efficacy in mitigating ischemic brain injury at the optimal dosages and therapeutic window of NADPH, NADH, and edaravone in ischemic stroke mice in the same experiment. The results showed that NADPH (7.5 mg/kg) significantly reduced infarct volume from 62.36% ± 7.269% (vehicle-treated) to 27.78% ± 8.465% (P < 0.001). The infarction volume of the mice injected with NADH (22.5 mg/kg) was reduced to 46.33% ± 8.058% (P < 0.001), while it was 41.62% ± 7.384% (P < 0.001) in edaravone (3 mg/kg)-treated mice (Fig. 4a, b). The results showed that all of them significantly reduced infarct volume in mice 24 h after reperfusion to a certain extent, while the magnitude of the effects of NADH and edaravone were significantly lower than that of NADPH (P < 0.001). These results suggested that NADPH is more effective than NADH or edaravone in reducing the infarct volume in mice. In addition, administration of NADH, NADPH, and edaravone all improved the neurological deficits and reduced brain water content in ischemic stroke mice 24 h after reperfusion. NADPH was better than NADH and edaravone in reducing brain water content in mice (Fig. 4c, d).
Mice were subjected to the t-MCAO for 2 h, NADPH (7.5 mg/kg) and NADH (22.5 mg/kg), or edaravone (3 mg/kg) were administered to mice immediately after reperfusion. a, b TTC staining was used to measure the cerebral infarct volume. c The neurological deficit score and d brain water content 24 h after t-MCAO/R. *P < 0.05, ***P < 0.001 vs Vehicle (n = 8–15); #P < 0.05, ##P < 0.01, ###P < 0.001 vs NADPH (7.5 mg/kg). Data were expressed as the Mean ± SD. Statistical comparisons were carried out with a one-way ANOVA followed by Tukey’s multiple comparisons test.
NADPH shows overall better long-term benefits in ischemic stroke model than NADH or edaravone
To further compare the long-term effects of NADPH, NADH, and edaravone on ischemic stroke in mice, we evaluated the long-term brain histology changes and functional recovery after ischemia-reperfusion injury in mice. NADPH (7.5 mg/kg), NADH (22.5 mg/kg), and edaravone (3 mg/kg) were injected into the tail vein of mice immediately after reperfusion and the neurological recovery was measured 28 days after surgery. One month later, brains were taken to observe the brain atrophy of the mice in each group (Fig. 5a). The long-term survival curves showed that the long-term survival rate of mice subjected to ischemic stroke was increased in all treated groups, and the survival rate of NADPH and NADH groups was higher than that of edaravone, reaching 40.0% and 41.6%, respectively. Edaravone was only around 32.0% (Fig. 5b). Moreover, NADPH, NADH, and edaravone all markedly alleviated the brain atrophy of ischemic hemisphere 30 days after t-MCAO/R. In NADPH-treated group there was 27.46% ± 5.844% of brain atrophy and was the smallest among the three tested drugs (Fig. 5c). These results confirmed that NADPH and edaravone were better than NADH in improving brain atrophy, while NADH and NADPH were better than edaravone in increasing the survival rate. Besides, administration of NADPH, NADH or Edaravone also significantly improved the recovery of neurological functions 28 days post-t-MCAO/R, while NADPH showed more robust effects than that of NADH and Edaravone (Fig. 5d–f).
a Experimental design. Mice were subjected to the t-MCAO for 2 h and NADPH (7.5 mg/kg), NADH (22.5 mg/kg), and edaravone (3 mg/kg) were administered to mice immediately after reperfusion. Neurobehavioral tests were performed in mice 28 days after post-stroke, and brain atrophy was measured one month after t-MCAO. b Post-stroke survival rate 30 day after t-MCAO/R. c Representative images of morphology of mice brains and quantitative analysis of the brain shrinkage 30 days post-stroke. *P < 0.05, **P < 0.01, ***P < 0.001. d–f Beam balance test, Rota-rod test, and Y-maze test 28 days after t-MCAO/R. *P < 0.05, ***P < 0.001 vs Vehicle (n = 6); ###P < 0.001 vs Sham (n = 10). NADPH group (n = 12), NADH group (n = 10), edaravone group (n = 8). Data were expressed as the Mean ± SD. Statistical comparisons were carried out with a one-way ANOVA followed by Tukey’s multiple comparisons test.
