Abstract
Adolescence is marked by the maturation of systems involved in emotional regulation and by an increased risk for internalizing disorders (anxiety/depression), especially in females. Hypothalamic-pituitary-adrenal (HPA)-axis function and redox homeostasis (balance between reactive oxygen species and antioxidants) have both been associated with internalizing disorders and may represent critical factors for the development of brain networks of emotional regulation. However, sex-specific interactions between these factors and internalizing symptoms and their link with brain maturation remain unexplored. We investigated in a cohort of adolescents aged 13–15 from the general population (n = 69) whether sex-differences in internalizing symptoms were associated with the glutathione (GSH)-redox cycle homeostasis and HPA-axis function and if these parameters were associated with brain white matter microstructure development. Female adolescents displayed higher levels of internalizing symptoms, GSH-peroxidase (GPx) activity and cortisol/11-deoxycortisol ratio than males. There was a strong correlation between GPx and GSH-reductase (Gred) activities in females only. The cortisol/11-deoxycortisol ratio, related to the HPA-axis activity, was associated with internalizing symptoms in both sexes, whereas GPx activity was associated with internalizing symptoms in females specifically. The cortisol/11-deoxycortisol ratio mediated sex-differences in internalizing symptoms and the association between anxiety and GPx activity in females specifically. In females, GPx activity was positively associated with generalized fractional anisotropy in widespread white matter brain regions. We found that higher levels of internalizing symptoms in female adolescents than in males relate to sex-differences in HPA-axis function. In females, our results suggest an important interplay between HPA-axis function and GSH-homeostasis, a parameter strongly associated with brain white matter microstructure.
Similar content being viewed by others
Introduction
Adolescence is marked by important brain morphological and functional changes of affect-related systems, such as the corticolimbic network. Among maturational processes, synaptic refinement and axonal myelination in prefrontal and limbic areas may promote adult-like emotion and affect regulation strategies [1]. This protracted period of growth is characterized by a heightened sensitivity to the environment, representing both an opportunity for increased adaptability to positive experiences, but also a higher vulnerability to environmental insults, which may impact developmental trajectories [2]. The period of adolescence also witnesses a steep rise in the incidence of internalizing disorders, encompassing anxiety and affective disorders [3], with a worse outcome compared to adult onset [4, 5]. Importantly, puberty appears to set the stage for a higher susceptibility to stress-related disorders in females, as they display an important increase in internalizing disorders [6, 7], setting the well-established 1:2 male:female ratio of depression observed in adults [8]. Longitudinal imaging studies exploring the dynamic of brain changes during adolescence also indicate sex-specific trajectories regarding volumes [9] and microstructure [10]. Puberty seems to be an important factor underlying sexually divergent trajectories, suggesting that physiological changes associated with sexual maturation, such as the rising levels of gonadal hormones, may play sex-specific roles in brain maturation [11]. However, the biological mechanisms promoting sex-difference in brain maturation trajectories as well as differential vulnerability to internalizing disorders remain poorly understood. Both clinical and preclinical findings indicate that the hypothalamic-pituitary-adrenal (HPA)-axis shows a shift towards heightened reactivity and sexually-dimorphic response along with sexual maturation [12,13,14,15]. This surge of heightened stress-reactivity has been postulated to yield increased sensitivity to environmental stress, which could impact the maturation of the corticolimbic network implicated in emotion regulation, lastingly increasing the risk for anxiety and affective disorders [16, 17].
Adolescence is a highly metabolically demanding period [18], rendering the brain potentially sensitive to the imbalance between antioxidant capacity and reactive oxygen species (ROS) leading to oxidative stress (OxS) [19]. Preclinical findings indicate increased vulnerability of the brain to redox dysregulation during peripuberty, compared to later developmental stages [20, 21]. Growing evidence indicates that impaired redox homeostasis, as well as subsequent OxS may be critically involved in the development of anxiety and affective disorders [22,23,24,25]. Preclinical findings have evidenced a causal link between brain oxidative damages and the emergence of anxiety and depressive-like behavior [25,26,27,28]. On the other hand, multiple evidence indicates that chronic stress promotes OxS in stress-sensitive areas such as the prefrontal cortex (PFC) [29], amygdala [30] and hippocampus [25]. While antioxidants can diminish anxiety-like behavior in rodents [28, 31, 32], the expression of two enzymes associated with the antioxidant glutathione (GSH) system (glyoxalase 1 and GSH reductase 1) in emotion-related brain regions modulates anxiety-like behavior [26]. Noteworthy, mice with a limited capacity to synthetize GSH are more vulnerable to adolescent stress-induced anxiety [20]. As one of the major non-enzymatic antioxidant and redox regulator in the brain, GSH acts largely via the GSH-redox cycle (Supplementary Fig. S1) which involves two GSH-dependent enzymes, GSH peroxidase (GPx) and GSH reductase (Gred). The activity of GPx catalyzes the reduction of peroxides by oxidizing GSH into GSSG, while the activity of Gred reduces GSSG back to GSH. The activity of these two enzymes may reflect GSH-redox cycle regulation and were shown to be altered in patients with anxiety and affective disorders [22, 33,34,35]. Furthermore, glucocorticoid sensitivity [36] and HPA-axis reactivity [28, 37] are influenced by the oxidoreductive status and were shown to normalize upon antioxidant treatment [28, 38]. On the other hand, high doses and/or long-term exposition to glucocorticoids was shown to repress the antioxidant response in the brain and promote oxidative stress [28, 39,40,41]. However, to our knowledge, no clinical study has investigated the association between peripheral GSH-redox cycle regulation and markers of the HPA-axis, especially during adolescence. Additionally, animal studies suggest that both glucocorticoids and the GSH-antioxidant balance may play a role in modulating myelin maturation [42, 43]. Several clinical studies report negative association between morning cortisol levels and neuroimaging indices of white matter microstructure such as fractional anisotropy (FA) [44, 45], whereas others report developmental exposure to glucocorticoids and stress to be associated with increased FA [46, 47]. Regarding the role of redox regulation, we previously reported a positive association between central levels of GSH in the prefrontal cortex measured by magnetic resonance spectroscopy, and structural and functional connectivity along the cingulum bundle in healthy young adults, suggesting that redox regulation supported by the GSH-redox cycle may be involved in white matter maturation [42]. Association between GSH and FA measured in the limbic system was also evidenced in young adults, with diagnosis of mood disorder and low hippocampal GSH associated with lower FA in the stria terminalis [48]. Given the important changes in HPA-axis reactivity and sensitivity occurring during adolescence and the greater susceptibility to redox dysregulation conferred by the metabolic changes occurring during this period, we aimed to investigate the associations between the homeostasis of the GSH-redox cycle, and markers of HPA-axis activity in blood, as well as their relationship with internalizing symptoms and brain white matter microstructure in young adolescents. In a well characterized sample of adolescents from the general population, we investigated whether adolescents show sex-differences in (1) the levels of self-rated anxiety and depression symptoms, (2) markers of GSH homeostasis, and if this difference is related to internalizing symptoms severity, (3) markers of the HPA axis activity, and if this difference is related to internalizing symptoms severity, (4) the association between markers of HPA axis activity and GSH-homeostasis. Lastly, (5) we investigated whether markers of the HPA axis and the GSH-antioxidant balance were associated with cerebral white matter microstructure in a sex-dependent manner. Given the extended literature reporting the influence of pubertal hormones on HPA-axis [49], redox regulation [50, 51] and brain maturation [11], we explored in a secondary hypothesis whether the aforementioned associations were related to pubertal status and gonadal hormones (estradiol and testosterone).
Methods
Subjects
This study was nested in the Mindfulteen study, which is a randomized controlled cross-over clinical trial investigating the impact of a stress-reduction intervention based on mindfulness meditation on adolescent well-being [52]. Briefly, the Mindfulteen study recruited adolescents between 13 and 15 years old. They were excluded if they had a chronic somatic disease or a significant medical condition, if they benefited from psychotherapy in the last 6 months, if they received a psychotropic medication in the past month and if they met criteria for any psychiatric disorder of the DSM-IV except for a current anxiety disorder and/or a past (>6 months) episode of major depressive disorder. The study protocol was approved by the Geneva Regional Ethical Committee (CCER 2018–01731). For the current study, we included only data drawn from the baseline evaluation and blood sampling (Visit (V)0 and V1), prior to the intervention. Detailed information concerning recruitment, inclusion and exclusion criteria are available in the published protocol [52].
Clinical evaluation
Participants underwent a thorough psychiatric evaluation by trained psychologists using the K-SADS [53] during the baseline evaluation (V0). Trait anxiety was assessed using the State and Trait Anxiety Inventory for Children (STAIC-T) [54] and depressive symptoms were assessed with the Beck Depression Inventory (BDI) [55]. Information on the pubertal status (PS) was drawn from the K-SADS’ item assessing attainment of puberty (based on the apparition of menarche in females and changes in voice and pilosity in males). One male did not provide information on the PS.
Blood analysis
Blood was collected within 7 days before or after the MRI (V1), in the morning (between 7 and 8.30 a.m.) after an overnight fast. Enzymatic activity of GPx and Gred were assessed in red blood cell lysates using an in-house protocol described in the Supplementary Information. Levels of reduced GSH were measured in red blood cell lysates using a diagnostic kit (Calbiochem) and normalized to blood volume (see Supplementary Information). Steroids were measured in the serum by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in the laboratory of Clinical Chemistry of the Lausanne University Hospital. Gonadal steroids of interest included levels of estradiol (E2) and testosterone (T). We also focused on glucocorticoids directly involved in cortisol synthesis and metabolism, namely 11-deoxycortisol, cortisol and cortisone, as well as the ratio between cortisol and its precursor and metabolite (cortisol/11-deoxycortisol and cortisol/cortisone) (Fig. 3F).
One female subject missed the blood test. One male subject had an extreme Gred activity value (beyond 3 SD of the mean) which was considered as a technical outlier and was removed from all analyses involving Gred activity. Only one female subject was taking the contraceptive pill at the time of the first visit and was removed from the analysis involving steroids due to cortisol value >2 SD. This resulted in 67 subjects with measures of GSH markers, 66 subjects with measures of steroids and a total 65 subjects with measures of GSH markers and steroids.
Image acquisition
Participants underwent a brain scan on a 3T Magnetom TIM Trio scanner (Siemens, Germany) equipped with a 32-channel head coil. Each scanning session included a magnetization-prepared rapid acquisition gradient echo (MPRAGE) T1-weighted sequence with 1 mm in-plane resolution and 1 mm slice thickness and a diffusion spectrum imaging (DSI) sequence including 128 diffusion-weighted images with a maximum b-value of 8000 s/mm2 and one b0 reference image. Detailed specifications of each sequence are provided in the Supplementary Information.
