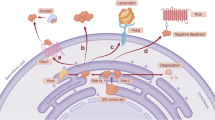
Abstract
Overexpression of nuclear coactivator steroid receptor coactivator 1 (SRC-1) and aberrant activation of the Hedgehog (Hh) signaling pathway are associated with various tumorigenesis; however, the significance of SRC-1 in colorectal cancer (CRC) and its contribution to the activation of Hh signaling are unclear. Here, we identified a conserved Hh signaling signature positively correlated with SRC-1 expression in CRC based on TCGA database; SRC-1 deficiency significantly inhibited the proliferation, survival, migration, invasion, and tumorigenesis of both human and mouse CRC cells, and SRC-1 knockout significantly suppressed azoxymethane/dextran sodium sulfate (AOM/DSS)-induced CRC in mice. Mechanistically, SRC-1 promoted the expression of GLI family zinc finger 2 (GLI2), a major downstream transcription factor of Hh pathway, and cooperated with GLI2 to enhance multiple Hh-regulated oncogene expression, including Cyclin D1, Bcl-2, and Slug. Pharmacological blockages of SRC-1 and Hh signaling retarded CRC progression in human CRC cell xenograft mouse model. Together, our studies uncover an SRC-1/GLI2-regulated Hh signaling looping axis that promotes CRC tumorigenesis, offering an attractive strategy for CRC treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
TCGA CRC RNA-seq data is available at UCSC XENA website. All other remaining data are included in the article and Supplemental Information files, or available from the authors upon reasonable request.
References
O’Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217–22.
Lonard DM, O’Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8:598–604.
Mark M, Yoshida-Komiya H, Gehin M, Liao L, Tsai MJ, O’Malley BW, et al. Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proc Natl Acad Sci USA. 2004;101:4453–8.
Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 1998;279:1922–5.
Picard F, Géhin M, Annicotte J, Rocchi S, Champy MF, O’Malley BW, et al. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 2002;111:931–41.
Louet JF, Chopra AR, Sagen JV, An J, York B, Tannour-Louet M, et al. The coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell Metab. 2010;12:606–18.
Duteil D, Chambon C, Ali F, Malivindi R, Zoll J, Kato S, et al. The transcriptional coregulators TIF2 and SRC-1 regulate energy homeostasis by modulating mitochondrial respiration in skeletal muscles. Cell Metab. 2010;12:496–508.
Walsh CA, Bolger JC, Byrne C, Cocchiglia S, Hao Y, Fagan A, et al. Global gene repression by the steroid receptor coactivator SRC-1 promotes oncogenesis. Cancer Res. 2014;74:2533–44.
Qin L, Wu YL, Toneff MJ, Li D, Liao L, Gao X, et al. NCOA1 directly targets M-CSF1 expression to promote breast cancer metastasis. Cancer Res. 2014;74:3477–88.
Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, O’Malley BW. Small molecule inhibition of the steroid receptor coactivators, SRC-3 and SRC-1. Mol Endocrinol. 2011;25:2041–53.
Qin L, Chen X, Wu Y, Feng Z, He T, Wang L, et al. Steroid receptor coactivator-1 upregulates integrin αs expression to promote breast cancer cell adhesion and migration. Cancer Res. 2011;71:1742–51.
Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–30.
Giudici M, Goni S, Fan R, Treuter E. Nuclear receptor coregulators in metabolism and disease. Handb Exp Pharm. 2016;233:95–135.
Kato S, Yokoyama A, Fujiki R. Nuclear receptor coregulators merge transcriptional coregulation with epigenetic regulation. Trends Biochem Sci. 2011;36:272–81.
Hsia EY, Goodson ML, Zou JX, Privalsky ML, Chen HW. Nuclear receptor coregulators as a new paradigm for therapeutic targeting. Adv Drug Deliv Rev. 2010;62:1227–37.
Na SY, Lee SK, Han SJ, Choi HS, Im SY, Lee JW. Steroid receptor coactivator-1 interacts with the p50 subunit and coactivates nuclear factor kappaB-mediated transactivations. J Biol Chem. 1998;273:10831–4.