The effects of NADPH, NADH, and edaravone on NADP+/NADPH, NAD+/NADH in the brain
According to our previous study, tail vein injection of NADPH can significantly increase the level of NADPH in the brain [15]. It has been reported that exogenous NADH can significantly increase the intracellular NAD+ level [31]. NADPH (7.5 mg/kg), NADH (22.5 mg/kg) or edaravone (3 mg/kg) were administered at the beginning of reperfusion. After 3 h of reperfusion, the level of NAD(P)H, NAD(P)+ in brain was detected. We found that NADPH treatment significantly inhibited the I/R-induced decrease in NADPH levels and the decrease in the ratio of NADPH/NADP+ (Fig. 6a, b). Tail vein injection of NADH can significantly increase the levels of NADH and NAD+ in the brain after I/R (Fig. 6c, d). Edaravone has no significant effect on the decrease of NAD(P)H/NAD(P)+ metabolism in the brain induced by I/R.
Mice were subjected to the t-MCAO for 2 h and NADPH (7.5 mg/kg), NADH (22.5 mg/kg), or edaravone (3 mg/kg) were administered to mice immediately after reperfusion. After reperfusion for 3 h and then mice were perfused with saline to remove blood in brains. NADPH, NADP+, NADH, NAD+ concentration was measured in brain homogenate. a–d The changes of NADPH, NADPH/NADP+, NADH, NAD+ levels in ischemic cortex of mouse brain 3 h after reperfusion. n = 6. ***P < 0.001 vs Sham; ###P < 0.001 vs Vehicle. Data were expressed as the Mean ± SD. Statistical comparisons were carried out with a one-way ANOVA followed by Tukey’s multiple comparisons test.
NADPH, NADH, and edaravone improve mitochondrial function and inhibit apoptosis in vitro
Under the ischemic condition, energy metabolism will be impaired. In current studies, NADPH (7.5 mg/kg), NADH (22.5 mg/kg), or edaravone (3 mg/kg) were administered after t-MCAO/R, and the ATP levels in cortical tissues were detected 3 h after reperfusion. We found that the decrease of ATP level induced by ischemic stroke was significantly reversed in all the treatment groups, and the increase in ATP level in NADH group (138.6% ± 16.94%) was the most robust, which was statistically different from that in NADPH (82.13% ± 30.24%) or Edaravone-treated (83.99% ± 37.50%) group (Fig. 7a).
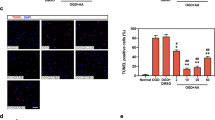
a The levels of ATP in the cortical tissues 3 h after t-MCAO/R in mice. ***P < 0.001 vs Sham; #P < 0.05, ###P < 0.001 vs Vehicle; &&&P < 0.001 vs NADH-treated group. n = 8. b The levels of ATP in the primary cortical neurons 3 h after reoxygenation. ***P < 0.001 vs Non-OGD/R-Con; ###P < 0.001 vs OGD/R-Con; &&&P < 0.001 vs OGD/R-NADH. n = 6. c MMP 3 h after OGD/R. R/G represents the JC-1 polymer/monomer ratio. d Representative fluorescence images of MMP in cells treated with NADPH, NADH, and edaravone 3 h after reoxygenation. Scale bar = 50 µm. ***P < 0.001 vs Non-OGD/R-Con; ###P < 0.001 vs OGD/R-Con. n = 6. e The levels of intracellular Ca2+ in the primary cortical neurons. ***P < 0.001 vs Non-OGD/R-Con; ###P < 0.001 vs OGD/R-Con. f The primary cortical neurons were treated with NADPH (10 μM), NADH (20 μM), or edaravone (100 μM) at the onset of reoxygenation, and the cell viability was detected with CCK-8 assay kit 24 h after reoxygenation. *P < 0.05, **P < 0.01 and ***P < 0.001 vs OGD/R-Con. n = 6. g The effects of NADPH (10 μM), NADH (20 μM), or edaravone (100 μM) on the LDH release (lactate dehydrogenase) from primary cortical neurons 24 h after OGD/R. ***P < 0.001 vs Non-OGD/R-Con; ###P < 0.001 vs OGD/R-Con; $$P < 0.01 vs OGD/R-NADPH. n = 12. h The effect of NADPH (10 μM), NADH (20 μM), or edaravone (100 μM) on the apoptosis of primary cortical neurons 24 h after OGD/R. Scale bar = 50 µm. ***P < 0.001 vs Non-OGD/R-Con; ###P < 0.001 vs OGD/R-Con; $$P < 0.01, $$$P < 0.001 vs OGD/R-NADPH. n = 12. Data were expressed as the Mean ± SD. Statistical comparisons were carried out with a one-way ANOVA followed by Tukey’s multiple comparisons test.