Generalized fractional anisotropy computation
QUAD (QUality Assessment for DMRI) was used to quantitatively assess the quality of the diffusion-weighted images and extract different quality control metrics for each subject [56]. This tool computes the quality control metrics using the results provided by the EDDY tool after correcting each individual dMRI for motion and induced currents. Total outliers, average absolute motion, average relative motion, signal-to noise ratio and contrast-to-noise ratio were used to assess the quality control of the DWIs. An automatic image correction and processing workflow was applied over the individual diffusion-weighted images. Briefly, the workflow employed mrtrix (v.3.0.3) [57] and FSL (v.6.0.3) [58] for performing the following correction steps: denoising, bias correction, intensity normalization, head motion correction (with gradient table rotation), eddy current and distortion correction. A registration-based approach using Advanced Normalization Tools (ANTs, v.2.4.1) [59] was implemented to correct the geometrical distortion along the phase-encoding direction. Dipy (v.1.5.0) [60] was applied over the corrected DWIs to fit both second order tensors and intravoxel orientation distribution functions (ODF) via the Simple Harmonic Oscillator-based Reconstruction and Estimation method (SHORE) [61]. The resulting ODFs derived from the corrected DWIs were employed to compute the generalized fractional anisotropy (gFA). Similar to FA, gFA values range from 0 to 1, indicating zero to maximal orientational anisotropy in the ODF. Each individual gFA map was inspected manually for gross abnormalities and/or artifacts. Additional information on the generation of gFA maps is provided in the Supplementary Data.
Statistical analysis
Statistical analysis was run using R v. 4.0.5. We examined gender differences in demographic and clinical characteristics using t-test for continuous outcomes and chi-square analysis for categorical outcomes. When normality was not assumed, permutation tests were performed using 5000 random permutations. Standard ordinary least square (OLS) regressions were used to investigate sex-differences in GSH-metabolizing enzymes, GSH levels and adrenal steroids while adjusting for age and Body Mass Index (BMI), based on the reported influence of these covariates on the activity of the GSH-system enzymes and steroid levels [62, 63]. Anxiety and depression scores were added in the OLS model to explore the association between GSH-homeostasis, steroid levels and internalizing symptoms. As females and males differed significantly in the levels of the main outcomes (internalizing symptoms, glucocorticoid levels and GPx activity), we added a sex-interaction term to explore sex-specific associations. In secondary analyses, we added PS and the levels of gonadal hormones (E2 and T) as covariates to explore the independent effect of pubertal maturation and gonadal hormones. Spearman correlation was used to investigate the relation between the activity of the two enzymes GPx and Gred in boys and girls separately. Statistical difference between the two independent correlation coefficients was calculated using the Fisher test. For all statistical analyses, the effect was considered significant at a threshold of p value < 0.05. Causal mediation analysis was performed using the “mediation” package in R. As a prerequisite, mediation analysis was run only when both the presupposed mediator and independent variable were significantly associated with the dependent variable. Sex, age, and BMI were entered as covariates in each analysis. Confidence intervals of the estimated mediation effect was inferred following non-parametric bootstrap resampling with 5000 simulations. Causal mediation effect was considered when the 95% confidence interval did not include 0.
Image analyses
Associations between blood markers and white matter microstructure (gFA) were tested across the entire brain white matter volume. Whole brain voxel-based-analysis (VBA) in white matter was performed for each sex separately on the diffusion images-derived gFA maps using generalized linear models, corrected for family-wise error rate using Threshold-Free Cluster Enhancement as implemented in FSL v.6.0.3 software package. Linear models included age as covariate.
Results
Demographics
A table recapitulating demographics according to sex is displayed in Table 1. Briefly, the sample comprised 68 adolescents of whom 39 were females (57.4%) and 29 were males (42.6%). Sex groups did not differ in age, BMI, pubertal status, and tobacco use. Ten adolescents (14.7%) met the criteria for a current anxiety disorder, of whom 3 boys and 6 girls had a diagnosis of generalized anxiety disorder and 1 girl had a diagnosis of panic disorder. Twelve adolescents (18%) had a history of previous major depressive episode.
Internalizing symptoms
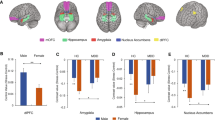
Adolescent females were significantly more anxious (Mean = 38.5, SD = 7.28) than males (Mean = 33.2, SD = 6.12) according to the STAIC-T (t(66) = 3.14, p = 0.003) and more depressed than males according to the BDI (females: median = 9, interquartile range = 9; males: median = 7, interquartile range = 6, p = 0.021) (Fig. 1).
GSH-redox cycle system
Regarding sex-differences in GSH-redox cycle system, GPx activity was higher in erythrocytes of females than males (ß = 4.09545, p = 0.001) (Fig. 2). Gred activity and levels of reduced GSH did not differ between sexes. Secondary analyses indicated that GPx and Gred activities were higher in post-pubertal compared to pre-pubertal adolescents (respectively: ß = 4.22081, p = 0.012; ß = 0.432078, p = 0.037) but were not associated with gonadal hormones. As the measure of the relation between activity of complementary enzymes GPx and Gred may reflect GSH system redox status, we looked at the correlation between GPx and Gred activity in each sex. In females, there was a positive correlation between GPx and Gred activities (r = 0.55, p < 0.001), indicating a highly regulated oxidoreductive balance of the GSH-redox cycle, whereas in males GPx and Gred did not correlate (r = 0.016, p = 0.940). The two correlation coefficients were significantly different (two-tailed Fisher test, z = 2.341, p = 0.021).
Females display higher glutathione peroxidase (GPx) activity compared to males (A) and a strong correlation between GPx and glutathione reductase (Gred) activities (r = 0.55, p < 0.001) compared to males in which no correlation is found (B). The two correlation coefficients are significantly different (Fisher test p < 0.05). No sex-differences are observed for Gred activity (C) and levels of reduced glutathione (GSH) (D).