Lee SK, Kim HJ, Kim JW, Lee JW. Steroid receptor coactivator-1 and its family members differentially regulate transactivation by the tumor suppressor protein p53. Mol Endocrinol. 1999;13:1924–33.
Myers E, Hill AD, Kelly G, McDermott EW, O’Higgins NJ, Buggy Y, et al. Associations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin Cancer Res. 2005;11:2111–22.
Russo L, Giller K, Pfitzner E, Griesinger C, Becker S. Insight into the molecular recognition mechanism of the coactivator NCoA1 by STAT6. Sci Rep. 2017;7:16845.
Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O’Malley BW, et al. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci USA. 2009;106:151–6.
Qin L, Liu Z, Chen H, Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009;69:3819–27.
Meerson A, Yehuda H. Leptin and insulin up-regulate miR-4443 to suppress NCOA1 and TRAF4, and decrease the invasiveness of human colon cancer cells. BMC Cancer. 2016;16:882.
Tong Z, Li M, Wang W, Mo P, Yu L, Liu K, et al. Steroid receptor coactivator 1 promotes human hepatocellular carcinoma progression by enhancing Wnt/β-Catenin signaling. J Biol Chem. 2015;290:18596–608.
Walsh CA, Qin L, Tien JC, Young LS, Xu J. The function of steroid receptor coactivator-1 in normal tissues and cancer. Int J Biol Sci. 2012;8:470–85.
Zeng X, Goetz JA, Suber LM, Scott WJ Jr., Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–20.
Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–29.
Zhao C, Cai S, Shin K, Lim A, Kalisky T, Lu WJ, et al. Stromal Gli2 activity coordinates a niche signaling program for mammary epithelial stem cells. Science. 2017;356.
Du K, Hyun J, Premont RT, Choi SS, Michelotti GA, Swiderska-Syn M, et al. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology. 2018;154:1465–79.e1413.
Sari IN, Phi LTH, Jun N, Wijaya YT, Lee S, Kwon HY. Hedgehog signaling in cancer: a prospective therapeutic target for eradicating cancer stem cells. Cells. 2018;7:208.
Sang Y, Li Y, Song L, Alvarez AA, Zhang W, Lv D, et al. TRIM59 promotes gliomagenesis by inhibiting TC45 dephosphorylation of STAT3. Cancer Res. 2018;78:1792–804.
Raleigh DR, Choksi PK, Krup AL, Mayer W, Santos N, Reiter JF. Hedgehog signaling drives medulloblastoma growth via CDK6. J Clin Invest. 2018;128:120–4.
Fattahi S, Pilehchian Langroudi M, Akhavan-Niaki H. Hedgehog signaling pathway: epigenetic regulation and role in disease and cancer development. J Cell Physiol. 2018;233:5726–35.
Zeng X, Ju D. Hedgehog signaling pathway and autophagy in cancer. Int J Mol Sci. 2018;19:2279.
Papadopoulos V, Tsapakidis K, Riobo Del Galdo NA, Papandreou CN, Del Galdo F, Anthoney A, et al. The prognostic significance of the hedgehog signaling pathway in colorectal cancer. Clin Colorectal Cancer. 2016;15:116–27.
Kangwan N, Kim YJ, Han YM, Jeong M, Park JM, Go EJ, et al. Sonic hedgehog inhibitors prevent colitis-associated cancer via orchestrated mechanisms of IL-6/gp130 inhibition, 15-PGDH induction, Bcl-2 abrogation, and tumorsphere inhibition. Oncotarget 2016;7:7667–82.
Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46:D956–63.
Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681–92.
Hui CC, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–37.
Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, Wang J, et al. Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res. 2014;74:1506–17.
Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Disco. 2012;11:437–8.
