Abstract
Hedgehog plays an important role in a wide range of physiological and pathological conditions. Paracrine activation of Hedgehog pathway in stromal cells increases the expression of VEGF, which promotes neovascularization in colorectal cancer and ultimately the growth of colorectal cancer. Berberine (BBR) has anticancer activity. In this study we investigated whether BBR inhibited the growth of colon cancer through suppressing the paracrine sonic hedgehog (SHH) signaling in vitro and in vivo. We showed that BBR (1–10 μM) dose-dependently inhibited the secretion and expression of SHH protein in HT-29 and SW480 cells. BBR did not influence the transcription of SHH, but promoted the degradation of SHH mRNA, thus decreased the SHH mRNA expression in the colorectal cancer cells. In nude mice bearing HT-29 xenograft, oral administration of BBR (100 mg · kg−1 · d−1) or a positive control drug GDC-0449 (100 mg · kg−1 · d−1) for 4 weeks markedly suppressed the growth of HT-29 tumor with BBR exhibiting a better antitumor efficacy. The tumor growth inhibition caused by BBR or GDC-0449 was comparable to their respective inhibitory effect on the mouse-specific Gli mRNA expression in the tumor. However, BBR (20 μM) did not affect the expression of human transcription factor Gli1 mRNA in HT-29 and SW480 cells. In conclusion, BBR promotes the degradation of SHH mRNA in colorectal cancer cells, interrupting the paracrine Hedgehog signaling pathway activity thus suppresses the colorectal cancer growth. This study reveals a novel molecular mechanism underlying the anticancer action of BBR.
Similar content being viewed by others
Introduction
Hedgehog (Hh) is a type of segmented gene that was first identified by genetic screens in Drosophila melanogaster [1]. Vertebrates have three homologous Hh genes: sonic Hh (SHH), Indian Hh, and desert Hh [1,2,3]. In the absence of Hh ligand binding, the patched (PTCH) receptor, which has 12 transmembrane domains, inhibits the activity of the SMO protein, which is another receptor possessing 7 transmembrane domains, thereby suppressing the transcription of downstream target genes [1, 4]. Upon binding by ligands, the inhibitory effect of PTCH on SMO is relieved, subsequently eliminating the inhibitory effect of sufu on GLI protein. This ultimately causes the translocation of the transcription factor GLI into the nucleus to initiate the transcription of target genes such as Gli1, Ptch1, twist1, and VEGF. Hh plays an important role in a wide range of physiological and pathological conditions, such as embryonic development, organ formation, injury and repair, and tumor cell proliferation, survival, angiogenesis, and metastasis [5].
It has been confirmed that Hh activity in colorectal cancer is mainly promoted in a paracrine ligand-dependent manner [6,7,8]. Colorectal cancer cells show aberrant expression of Hh signaling ligands. Hh ligands secreted by colorectal cancer cells activate the Hh transcription factor Gli in stromal cells of colorectal cancer tissues. The elevated Hh activity in stromal cells increases the expression of VEGF, which further promotes neovascularization in colorectal cancer and ultimately fuels the growth of colorectal cancer.
Berberine (BBR), an isoquinoline alkaloid, is an active component of numerous medicinal plants [9] and it comprises up to 2.58%–5.74% of the content of various tissues of Coptischinensis Franch [10]. Accumulating evidence has shown that it also has antitumor effects [9, 11]. Wang et al. reported that BBR could potentially suppress the Hh pathway and Hh-dependent medulloblastoma growth by targeting the critical component Smo of the Hh signaling pathway [12]. This study aimed to investigate whether BBR can inhibit colon cancer growth by inhibiting paracrine Hh signaling pathway activity.
Materials and methods
Reagents and antibodies
BBR (purity > 98%, B107341) was purchased from Aladdin; GDC-0449 and Robotnikinin were purchased from Selleck, USA. BAY11–8072 was purchased from Beyotime (Suzhou, China). The ELISA kit was purchased from Abnova, Taipei (China); the dual-luciferase reporter assay kit was purchased from Promega, USA. The 8 × 3 Gli-binding luciferase reporter was a gift from Dr Hiroshi Sasaki; the pRL-Renilla luciferase plasmid was purchased from Promega, USA, and the Lipo2000 liposome transfection kit was purchased from Life Tech, USA. The SHH antibody was purchased from Santa Cruz, USA, and the GAPDH antibody was purchased from Bioworld.