HPA-axis markers
To assess whether the sex-difference in internalizing symptoms was associated with a difference in HPA-axis related adrenal steroids, we first compared the basal levels of glucocorticoids (11-deoxycortisol, cortisol, cortisone) involved in cortisol metabolism as well as relevant steroid ratios (i.e., reflecting indirect activity of key enzymes), between males and females. Significant sex-differences were found for cortisone with lower levels in females compared to males (ß = −7.04832, p = 0.045), as well as higher cortisol/11-deoxycortisol ratio (ß = 89.302, p = 0.041) and cortisol/cortisone ratio (ß = 0.871113, p = 0.043) in females compared to males (Fig. 3). No difference was found for levels of cortisol, nor 11-deoxycortisol. There was no effect of PS on glucocorticoid levels, however a positive association was found between cortisone and testosterone (β = 1.5991, p = 0.002), and a negative association between cortisol/11-deoxycortisol and testosterone (β = −15.780, p = 0.015).
No differences in basal serum cortisol (A) and 11-deoxycortisol (B) between males and females. Females show lower levels of cortisone (C) and higher levels of cortisol/cortisone ratio (D) and cortisol/11-deoxycortisol ratio (E) than males. F 11β-hydroxylase catalyzes the conversion of 11-deoxycortisol to cortisol in the adrenal cortex, under the influence of adrenocorticotropic hormone (ACTH). Cortisol is converted to its inactive metabolite cortisone in peripheral tissues by the 11β-hydroxysteroid-dehydrogenase type II. Image created in Biorender.com. *p value <0.05.
Association between internalizing symptoms and the GSH-antioxidant balance
We next investigated whether levels of trait anxiety (STAI-T) and depression (BDI), which were higher in females than in males, were associated with markers of GSH-redox cycle. GSH levels as well as GPx and Gred activities were regressed on STAI-T and BDI with sex, BMI and age as covariates. Further, sex was added as an interaction term to investigate sex-specific associations. No significant association between STAI-T and GPx or BDI and GPx were found in the whole cohort. However, there was a significant STAI-T by sex interaction (F(5,61) = 4.596, p = 0.002) as well as significant BDI by sex interaction (F(5,61) = 3.959, p = 0.026) in predicting GPx activity (Fig. 4). Simple slopes indicated that in females, GPx activity was positively associated with STAI-T (ß = 0.2415, p = 0.031) and BDI (ß = 0.23509, p = 0.017) whereas in males GPx activity was negatively associated with STAI-T (ß = −0.3441, p = 0.026) and not significantly associated with BDI (ß = −0.20981, p = 0.225). No significant association between internalizing symptoms and Gred activity nor GSH levels were found neither in the whole cohort nor in a sex-specific way.
Ordinary least square (OLS) regression showing a positive association between cortisol/11-deoxycortisol ratio and State-Trait Anxiety Inventory Trait (STAIC-T) scores (A) (p < 0.001) and BDI (B) (p < 0.01) scores while controlling for sex, age and body mass index (BMI). Regression lines are displayed by sex. Sex-by-trait anxiety (STAIC-T) (p < 0.01) (C), sex-by-depression (BDI) (p < 0.05) (D) and sex-bycortisol/11-deoxycortisol (p < 0.01) (E) interaction in association with GPx activity. In females, significant positive association between STAI-T and GPx activity (p < 0.05) (C), BDI and GPx activity (p < 0.05) (D) and cortisol/11-deoxycortisol ratio and GPx activity (p < 0.0001) (E). In males, negative association between STAI-T and GPx activity (p < 0.05) (C). F Mediation of the cortisol/11-deoxycortisol ratio on sex-differences in STAIC-T and Beck Depression Inventory (BDI) scores using causal mediation analysis. G Mediation of cortisol/11-deoxycortisol ratio on the relationship between STAI-T and GPx activity in females using causal mediation analysis.
Associations between internalizing symptoms and HPA-axis markers
To investigate if the sex-difference observed for cortisone, cortisol/11-deoxycortisol ratio and cortisol/cortisone ratio was related to the higher internalizing symptoms observed in females than males, we next regressed STAIC-T and BDI scores on the glucocorticoids and glucocorticoid ratios of interest. Sex was first added as covariate and then as an interaction term to investigate sex-specific associations. The cortisol/11-deoxycortisol ratio was strongly associated with STAI-T (ß = 0.0168, p < 0.001) and BDI (ß = 0.0165, p = 0.002) in the whole cohort (Fig. 4A, B). No sex by cortisol/11-deoxycortisol ratio interaction was found, suggesting that internalizing symptoms are positively associated with the cortisol/11-deoxycortisol ratio in both sexes. No other significant association was found between glucocorticoid levels/ratio (i.e., cortisol, cortisone, 11-deoxycortisol, cortisol/cortisone ratio) and internalizing symptoms (i.e., STAI-T and BDI) in the whole cohort. To address whether the sex-difference in internalizing symptoms was mediated by differences in the cortisol/11-deoxycortisol, we conducted causal mediation analysis. STAI-T and BDI were entered in a regression model as dependent variables with sex, age, BMI and Cortisol/11-deoxycortisol ratio as independent variables. Sex was entered as the treatment of the mediation and Cortisol/11-deoxycortisol ratio as the mediator. Cortisol/11-deoxycortisol ratio accounted for 29.75% (p = 0.018) of the total effect of sex on STAI-T, and 34.43% (p = 0.024) of the total effect of sex on BDI (Fig. 4C).