Chen Q, Zhuang S, Hong Y, Yang L, Guo P, Mo P, et al. Demethylase JMJD2D induces PD-L1 expression to promote colorectal cancer immune escape by enhancing IFNGR1-STAT3-IRF1 signaling. Oncogene 2022;41:1421–33.
Peng K, Zhuo M, Li M, Chen Q, Mo P, Yu C. Histone demethylase JMJD2D activates HIF1 signaling pathway via multiple mechanisms to promote colorectal cancer glycolysis and progression. Oncogene 2020;39:7076–91.
Zhuo M, Chen W, Shang S, Guo P, Peng K, Li M, et al. Inflammation-induced JMJD2D promotes colitis recovery and colon tumorigenesis by activating Hedgehog signaling. Oncogene 2020;39:3336–53.
Peng K, Kou L, Yu L, Bai C, Li M, Mo P, et al. Histone demethylase JMJD2D interacts with β-Catenin to induce transcription and activate colorectal cancer cell proliferation and tumor growth in mice. Gastroenterology 2019;156:1112–26.
Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 2005;132:279–89.
Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338–51.
Jain S, Song R, Xie J. Sonidegib: mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco Targets Ther. 2017;10:1645–53.
Xin M, Ji X, De La Cruz LK, Thareja S, Wang B. Strategies to target the Hedgehog signaling pathway for cancer therapy. Med Res Rev. 2018;38:870–913.
Berlin J, Bendell JC, Hart LL, Firdaus I, Gore I, Hermann RC, et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin Cancer Res. 2013;19:258–67.
Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, et al. Randomized Phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284–92.
Mo P, Zhou Q, Guan L, Wang Y, Wang W, Miao M, et al. Amplified in breast cancer 1 promotes colorectal cancer progression through enhancing notch signaling. Oncogene 2015;34:3935–45.
Datta A, Biswas K, Sommers JA, Thompson H, Awate S, Nicolae CM, et al. WRN helicase safeguards deprotected replication forks in BRCA2-mutated cancer cells. Nat Commun. 2021;12:6561.
Lin J, Liu H, Fukumoto T, Zundell J, Yan Q, Tang CA, et al. Targeting the IRE1α/XBP1s pathway suppresses CARM1-expressing ovarian cancer. Nat Commun. 2021;12:5321.
Seba V, de Lima GG, Pereira BL, Silva G, Reinhardt LS, Arantes PR, et al. Development, Characterization and Cell Viability Inhibition of PVA Spheres Loaded with Doxorubicin and 4’-Amino-1-Naphthyl-Chalcone (D14) for Osteosarcoma. Polymers (Basel) 2021;13:2611.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics 2012;16:284–7.
Acknowledgements
We would like to thank Shiwen Luo (Nanchang University) for providing GLI2 expression plasmid and Hedgehog signaling reporter 8×GLI. We also thank Dr. Mingxia Zhu for technical support. This work was supported by the National Natural Science Foundation of China (Nos. 81970485 and 81772942 to Chundong Yu, and No. 81772539 to Yongyou Zhang), and the Fundamental Research Funds for the Central Universities (No. 20720180048 to Yongyou Zhang).
Author information
Authors and Affiliations
Contributions
PM, CY, and YZ conceived and coordinated the study. PG, QC, KP, YZ, CY, and PM conceived and designed the experiments. PG, QC, KP, JX, JL, WR, ZT, ML, CY, YZ, and PM performed or analyzed the experiments. PG, QC, KP, PM, JXu, CY, and YZ wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were conducted under protocols approved by the Laboratory Animal Center of Xiamen University. For experiments using human specimens, all specimens were anonymously coded in accordance with the Declaration of Helsinki. The study protocol that conformed to the ethical guidelines was approved by the Medical Ethics Committee at school of medicine, Xiamen University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Guo, P., Chen, Q., Peng, K. et al. Nuclear receptor coactivator SRC-1 promotes colorectal cancer progression through enhancing GLI2-mediated Hedgehog signaling. Oncogene 41, 2846–2859 (2022). https://doi.org/10.1038/s41388-022-02308-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02308-8