Cell lines and cell culture
The HT-29, SW480, and NIH3T3-Light2 cell lines were purchased from ATCC (American Type Culture Collection). All these cells were routinely cultured according to the manufacturer’s instructions.
Quantitative reverse transcription–polymerase chain reaction analysis
Total RNA was extracted from cells or xenografted HT-29 tumors using TRIzol reagent (Takara; Dalian, China) following the manufacturer’s protocol. The primers used in this experiment were synthesized by Beijing Dingguo Co., Ltd. The sequences are as follows:
hGUSB: Forward: 5′-TGGTTGGAGAGCTCATTTGGA-3′,
Reverse: 5′-GCACTCTCGTCGGTGACTGTT-3′,
hSHH: Forward: 5′-CAAGCAGTTTATCCCCAATGTG-3′,
Reverse: 5′-TCACCCGCAGTTTCACTC-3′,
hGLI1: Forward: 5′-ATCCTTACCTCCCAACCTCTGT-3′,
Reverse: 5′-AACTTCTGGCTCTTCCTGTAGC-3′,
mGusb: Forward: 5′-CTGCCACGGCGATGGA-3′,
Reverse: 5′-ACTGCATAATAATGGGCACTGTTG-3′,
mGli1: Forward: 5′-GCAGTGGGTAACATGAGTGTCT-3′,
Reverse: 5′-AGGCACTAGAGTTGAGGAATTGT-3′.
Western blotting analysis
HT-29 and SW480 cells were harvested for Western blotting analysis of the expression of SHH according to the standard procedure. The blotting of GAPDH was used as a loading control.
Dual-luciferase assays
Cells transfected with the respective luciferase plasmids and Renilla-TK construct were seeded into 48-well plates. After various treatments were performed as indicated, the luciferase activities were detected using a dual-luciferase assay kit according to the manufacturer’s instructions (Promega) with a luminometer (Molecular Devices; Sunnyvale, CA). The firefly luciferase values were normalized to the Renilla luciferase values.
ELISA assay
Conditioned medium from HT-29 and SW480 cells was collected after the indicated treatment and stored at −80 °C until the assay was performed. According to the manufacturer’s instructions, SHH levels in the supernatants of the two colorectal cancer cell lines were detected with an SHH (N-terminal) (human) sandwich ELISA kit (Abnova, Taipei, China).
HT-29 xenograft model
Healthy BALB/c nude mice were purchased from Beijing Huafukang Biotechnology Co., Ltd (Beijing HFK Bio-Technology; Beijing, China). HT-29 cells were harvested and allografted subcutaneously into the right and left flanks of athymic nude mice. The well-developed tumors were harvested, cut into 10-mm3 fragments and inoculated subcutaneously into the right flank of athymic nude mice using a trocar. When the tumor volume reached 200 mm3, the mice were randomly divided into control and treatment groups (n = 8). The control group was given equivalent amounts of vehicle containing 0.5% CMC-Na, and the treatment groups were given BBR (100 mg · kg−1 · d−1, ig) or GDC-0449 (100 mg · kg−1 · d−1, ig) for 4 weeks. The volume of the tumors was measured every 3 days using a microcaliper. The tumor volume (V) was calculated by using the following formula: V = [length (mm) × width2 (mm2)]/2. The individual relative tumor volume (RTV) was calculated as follows: RTV = Vt/V0, where Vt = the volume measured each day and V0 = the volume at the beginning of the treatment. All animal experimental protocols in this study were preapproved by the Animal Care and Use Committee of Fudan University and performed according to institutional policies.
Statistical analysis
All data were expressed as the mean ± SD and analyzed by the statistical analysis software SAS 9.2. The data were statistically analyzed by one-way analysis of variance (ANOVA), and multiple comparisons between the groups were performed using Dunnett’s method. P < 0.05 was considered statistically significant.