Associations between GSH-antioxidant system and the HPA-axis
Following the hypothesis of a biological interaction between the GSH system and the HPA axis, we further explored how the cortisol/11-deoxycortisol ratio related to GPx activity and if the association was sex-specific. Cortisol/11-deoxycortisol ratio was significantly positively associated with GPx activity in the whole cohort (ß = 0.011254, p = 0.002). Moreover, there was a significant sex by Cortisol/11-deoxycortisol ratio interaction (F(5,60) = 6.236, p = 0.0011) (Fig. 4C). Cortisol/11-deoxycortisol ratio levels were positively associated with GPx activity in females (ß = 0.0160756, p < 0.0001) but not in males. Given the sex-specificity of this association, we next evaluated in females the directionality of the association between cortisol/11-deoxycortisol ratio, GPx activity and internalizing symptoms using causal mediation analysis: GPx did not mediate the association between cortisol/11-deoxycortisol ratio and STAI-T nor BDI, whereas the cortisol/11-deoxycortisol accounted for 73% (p = 0.048) of the association between STAI-T and GPx activity in females (Fig. 4D). The mediated effect of Cortisol/11-deoxycortisol ratio on the association between BDI and GPx activity did not reach significance (p = 0.109).
Association with white matter diffusion properties
Lastly, we investigated if higher levels of anxiety and depression in females and specific markers of the HPA-axis (cortisol/11-deoxycortisol ratio) and GSH-antioxidant balance (GPx) were related to alterations in brain white matter microstructure. We first ran a whole brain voxel-based analysis (VBA) in white matter looking at the association between regional gFA and STAI-T as well as between gFA and BDI scores. There was no white matter region showing a significant association between gFA and STAI-T or BDI, nor any sex effect. Next, VBA was conducted to estimate associations between gFA and both GPx and the cortisol/11-deoxycortisol ratio. In females, GPx was strongly and positively associated with gFA in widespread regions of white matter (Fig. 5). No association was found in males. Similarly, cortisol/11-deoxycortisol ratio was positively associated with widespread clusters in females but not in males. When cortisol/11-deoxycortisol ratio and GPx were added conjointly in the model, the significant association with cortisol/11-deoxycortisol was lost, whereas important clusters remained significant with GPx, suggesting that GPx activity mediated the association between cortisol/11-deoxycortisol ratio and gFA.
Widespread correlation in female subjects between (A) generalized fractional anisotropy (gFA) and glutathione peroxidase (GPx) activity, (B) gFA and cortisol/11-deoxycortisol ratio. All voxels in the cluster are significant at the whole brain level (non-parametric family-wise error corrected p < 0.05). Scatterplots display correlation between (A) GPx activity and (B) cortisol/11-deoxycortisol ratio on x-axis and mean gFA values in significant clusters on y-axis.
Discussion
In this study, we aimed to investigate the sex-specific associations between internalizing symptoms, GSH-redox cycle regulation and HPA-axis function during the peripubertal period. In young adolescents from the general population, we found increased anxiety and depressive symptoms, increased GSH-redox cycle regulation (e.g., increased GPx activity, strong correlation between GPx and Gred activity) as well as increased HPA-axis activity (e.g., increased cortisol/11-deoxycortisol) in females as compared to males. Increased GPx activity and cortisol/11-deoxycortisol ratio were not only associated with high scores of internalizing symptoms, but also with a widespread increase in white matter gFA in females’ brain only. Mediation analysis indicated a causal effect of the cortisol/11-deoxycortisol ratio on the sex-difference in internalizing symptoms. Moreover, the cortisol/11-deoxycortisol ratio mediated the association between anxiety and GPx activity in females, suggesting a causal role of HPA-axis function in mediating changes in redox homeostasis associated with anxiety. Lastly, changes in GPx activity appeared to account for the association between cortisol/11-deoxycortisol and gFA in females. Altogether, our results suggest the involvement of tight and sex-specific interactions between GSH-redox cycle homeostasis and the HPA-axis, in the occurrence of internalizing symptoms and maturation of brain white matter in adolescent females.
Higher levels of internalizing symptoms in female compared to male adolescents recapitulated well known epidemiological findings [6, 8]. Based on these results, we further explored the biological mechanisms that may underlie this sex-difference in internalizing symptoms. The HPA-axis matures during adolescence and its activity can be quantified by the blood measurement of several glucocorticoids related to the metabolism of cortisol, the main stress hormone. Importantly, the ratio of such glucocorticoids may reflect the activity of enzymes involved in cortisol metabolism and which are under the influence of the adrenocorticotropic hormone (ACTH) [64]. Although few studies have characterized the adrenal steroid profile of healthy adolescents and its changes throughout puberty, some have underlined the high specificity of some steroids as well as their ratio in depression, especially in adolescents [42, 43]. For example, basal corticosterone levels and the ratio of corticosterone to its precursor 11-deoxycorticosterone, indicative of 11β-hydroxylase activity, were reported to be increased in adolescent depression, with more discriminant features in girls [65]. In the present cohort of adolescents, we found that females exhibited a higher cortisol/11-deoxycortisol ratio than males, a difference that mediated in part the higher levels of internalizing symptoms observed in females, suggesting a causal effect of HPA-axis function on internalizing symptoms. 11β-hydroxylase catalyzes the conversion of 11-deoxycortisol to cortisol and is influenced by ACTH, therefore increased cortisol/11-deoxycortisol ratio may involve a higher ACTH drive and reflect heightened activity of the HPA-axis. In this regard, increased cortisol/11-deoxycortisol ratio was reported in depressed subjects following dexamethasone suppression test [66]. Genetic polymorphism in the 11β-Hydroxylase gene (CYP11B1) have been also found to be associated with increased risk for late-life depression in women specifically [67]. However, the cortisol/11-deoyxcortisol ratio should not be interpreted as a sole result of 11β-Hydroxylase activity, as it may be influenced by changes in the downstream metabolism of cortisol, as well as alterations in the levels of corticosteroid binding globulin (CBG), which may increase levels of serum bound (inactive) cortisol and affect levels of total cortisol measured in serum. To our knowledge, our study is the first to explore sex-specific associations between the cortisol/11-deoxycortisol ratio and dimensional symptoms of anxiety and depression in adolescents. In line with prospective studies, these results highlight the function of the HPA-axis during the peripubertal period as a possible factor associated with greater vulnerability to internalizing symptoms [13] and emphasizes that this vulnerability may be exacerbated in females due to the emergence of sexually dimorphic HPA-axis regulation [68, 69].