To investigate whether NADH, NADPH, or edaravone improves metabolism in neurons, cultured primary cortical neurons were treated with indicated concentrations of NADPH, NADH, or Edaravone at the onset of reoxygenation, and the cell viability was detected 24 h after reoxygenation with CCK-8. It was found that NADPH reduced cell death after OGD/R in a dose-dependent manner, with the best effect was observed at 10 µM. Similarly, the addition of 20 µM NADH and 100 µM Edaravone could significantly promote the cell survival (Supplementary Fig. S2). Then we measured the intracellular ATP level after OGD/R 3 h. The results of in vitro experiments are consistent with animal studies (Fig. 7b). The MMP was observed using the fluorescent probes JC-1 and Mito-Tracker Red CMXRos. The JC-1 polymer/monomer ratio was used to indicate the level of MMP. It was found that in the model group, the MMP was decreased 3 h after reoxygenation in primary cortical neurons. NADPH (10 μM), NADH (20 μM), and edaravone (100 μM) could significantly inhibit the reduction of MMP induced by OGD/R (Fig. 7c). Mito-Tracker Red CMXRos fluorescence studies revealed that the red fluorescence of mitochondria was attenuated 3 h after reoxygenation, indicating a decrease in the level of MMP. While NADPH, NADH, and edaravone all preserved MMP, and the preservative effect in MMP was most pronounced in the NADPH-treated group (Fig. 7d). Ca2+ plays an important role in maintaining cell membrane potential and nerve conductivity. NADPH, NADH, and edaravone can inhibit the Ca2+ influx induced by OGD/R (Fig. 7e).
Next, we evaluated the effects of NADPH, NADH, and edaravone on neuronal death induced by OGD/R. The results showed that after OGD/R 24 h, neuronal cell viability decreased, LDH release increased, and neuronal apoptosis increased. NADPH, NADH, and Edaravone treatment significantly improved the cell viability of neurons after reoxygenation, inhibited the release of LDH and reduced neuronal apoptosis (Fig. 7f–h). Among them, NADPH has the best effect.
NADPH, NADH, and edaravone reduce oxidative damage caused by ischemia/reperfusion
Oxidative stress is a key pathological factor of brain injury induced by ischemic stroke. In this work, we compared the antioxidant capacity of NADPH (7.5 mg/kg), NADH (22.5 mg/kg), and edaravone (3 mg/kg) by detecting the SOD vitality, the ratio of GSH/GSSG, and the levels of MDA and H2O2 in ischemic cortex of mice. We found that NAPDH, NADH, and edaravone all remarkably reversed the decrease of SOD vitality and the increase of MDA level in the brains of mice induced by ischemia/reperfusion. Among them, the level of increased SOD vitality and decreased MDA by NADPH was significantly better than that by NADH or Edaravone (Fig. 8a, b). The ratio of GSH/GSSG decreased after ischemic stroke and NADPH was effective in suppressing such change, but edaravone or NADH had no effect (Fig. 8c). In addition, NADH, NADPH, and edaravone all significantly reversed the increase of H2O2 level induced by ischemia/reperfusion in mice (Fig. 8d). Then, we labeled the ROS with DCFH2-DA fluorescent probe and the results showed that the intracellular ROS levels rapidly increased after OGD/R, while NADPH (10 µM), NADH (20 µM), and edaravone (100 µM) all markedly suppressed the rise in ROS in the primary cortical neurons (Fig. 8e).
a The total SOD vitality in the cortical tissues 3 h after reperfusion in mice. b The content of lipid peroxidation product MDA in the cortical tissues 3 h after reperfusion in mice. c The ratio of GSH/GSSG in the cortical tissues 3 h after reperfusion in mice. d The levels of H2O2 in the cortical tissues 3 h after reperfusion in mice. ***P < 0.001 vs Sham; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs Vehicle; $P < 0.05, $$P < 0.01 vs NADPH-treated group. n = 8. e The levels of ROS in primary cortical neurons 3 h after reoxygenation detected by DCFH2-DA staining. Representative fluorescence images of DCFH2-DA in cells treated with NADPH (10 µM), NADH (20 µM), or edaravone (100 µM) 3 h after reoxygenation. Scale bar = 50 µm. ***P < 0.001 vs Non-OGD/R-Con; ###P < 0.001 vs OGD/R-Con. n = 12. f Representative immunoblots and quantification of Nit, 4-HNE, and γ-H2AX levels in mice 24 h after t-MCAO/R. *P < 0.05, ***P < 0.001 vs Sham; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs Vehicle. n = 8. Data were expressed as the Mean ± SD. Statistical comparisons were carried out with a one-way ANOVA followed by Tukey’s multiple comparisons test.