Results
BBR inhibits SHH secretion from both HT-29 cells and SW480 cells
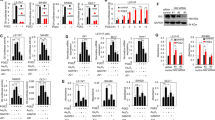
HT-29 cells and SW480 cells have been well documented to exhibit aberrant expression of SHH, which can activate Hh signaling pathway activity in stromal cells in colorectal cancer tissues in a paracrine manner [13, 14]. We then used these two colorectal cancer cell lines to determine whether BBR may influence the secretion of SHH. ELISA analysis showed that BBR treatment could significantly inhibit the secretion of SHH into the conditioned medium of HT-29 cells and SW480 cells in a dose-dependent manner (Fig. 1a, b). Furthermore, conditioned medium collected from HT-29 cells treated with BBR exhibited a decreased ability to provoke Hh activity in NIH3T3-Light2 cells (Fig. 1c), as revealed by a dual-luciferase reporter assay. However, conditioned medium collected from HT-29 cells treated with GDC-0449, an antagonist of SMO, and Robotnikinin, an inhibitor of SHH protein signaling transduction, was unable to influence Gli-induced luciferase activity, which served as a negative control. These observations indicated that BBR may reduce the secretion of SHH ligands from colorectal cancer cells and consequently inhibit paracrine Hh activity.
ELISA analysis of SHH protein production in HT-29 cells (a) or SW480 cells (b). Cells were treated with various concentrations of BBR for 48 h. c Gli-luciferase activity analysis in NIH3T3-Light2 cells after treatment for 36 h with various supernatants that were collected from HT-29 cells after treatment with the compounds for 48 h. The data are expressed as the mean ± SD, n = 3. ###P < 0.001 vs. HT-29 supernatant; *P < 0.05, **P < 0.01, ***P < 0.001 vs. con.
BBR suppresses the expression of SHH in colorectal cancer cells
Having determined that BBR may decrease the secretion of SHH from colorectal cancer cells, we continued to investigate its mechanisms. Western blotting analysis showed that BBR significantly inhibited the expression of SHH protein in a dose-dependent manner (Fig. 2a, b, e, f) and a time-dependent manner (Fig. 2c, d, g, h). Furthermore, we also observed that BBR obviously decreased the expression of SHH mRNA in a dose-dependent (Fig. 2i, j) and time-dependent manner (Fig. 2k, l). The results suggest that BBR decreases SHH secretion by downregulating SHH expression.
Western blotting analysis of SHH protein in HT-29 cells (a, c) or SW480 cells (b, d) after treatment with various concentrations of BBR (a, b) or various times (c, d) as indicated. e–h Quantitative analyses of a–d. i–l RT-qPCR analysis of SHH mRNA expression as indicated. The data are expressed as the mean ± SD, n = 3. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. BBR (0 µM); *P < 0.05, **P < 0.01, ***P < 0.001 vs. con.
BBR is unable to influence SHH transcription in colon cancer cells
To determine whether BBR downregulates SHH mRNA expression by acting at the transcriptional level, we used a SHH promoter-driven firefly luciferase vector to detect the effect of BBR on the transcription of SHH. Dual-luciferase analysis revealed that BBR had no effect on SHH-luciferase activity, while BAY11-7082, a small molecule that can block SHH transcription [15], obviously decreased SHH promoter-driven luciferase activity (Fig. 3), indicating that BBR may decrease SHH mRNA expression by acting at the posttranscriptional level.
BBR reduces the stability of SHH mRNA
To further explore the mechanism of the inhibition of SHH mRNA expression by BBR, we tested the effect of BBR on the stability of SHH mRNA in HT-29 colon cancer cells by indirectly analyzing the mRNA half-life following transcription inhibition with actinomycin D (ACD). We found that treatment with BBR obviously decreased the SHH mRNA half-life (Fig. 4), suggesting that BBR decreases SHH mRNA expression in colorectal cancer cells by promoting the degradation of mRNA.
BBR inhibits the growth of HT-29 tumors in vivo through the Hh paracrine pathway
Having demonstrated that BBR may decrease the stability of SHH mRNA and consequently the secretion of SHH from colorectal cancer cells, we sought to determine whether these observations may somehow reflect the inhibition of tumor growth in vivo. We engrafted HT-29 tumors into nude mice. BBR administered by daily gavage for 4 weeks exhibited better efficacy in inhibiting the growth of HT-29 cells in vivo than GDC-0449 (Fig. 5a). The tumor growth inhibition elicited by administration of BBR or GDC-0449 was comparable to their respective inhibitory effects on mouse-specific Gli mRNA expression (Fig. 5b). However, we observed that BBR failed to influence the expression of human Gli1 mRNA in HT-29 (Fig. 5c) and SW480 cells (Fig. 5d). GANT-61 [16,17,18], an antagonist of Gli, served as a positive control. Hence, these data demonstrated that BBR may inhibit the growth of HT-29 cells by decreasing paracrine Hh activity in HT-29 cancer cells.