In addition to HPA axis maturation, sexual dimorphism of the GSH metabolism and GSH-dependent responses have been reported [70] and may also be involved in sex-differences regarding internalizing symptoms. Accordingly, we found higher GPx activity in female adolescents as compared to males. This result is in line with previous studies which reported higher activity of GPx in pre-menopausal women compared to men, as well as in menopaused women under hormone replacement therapy, and in adolescent females compared to males [71, 72]. Importantly, females showed a strong correlation between GPx and Gred activities that may indicate a highly regulated oxidoreductive balance of the GSH-redox cycle during adolescence compared to males. Moreover, the female-specific association between GPx activity and anxiety, which appears mediated by the cortisol/11-deoxycortisol ratio, suggests a time and sex-specific interplay between HPA-axis function, GSH-antioxidant balance and the expression of internalizing symptoms. Based on experimental studies in preclinical models, two main hypotheses can be drawn. First, changes in the redox status occurring during the pubertal transition may show sex-specific patterns, as the rising gonadal hormones have known effects on the redox balance [50]. This change in redox status may in turn affect the synthesis of steroid hormones, as adrenal enzymes involved in steroid synthesis are highly sensitive to the redox state and the supply of oxidoreductive couples provided in great part by the GSH-system, such as NADP+/NADPH [73, 74]. The importance of a finely tuned redox balance required for proper HPA-axis function is further stressed in animal experiments, in which antioxidant treatment abolished the increased HPA-axis activity observed following chronic stress, but also promoted a hyperactivation of the HPA-axis activity in non-stressed animals [37]. Second, the increase in HPA-axis sensitivity and reactivity occurring during adolescence may promote an increase in metabolic demand and the generation of ROS, leading to a compensatory increase in the activity of detoxifying enzymes in females [25]. This hypothesis is supported by the causal mediation analysis, suggesting a causal role of HPA-axis function on changes of redox homeostasis in females. Consistently, experiments in rats show sex-specific antioxidant response to stress in the brain, with females showing an increased GPx activity compared to males [75], indicating sex-specific redox adaptations following stress exposure. Clinical studies report increased GPx activity in erythrocytes of patients with social phobia [34] and panic disorder [76]. Increased GPx activity was also reported in major depression [33] but with contrasting findings of decreased activity in another study [77]. Increased GPx activity may therefore represent compensatory mechanisms and may be influenced by sex, age, and the stage of illness. Together, our results suggest a tight interaction between stress, activity of the HPA-axis and redox homeostasis in females, that may underlie their increased vulnerability to internalizing disorders during the adolescent period.
In this study, we found no association between gFA and internalizing symptoms (i.e., STAI-T and BDI). While previous studies reported alterations in white matter microstructure in association with anxiety and depression self-ratings [78,79,80], we find this result not surprising given that this sample included adolescents from the general population with overall moderate levels of internalizing symptoms, as only ten adolescents met the criteria for a clinical anxiety disorder. On the other hand, our results indicate that increased GPx activity was specifically associated with widespread patterns of increased gFA in females and accounted for the association between cortisol/11-deoxycortisol ratio and gFA. While no previous study investigated the association between peripheral GSH-redox cycle regulation and white matter microstructure, our results, indicating higher integrity of white matter microstructure in association with increased peripheral antioxidant defenses in females, are in line with studies reporting a positive association between levels of brain GSH and FA in young adults [42, 48]. Oligodendrocytes have a high metabolism to build and maintain the myelin sheets around the axons [81] as well as high levels of iron necessary for some enzymes involved in the synthesis of myelin [82]. Therefore, these glial cells are particularly vulnerable to OxS and need to keep an appropriate redox homeostasis via the antioxidant systems, including the GSH redox cycle. Notably, Corcoba et al. [83] showed reduced FA along some white matter tracts (including the fornix) in mice with a weak GSH synthesis capacity. Interestingly, while oxidative stress can affect the viability of oligodendrocytes, an oxidized redox state can on the other hand favor the differentiation/maturation of oligodendrocytes [84, 85]. Therefore, further studies on redox modulating factors impacting myelin microstructure during development is needed. Last, developmental studies investigating the effects of chronic stress converge on evidence that stress may impact brain maturational trajectory and rate, especially accelerating the maturation of affective-related circuits [86, 87], which may both represent an advantage in adverse conditions but may also limit plasticity and raise vulnerability to subsequent anxiety and affective disorders. Accordingly, stress is associated with faster pubertal maturation [88] and precocious puberty increases the risk for internalizing disorders [89, 90]. Altogether, these results suggest that changes in the redox status associated with altered HPA-axis function and internalizing symptoms may play a role in the modulation of white matter microstructure maturation.