Furthermore, we compared NADH, NADPH, and edaravone on oxidative injury in macromolecules in ischemic tissue in mice. Nitrotyrosine (Nit) is a marker of oxidative protein injury, 4-hydroxynonenal (4-HNE) is a marker of lipid peroxidation, and γ-H2AX is related to oxidative DNA damage. The results suggested that in the ischemic cortex in mice, the protein levels of Nit, 4-HNE and γ-H2AX were all up-regulated after t-MCAO/R and reached a peak at 24 h after reperfusion (Supplementary Fig. S4). NADPH (7.5 mg/kg) or edaravone (3 mg/kg) significantly decreased the levels of those proteins, and there was no statistical significance in NADH (22.5 mg/kg) group (Fig. 8f). All these results suggest that NADPH, NADH, and edaravone all can reduce the oxidative damage caused by ischemic stroke. Overall, NADPH is more potent in fighting against oxidative stress.
Discussion
Stroke remains the leading cause of death and long-term disability in China. It has been reported that more than 6 million stroke patients die each year and 5 million have permanent disabilities worldwide. When cerebral ischemia occurs, the interruption of local cerebral blood supply leads to the damage of brain function, and the ischemic core area of the brain is surrounded by the penumbra infarcted area. The penumbra is supplied by a collateral artery that anastomoses with an occluded vascular branch. Therefore, timely recanalization of occluded blood vessels can save penumbra tissue and improve neurological function. Theoretically, the best treatment for ischemic stroke is to restore the blood perfusion in the ischemic area. However, many research data indicate that the rapid recovery of blood flow may increase the level of tissue oxygenation, and the reacquired O2 catalyzes the production of large amounts of ROS by intracellular enzymes, which exacerbates oxidative damage and even causes cell cycle arrest, cell necrosis and apoptosis [4, 32]. The typical drug capable of recanalization of occluded blood vessels is tPA, but this drug has a relative narrow therapeutic window and high risk of brain hemorrhage [28, 29]. Therefore, many studies have focused on the rescue of affected neurons after ischemia, that is, use of neuroprotective drugs, but such drugs often fail in clinical trials [7]. In addition to pathophysiological factors in stroke attack may limit the drug actions, lack of efficacy of neuroprotectants in clinical trials could also be due to unfavorable chemical or physical properties of these drugs.
At the present, edaravone is the one of clinically proved brain protectant, which can reduce the infarct volume in patients and improve the recovery of neurological function. Recently, abundant evidence has elucidated that NADPH and NADH play key roles in maintaining redox homeostasis, energy metabolism, mitochondrial function, gene expression, and signaling pathways in cells [6, 7]. Previous studies in our laboratory have shown that NADPH regenerates rGSH to reduce ROS, protects mitochondria and reduces neuronal inflammation [15, 20], thus protects neuronal cells in animal models of ischemic stroke. NADH is a reduced form of co-enzyme I, it is generated through transferring a H+ to a NAD+ by mitochondiral complex I [8, 14]. NADH in the cytoplasm can enter the mitochondrial oxidative respiratory chain through malate-aspartate shuttle and 3-phosphoglycerol shuttle to oxidize water and produce energy substance ATP, which is involved in cellular energy metabolism [33]. Therefore, we thought that NADH and NADPH share multiple biological functions, and NADH may have a therapeutic effect on ischemic stroke also. However, the similarity and difference of pharmacological actions between NADPH, NADH, and edaravone in the treatment of ischemic stroke need to be characterized. Our present results demonstrated that NADPH, NADH and Edaravone all reduced infarct volume in t-MCAO/R mice, thus confirming the therapeutic effect of NADH on ischemic stroke. Meanwhile, we found that NADPH had better efficacy than NADH or edaravone in the treatment of ischemic stroke in mice. However, there was no significant difference between NADH and Edaravone.