a Assessing the growth of HT-29 tumors in nude mice administered the vehicle control (0.5% CMC-Na), GDC-0449 (100 mg · kg−1 · d−1) or BBR (100 mg · kg−1 · d−1) by daily gavage for 4 weeks. The RTV for the indicated days is shown as the mean ± SD for each group of mice (n = 8). b RT-qPCR analysis of mouse Gli1 mRNA expression in tumors presented in a. RT-qPCR analysis of human Gli1 mRNA expression in HT-29 (c) or SW480 (d) cells after treatment with compounds for 48 h. The data are expressed as the mean ± SD, n = 3. #P < 0.05 vs. GDC-0449; *P < 0.05, **P < 0.05, ***P < 0.001 vs. vehicle control; &P < 0.05, &&&P < 0.001 vs. con.
Discussion
Secretion of SHH in some cancer cells facilitates the activity of the Hh signaling pathway in stromal cells, which ultimately promotes the proliferation of tumors [19]. BBR is a traditional Chinese herbal medicine that has been used to treat diarrhea and enteritis for many years [11, 20, 21]. Recent studies have shown its inhibitory effects in various tumors [22,23,24,25]. Previous reports about its antitumor mechanisms showed that they were associated with senescence [26], sterol regulation [27], or the Wnt signaling pathway [28]. However, the mechanism of the effects of BBR on de novo SHH in colorectal cancer cells is still far from clear.
In this study, we showed that BBR decreased the secretion of SHH from two colorectal cancer cell lines, HT-29 and SW480. Mechanistically, we further found that BBR inhibited the protein and mRNA expression of SHH in HT-29 and SW480 cells in a dose-dependent and time-dependent manner. However, BBR did not influence the transcription of SHH, as SHH promoter activity was not inhibited by this compound. Moreover, we discovered that BBR suppressed SHH secretion through a posttranscriptional regulation mechanism, as it reduced the stability of SHH mRNA. Interestingly, Kong et al. verified that BBR could lower cholesterol levels through a posttranscriptional mechanism that stabilized mRNA [29], which suggests the versatile characteristics of BBR. Zhu et al. demonstrated that BBR inhibits colon cancer cell proliferation via the SCAP/SREB-1 pathway [27] at high concentrations. However, BBR had minimal effect on colon cell growth in our study. The contradictory results may be attributed to the different carcinoma cell lines that were used or the differences in the concentrations used by the two independent studies. In this context, we could not rule out the possibility that BBR indirectly inhibited SHH, which remains to be investigated in further studies.
In conclusion, these results strongly support our hypothesis that BBR may inhibit colon tumor growth by inhibiting the paracrine Hh pathway in vivo and therefore shed light on the novel therapeutic mechanism of BBR for HT-29 tumor intervention.
References
Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–29.
Akhavan-Sigari R, Schulz-Schaeffer W, Angelika Harcej A, Rohde V. The importance of the hedgehog signaling pathway in tumorigenesis of spinal and cranial chordoma. J Clin Med. 2019;8:248–63.
Salaritabar A, Berindan-Neagoe I, Darvish B, Hadjiakhoondi F, Manayi A, Devi KP, et al. Targeting hedgehog signaling pathway: paving the road for cancer therapy. Pharmacol Res. 2019;141:466–80.
Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical hedgehog signaling. Vitam Horm. 2012;88:55–72.
Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801.
Scales SJ, de Sauvage FJ. Mechanisms of hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–12.
Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–33.
Mills LD, Zhang Y, Marler RJ, Herreros-Villanueva M, Zhang L, Almada LL, et al. Loss of the transcription factor GLI1 identifies a signaling network in the tumor microenvironment mediating KRAS oncogene-induced transformation. J Biol Chem. 2013;288:11786–94.
Tillhon M, Guaman Ortiz LM, Lombardi P, Scovassi AI. Berberine: new perspectives for old remedies. Biochem Pharmacol. 2012;84:1260–7.
Yang Y, Peng J, Li F, Liu X, Deng M, Wu H. Determination of alkaloid contents in various tissues of Coptis chinensis Franch. by reversed phase-high performance liquid chromatography and ultraviolet spectrophotometry. J Chromatogr Sci. 2017;55:556–63.