The main limitation of the study is the relatively low number of participants when stratified by sex. The results obtained must be interpreted with caution and should be replicated in a wider population of adolescents. Although all analyses investigating sex-differences were adjusted for internalizing symptoms, sex-specific findings may be influenced by the important difference in baseline levels of anxiety and depression levels between males and females in this cohort. Additionally, a main limitation is the lack of pubertal assessment by Tanner-staging, which allows a finer definition of the pubertal transition. Nevertheless, we reinforced the accuracy of the pubertal status estimation by adding T and E2 levels as covariates in the secondary analysis. Another main limitation is the fact that only the blood antioxidant system of GPx/Gred activity couple and GSH levels were analyzed, which may not reflect brain GSH-antioxidant regulation. Similarly, while the cortisol/11-deoxycortisol may reflect ACTH tone, it is not yet established how this ratio relates to HPA-axis reactivity, which would require a dynamic measure of cortisol secretion assessed over serial blood samples. Finally, the correlational nature of these findings, as well as the cross-sectional design used to conduct mediation analyses prevent from drawing any strong causal biological inference. Temporal contingency between these constructs should be investigated in longitudinal settings, considering important factors influencing HPA-axis activity such as adverse life events.
To conclude, these results highlight important interactions between HPA-axis and redox status as well as their potential role in the maturational trajectory of white matter microstructure. It lends support to the hypothesis that sexual dimorphism in HPA-axis and redox regulation may underly sex-differences in vulnerability to internalizing disorders. Longitudinal studies are warranted to investigate the association between peripheral redox status and brain maturation throughout the adolescent transition.
Data availability
The dataset analyzed in the current study is available from the corresponding author upon reasonable request.
References
Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52:S7–13.
Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77.
Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57.
Colman I, Wadsworth MEJ, Croudace TJ, Jones PB. Forty-year psychiatric outcomes following assessment for internalizing disorder in adolescence. Am J Psychiatry. 2007;164:126–33.
Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40:1086–93.
Patton GC, Hibbert ME, Carlin J, Shao Q, Rosier M, Caust J, et al. Menarche and the onset of depression and anxiety in Victoria. Aust J Epidemiol Community Health. 1996;50:661–6.
Angold A, Rutter M. Effects of age and pubertal status on depression in a large clinical sample. Dev Psychopathol. 1992;4:5–28.
Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29:145–58.
Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–73.
Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage. 2014;92:356–68.
Wierenga LM, Bos MGN, Schreuders E, vd Kamp F, Peper JS, Tamnes CK, et al. Unraveling age, puberty and testosterone effects on subcortical brain development across adolescence. Psychoneuroendocrinology. 2018;91:105–14.
Klein ZA, Romeo RD. Changes in hypothalamic–pituitary–adrenal stress responsiveness before and after puberty in rats. Horm Behav. 2013;64:357–63.
Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21:69.
Kiess W, Meidert A, Dressendórfer RA, Schriever K, Kessler U, Kónig A, et al. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37:502–6.
Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology. 2011;36:1226–38.
Malter Cohen M, Tottenham N, Casey BJ. Translational developmental studies of stress on brain and behavior: implications for adolescent mental health and illness? Neuroscience. 2013;249:53.
Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–71.
Harris LW, Lockstone HE, Khaitovich P, Weickert CS, Webster MJ, Bahn S. Gene expression in the prefrontal cortex during adolescence: implications for the onset of schizophrenia. BMC Med Genomics. 2009;2:1–14.
Dwir D, Khadimallah I, Xin L, Rahman M, Du F, Öngür D, et al. Redox and immune signaling in schizophrenia: new therapeutic potential. Int J Neuropsychopharmacol. 2023;26:309–21.
Schnider M, Jenni R, Ramain J, Camporesi S, Golay P, Alameda L, et al. Time of exposure to social defeat stress during childhood and adolescence and redox dysregulation on long-lasting behavioral changes, a translational study. Transl Psychiatry. 2022;26:12:1–12.
Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry. 2013;73:574–82.
Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68:261–75.
Salim S, et al. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res 2011;1404:63–71.
Distler MG, Palmer AA. Role of glyoxalase 1 (Glo1) and methylglyoxal (MG) in behavior: recent advances and mechanistic insights. Front Genet. 2012;3:250.
Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal JH, Cresto N, et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry. 2017;22:1701–13.
Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–6.
Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010;208:545–52.
Colaianna M, Schiavone S, Zotti M, Tucci P, Morgese MG, Bä L, et al. Neuroendocrine profile in a rat model of psychosocial stress: relation to oxidative stress. Antioxid Redox Signal. 2013;18:1385–99.
Zlatković J, Todorović N, Bošković M, Pajović SB, Demajo M, Filipović D. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol Cell Biochem. 2014;393:43–57.
Mejia-Carmona GE, Gosselink KL, Pérez-Ishiwara G, Martínez-Martínez A. Oxidant/antioxidant effects of chronic exposure to predator odor in prefrontal cortex, amygdala, and hypothalamus. Mol Cell Biochem. 2015;406:121–9.
Mocelin R, Marcon M, D’ambros S, Mattos J, Sachett A, Siebel AM, et al. N-Acetylcysteine reverses anxiety and oxidative damage induced by unpredictable chronic stress in zebrafish. Mol Neurobiol. 2019;56:1188–95.
Chakraborty S, Tripathi SJ, Raju TR, Shankaranarayana Rao BS. Mechanisms underlying remediation of depression-associated anxiety by chronic N-acetyl cysteine treatment. Psychopharmacology. 2020;237:2967–81.
Bilici M, Efe H, Köroğlu MA, Uydu HA, Bekaroğlu M, Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51.
Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Antioxidant enzyme and malondialdehyde levels in patients with social phobia. Psychiatry Res. 2008;159:95–100.
Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143:34–8.
Asaba K, Iwasaki Y, Yoshida M, Asai M, Oiso Y, Murohara T, et al. Attenuation by reactive oxygen species of glucocorticoid suppression on proopiomelanocortin gene expression in pituitary corticotroph cells. Endocrinology. 2004;145:39–42.