Thrombolytic therapy is currently a clinical method for the treatment of ischemic stroke. In addition to increasing the risk of hemorrhagic stroke, the biggest disadvantage of thrombolytic therapy is that the therapeutic time window is narrow, which is only effective in the early stage of ischemia/reperfusion. However, as we all know that patients with ischemic stroke often past their optimal thrombolytic period when they are admitted to hospital, which greatly limits thrombolytic therapy and its clinical benefits. Therefore, we aimed to find drugs that have a longer therapeutic time window to break the limitations of thrombolytic therapy. In the present study, we found that NADPH could maintain efficacy for 5 h after reperfusion, while NADH and edaravone could only maintain effectiveness for 2 h after reperfusion. This suggests that NADPH has a more significant therapeutic advantage in strokes with advanced ischemia/reperfusion. Due to the sudden onset of stroke, inconvenient transportation and the need for differential diagnosis in hospital, patients from the onset to treatment is often two hours later, so the clinical application value of drugs with longer therapeutic time window is advantageous.
The long-term survival and neurological function recovery in patients with ischemic stroke are important indicators of clinical benefits of stroke treatment. In this study, we found that NADPH and NADH were better than edaravone in increasing the survival rate, which was consistent with the lack of evidence that edaravone could reduce the death rate of patients with ischemic stroke [29]. Moreover, the present results demonstrated that NADPH was more effective than NADH and edaravone in reducing brain atrophy, improving the motor and cognitive functions in mice about one month post-t-MCAO/R. The current results suggest that NADPH is superior to NADH or edaravone in ameliorating brain injury in ischemic stroke mice, both in acute outcomes and in long-term prognosis.
The current study demonstrated that exogenous intake of NADH can significantly increase the level of intracellular NAD+ [31]. NADH has stronger protective effect on cells and can significantly reduce cell mortality by treating cells with NADH and NAD+ at the same concentration [10]. NADH is relatively unstable, and after entering the cell, it will quickly decompose into NAD+, hydrogen (H+). Recent studies have also found that by adding H+ to NAD+ precursors NR and NMN to turn them into NRH and NMNH, the efficiency of their conversion to NAD+ can be significantly improved [34]. Regardless of whether it is NRH or NMNH, it needs to be converted into NADH first, and then into NAD+. Therefore, NADH is the most effective NAD+ supplement. We measured the levels of NAD(P)H and NAD(P)+ in the brain of mice after I/R. The results showed that exogenous NADH could significantly increase the levels of NADH and NAD+ in the brain. NADPH treatment can also significantly increase the level of NADPH in the brain. We also tested the effects of NADH, NADPH, and edaravone on OGD/R-induced mitochondrial dysfunction in neurons. The results showed that NADH, NADPH, and edaravone could significantly inhibit OGD/R-induced Ca2+ influx, increase MMP, inhibit ROS production and increase ATP, thus protecting mitochondrial function. NADH, NADPH, and edaravone can also inhibit the decline of neuronal cell viability induced by OGD/R, inhibit the release of LDH and reduce neuronal apoptosis. Therefore, NADH can not only improve cell energy metabolism after ischemic stroke, but also can inhibit oxidative stress by decomposing into NAD+, protect mitochondrial function, and reduce cerebral ischemia-reperfusion injury.
It has been shown that after ischemic stroke, the endogenous ROS of cells are mainly derived from NADPH oxidase (NOX) and mitochondrial oxidative respiratory chain [35]. As an H+ donor, NADH provides electrons to participate in mitochondrial oxidative phosphorylation and promotes the production of ATP. As an antioxidant co-enzyme, NADPH in mitochondrial is essential for the maintaining reductive power of mitochondria, while NADPH also plays an important role in the biosynthesis of many substances, such as nucleotides, lipids, and amino acids [8, 15, 36]. The current results indicated that NADH was better than NADPH or edaravone in maintaining energy metabolism, whereas the NADPH was better than that of NADH or edaravone in antioxidant effects. Moreover, studies have shown that after ischemia/reperfusion, excessive ROS can react with protein molecules, resulting in peptide bond breakage and protein denaturation [37]; under severe condition, it will lead to oxidative degradation of lipids, which is more harmful. In addition, ROS can also destroy the DNA double strand, resulting in DNA oxidative damage [38, 39]. Our results found that oxidative damage related proteins Nit, 4-HNE and γ-H2AX were all activated after ischemic stroke. Compared with NADH and edaravone, the level of these proteins in NADPH-treated groups decreased more robustly, which also confirmed that the antioxidant effects of NADPH were stronger than that of NADH or edaravone. However, NADH was more effective in recovering energy levels.