Mirhadi E, Rezaee M, Malaekeh-Nikouei B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed Pharmacother. 2018;104:465–73.
Wang J, Peng Y, Liu Y, Yang J, Ding N, Tan W. Berberine, a natural compound, suppresses Hedgehog signaling pathway activity and cancer growth. BMC Cancer. 2015;15:595.
Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10.
Theunissen JW, de Sauvage FJ. Paracrine hedgehog signaling in cancer. Cancer Res. 2009;69:6007–10.
Wang TP, Hsu SH, Feng HC, Huang RF. Folate deprivation enhances invasiveness of human colon cancer cells mediated by activation of sonic hedgehog signaling through promoter hypomethylation and cross action with transcription nuclear factor-kappa B pathway. Carcinogenesis. 2012;33:1158–68.
Chakrabarti J, Holokai L, Syu L, Steele NG, Chang J, Wang J, et al. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget. 2018;9:37439–57.
Benvenuto M, Masuelli L, De Smaele E, Fantini M, Mattera R, Cucchi D, et al. In vitro and in vivo inhibition of breast cancer cell growth by targeting the hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget. 2016;7:9250–70.
Park S, Kim H, Kim K, Roh S. Sonic hedgehog signalling regulates the self-renewal and proliferation of skin-derived precursor cells in mice. Cell Prolif. 2018;51:e12500.
Iwamoto H, Matsuhisa K, Saito A, Kanemoto S, Asada R, Hino K, et al. Promotion of cancer cell proliferation by cleaved and secreted luminal domains of ER stress transducer BBF2H7. PLoS One. 2015;10:e0125982.
Lin X, Zhang N. Berberine: pathways to protect neurons. Phytother Res. 2018;32:1501–10.
Yue SJ, Liu J, Wang WX, Wang AT, Yang XY, Guan HS, et al. Berberine treatment-emergent mild diarrhea associated with gut microbiota dysbiosis. Biomed Pharmacother. 2019;116:109002.
Mao L, Chen Q, Gong K, Xu X, Xie Y, Zhang W, et al. Berberine decelerates glucose metabolism via suppression of mTORdependent HIF1alpha protein synthesis in colon cancer cells. Oncol Rep. 2018;39:2436–42.
Huang Y, Wang K, Gu C, Yu G, Zhao D, Mai W, et al. Berberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenib. Oncol Rep. 2018;40:1525–32.
Hesari A, Ghasemi F, Cicero AFG, Mohajeri M, Rezaei O, Hayat SMG, et al. Berberine: a potential adjunct for the treatment of gastrointestinal cancers? J Cell Biochem. 2018;119:9655–63.
Mortazavi H, Nikfar B, Esmaeili SA, Rafieenia F, Saburi E, Chaichian S, et al. Potential cytotoxic and anti-metastatic effects of berberine on gynaecological cancers with drug-associated resistance. Eur J Med Chem. 2020;187:111951.
Kumar R, Awasthi M, Sharma A, Padwad Y, Sharma R. Berberine induces dose-dependent quiescence and apoptosis in A549 cancer cells by modulating cell cyclins and inflammation independent of mTOR pathway. Life Sci. 2020;244:117346.
Liu Y, Hua W, Li Y, Xian X, Zhao Z, Liu C, et al. Berberine suppresses colon cancer cell proliferation by inhibiting the SCAP/SREBP-1 signaling pathway-mediated lipogenesis. Biochem Pharmacol. 2020;174:113776.
Zhang J, Cao H, Zhang B, Cao H, Xu X, Ruan H, et al. Berberine potently attenuates intestinal polyps growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signalling. J Cell Mol Med. 2013;17:1484–93.
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51.
Acknowledgements
This study was financially supported by the Science and Technology Commission of Shanghai Municipality (No. 19401900600).
Author information
Authors and Affiliations
Contributions
TMH, WFT, and ZQS conceived and designed the project; ZQS and JW performed experiments and analyzed data; WFT and ZQS wrote the manuscript; TMH and WFT supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Shen, Zq., Wang, J., Tan, Wf. et al. Berberine inhibits colorectal tumor growth by suppressing SHH secretion. Acta Pharmacol Sin 42, 1190–1194 (2021). https://doi.org/10.1038/s41401-020-00514-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-00514-2