In conclusions, we have demonstrated that NADPH, NADH, and edaravone have protective effects on ischemia-reperfusion injury in both rodent and cellular stroke models. Our current studies suggest that NADPH is superior to NADH or edaravone in the treatment of ischemic stroke, whether in acute efficacy trials, therapeutic time window studies, or long-term neurological function recovery. Meanwhile, NADPH has better antioxidant effects than NADH, and in terms of energy metabolism, NADH is better than NADPH. Overall, NADPH shows better neuroprotective effects in ischemic stroke. This study may open a new avenue for the development of novel drugs for ischemic stroke.
References
Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Matamala JM, Carrasco R, Miranda-Merchak A, et al. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug targets. 2013;12:698–714.
Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38:1167–86.
Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–70.
Yang J, Qi J, Xiu B, Yang B, Niu C, Yang H. Reactive oxygen species play a biphasic role in brain ischemia. J Invest Surg. 2019;32:97–102.
Knecht T, Story J, Liu J, Davis W, Borlongan CV, Dela Peña IC. Adjunctive therapy approaches for ischemic stroke: innovations to expand time window of treatment. Int J Mol Sci. 2017;18:2756.
Enomoto M, Endo A, Yatsushige H, Fushimi K, Otomo Y. Clinical effects of early edaravone use in acute ischemic stroke patients treated by endovascular reperfusion therapy. Stroke. 2019;50:652–8.
Yang J, Cui X, Li J, Zhang C, Zhang J, Liu M. Edaravone for acute stroke: Meta-analyses of data from randomized controlled trials. Dev Neurorehabil. 2015;18:330–5.
Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206.
Goodman RP, Calvo SE, Mootha VK. Spatiotemporal compartmentalization of hepatic NADH and NADPH metabolism. J Biol Chem. 2018;293:7508–16.
Lu H, Burns D, Garnier P, Wei G, Zhu K, Ying W. P2X7 receptors mediate NADH transport across the plasma membranes of astrocytes. Biochem Biophys Res Commun. 2007;362:946–50.
Yang L, Lin X, Tang H, Fan Y, Zeng S, Jia L, et al. Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD+ redox. Aging Cell. 2020;19:e13206.
Bertoldo MJ, Listijono DR, Ho WJ, Riepsamen AH, Goss DM, Richani D, et al. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep. 2020;30:1670–81.e7.
Buettner GR, Wagner BA, Rodgers VG. Quantitative redox biology: an approach to understand the role of reactive species in defining the cellular redox environment. Cell Biochem Biophys. 2013;67:477–83.
Xiao W, Wang RS, Handy DE, Loscalzo J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid Redox Signal. 2018;28:251–72.
Li M, Zhou ZP, Sun M, Cao L, Chen J, Qin YY, et al. Reduced nicotinamide adenine dinucleotide phosphate, a pentose phosphate pathway product, might be a novel drug candidate for ischemic stroke. Stroke. 2016;47:187–95.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91.
Marutani E, Kosugi S, Tokuda K, Khatri A, Nguyen R, Atochin DN, et al. A novel hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonist prevents ischemic neuronal death. J Biol Chem. 2012;287:32124–35.
Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, et al. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8:310–25.
Chen J, Zhang DM, Feng X, Wang J, Qin YY, Zhang T, et al. TIGAR inhibits ischemia/reperfusion-induced inflammatory response of astrocytes. Neuropharmacology. 2018;131:377–88.
Huang Q, Sun M, Li M, Zhang D, Han F, Wu JC, et al. Combination of NAD+ and NADPH offers greater neuroprotection in ischemic stroke models by relieving metabolic stress. Mol Neurobiol. 2018;55:6063–75.
Wu JY, Li M, Cao LJ, Sun ML, Chen D, Ren HG, et al. Protease Omi cleaving Hax-1 protein contributes to OGD/R-induced mitochondrial damage in neuroblastoma N2a cells and cerebral injury in MCAO mice. Acta Pharmacol Sin. 2015;36:1043–52.
Crupi R, Di Paola R, Esposito E, Cuzzocrea S. Middle cerebral artery occlusion by an intraluminal suture method. Methods Mol Biol. 2018;1727:393–401.
Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–8.
Meyer M, Kruse MS, Garay L, Lima A, Roig P, Hunt H, et al. Long-term effects of the glucocorticoid receptor modulator CORT113176 in murine motoneuron degeneration. Brain Res. 2020;1727:146551.
Kraeuter AK, Guest PC, Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 2019;1916:105–11.
Yamada K, Tanaka T, Zou LB, Senzaki K, Yano K, Osada T, et al. Long-term deprivation of oestrogens by ovariectomy potentiates beta-amyloid-induced working memory deficits in rats. Br J Pharmacol. 1999;128:419–27.
Zhou Y, Wu J, Sheng R, Li M, Wang Y, Han R, et al. Reduced nicotinamide adenine dinucleotide phosphate inhibits MPTP-induced neuroinflammation and neurotoxicity. Neuroscience. 2018;391:140–53.
Herbet M, Natorska-Chomicka D, Ostrowska M, Gawrońska-Grzywacz M, Izdebska M, Piątkowska-Chmiel I, et al. Edaravone presents antidepressant-like activity in corticosterone model of depression in mice with possible role of Fkbp5, Comt, Adora1 and Slc6a15 genes. Toxicol Appl Pharmacol. 2019;380:114689.
Matsumoto S, Murozono M, Kanazawa M, Nara T, Ozawa T, Watanabe Y. Edaravone and cyclosporine A as neuroprotective agents for acute ischemic stroke. Acute Med Surg. 2018;5:213–21.
Hua K, Sheng X, Li TT, Wang LN, Zhang YH, Huang ZJ, et al. The edaravone and 3-n-butylphthalide ring-opening derivative 10b effectively attenuates cerebral ischemia injury in rats. Acta Pharmacol Sin. 2015;36:917–27.
Zhu K, Swanson RA, Ying W. NADH can enter into astrocytes and block poly(ADP-ribose) polymerase-1-mediated astrocyte death. Neuroreport. 2005;16:1209–12.
Bylicky MA, Mueller GP, Day RM. Mechanisms of endogenous neuroprotective effects of astrocytes in brain injury. Oxid Med Cell Longev. 2018;2018:6501031.
Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223.
Giroud-Gerbetant J, Joffraud M, Giner MP, Cercillieux A, Bartova S, Makarov MV, et al. A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Mol Metab. 2019;30:192–202.
Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–8.
Cao L, Zhang D, Chen J, Qin YY, Sheng R, Feng X, et al. G6PD plays a neuroprotective role in brain ischemia through promoting pentose phosphate pathway. Free Radic Biol Med. 2017;112:433–44.
Niatsetskaya ZV, Sosunov SA, Matsiukevich D, Utkina-Sosunova IV, Ratner VI, Starkov AA, et al. The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice. J Neurosci. 2012;32:3235–44.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317.
Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu W, et al. Oxidative stress and DNA damage after cerebral ischemia: potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2018;134:208–17.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81730092), the Natural Science Foundation of Jiangsu Province of China (BK20180207), Jiangsu Key Laboratory of Neuropsychiatric Diseases (BM2013003) and the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD).
Author information
Authors and Affiliations
Contributions
ZHQ contributed to the conception and design; XXW, FW, GHM, JCW, ML, RH, JS and RZ contributed to the acquisition of data or analysis and interpretation of data; XXW and FW drafted the paper; RS, ZC, and ZHQ supervised the experiments. All authors read and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Xx., Wang, F., Mao, Gh. et al. NADPH is superior to NADH or edaravone in ameliorating metabolic disturbance and brain injury in ischemic stroke. Acta Pharmacol Sin 43, 529–540 (2022). https://doi.org/10.1038/s41401-021-00705-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00705-5
Keywords
This article is cited by
-
NADH intraperitoneal injection prevents massive pancreatic beta cell destruction in a streptozotocin-induced diabetes in rats
Histochemistry and Cell Biology (2024)
-
Monocyte, neutrophil, and whole blood transcriptome dynamics following ischemic stroke
BMC Medicine (2023)
-
RIPK1 inhibition contributes to lysosomal membrane stabilization in ischemic astrocytes via a lysosomal Hsp70.1B-dependent mechanism
Acta Pharmacologica Sinica (2023)
-
Angiogenesis after ischemic stroke
Acta Pharmacologica Sinica (2023)
-
Humanized cerebral organoids-based ischemic stroke model for discovering of potential anti-stroke agents
Acta Pharmacologica Sinica (2023)