Prevatto JP, Torres RC, Diaz BL, Silva PMRE, Martins MA, Carvalho VF. Antioxidant treatment induces hyperactivation of the HPA axis by upregulating ACTH receptor in the adrenal and downregulating glucocorticoid receptors in the pituitary. Oxid Med Cell Longev. 2017;2017:4156361.
Mitani A, Azam A, Vuppusetty C, Ito K, Mercado N, Barnes PJ. Quercetin restores corticosteroid sensitivity in cells from patients with chronic obstructive pulmonary disease. Exp Lung Res. 2017;43:417.
McIntosh LJ, Hong KE, Sapolsky RM. Glucocorticoids may alter antioxidant enzyme capacity in the brain: baseline studies. Brain Res. 1998;791:209–14.
Beytut E, Yilmaz S, Aksakal M, Polat S. The possible protective effects of vitamin E and selenium administration in oxidative stress caused by high doses of glucocorticoid administration in the brain of rats. J Trace Elem Med Biol. 2018;45:131–5.
Alam MM, Okazaki K, Nguyen LTT, Ota N, Kitamura H, Murakami S, et al. Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J Biol Chem. 2017;292:7519–30.
Monin A, Baumann PS, Griffa A, Xin L, Mekle R, Fournier M, et al. Glutathione deficit impairs myelin maturation: relevance for white matter integrity in schizophrenia patients. Mol Psychiatry. 2015;20:827–38.
Chetty S, Friedman AR, Taravosh-Lahn K, Kirby ED, Mirescu C, Guo F, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry. 2014;19:1275–83.
Liu X, Watanabe K, Kakeda S, Yoshimura R, Abe O, Ide S, et al. Relationship between white matter integrity and serum cortisol levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Br J Psychiatry. 2016;208:585–90.
Echouffo-Tcheugui JB, Conner SC, Himali JJ, Maillard P, Decarli CS, Beiser AS, et al. Circulating cortisol and cognitive and structural brain measures. Neurology. 2018;91:e1961–70.
Simon KR, Merz EC, He X, Desai PM, Meyer JS, Noble KG. Socioeconomic factors, stress, hair cortisol, and white matter microstructure in children. Dev Psychobiol. 2021;63:e22147.
Vestergaard M, Baaré WFC, Holm SK, Madsen CG, Paulson OB, Born AP, et al. Glucocorticoid treatment for non-cerebral diseases in children and adolescents is associated with differences in uncinate fasciculus microstructure. Pediatr Res. 2022;91:879–87.
Hermens DF, Hatton SN, Lee RSC, Naismith SL, Duffy SL, Paul Amminger G, et al. In vivo imaging of oxidative stress and fronto-limbic white matter integrity in young adults with mood disorders. Eur Arch Psychiatry Clin Neurosci. 2018;268:145–56.
Heck AL, Handa RJ. Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44:45.
Ruszkiewicz JA, Miranda-Vizuete A, Tinkov AA, Skalnaya MG, Skalny AV, Tsatsakis A, et al. Sex-specific differences in redox homeostasis in brain norm and disease. J Mol Neurosci. 2019;67:312–42.
Massafra C, Gioia D, De Felice C, Picciolini E, De Leo V, Bonifazi M, et al. Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. J Endocrinol. 2000;167:447–52.
Piguet C, Klauser P, Celen Z, James Murray R, Magnus Smith M, Merglen A. Randomized controlled trial of a mindfulness-based intervention in adolescents from the general population: the Mindfulteen neuroimaging study protocol. Early Inter Psychiatry. 2021;16:891–901.
Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39:1208.
Turgeon L, Chartrand É. Psychometric properties of the French Canadian version of the state-trait anxiety inventory for children. Educ Psychol Meas. 2016;63:174–85. https://doi.org/10.1177/0013164402239324.
Osman A, Barrios FX, Gutierrez PM, Williams JE, Bailey J. Psychometric properties of the Beck Depression Inventory-II in nonclinical adolescent samples. J Clin Psychol. 2008;64:83–102.
Alemán-Gómez Y, Baumgartner T, Klauser P, Cleusix M, Jenni R, Hagmann P, et al. Multimodal magnetic resonance imaging depicts widespread and subregion specific anomalies in the Thalamus of early-psychosis and chronic schizophrenia patients. Schizophr Bull. 2023;49:196–207.
Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90.
Avants BB, Tustison NJ, Stauffer M, Song G, Wu B, Gee JC. The Insight ToolKit image registration framework. Front Neuroinform. 2014;8:44.
Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 2014;8:8.
Özarslan, E., Koay, C. G., & Basser, P. J. Simple Harmonic Oscillator Based Reconstruction and Estimation for One-Dimensional q-Space Magnetic Resonance (1D-SHORE). Applied and Numerical Harmonic Analysis, 2012;373–99. https://doi.org/10.1007/978-0-8176-8379-5_19.
Alkazemi D, Rahman A, Habra B. Alterations in glutathione redox homeostasis among adolescents with obesity and anemia. Sci Rep. 2021;11:3034.
Eisenhofer G, Peitzsch M, Kaden D, Langton K, Pamporaki C, Masjkur J, et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin Chim Acta. 2017;470:115–24.
Holsboer F, Müller OA, Doerr HG, Sippell WG, Stalla GK, Gerken A, et al. ACTH and multisteroid responses to corticotropin-releasing factor in depressive illness: relationship to multisteroid responses after ACTH stimulation and dexamethasone suppression. Psychoneuroendocrinology. 1984;9:147–60.
Hirtz R, Libuda L, Hinney A, Föcker M, Bühlmeier J, Holterhus PM, et al. The adrenal steroid profile in adolescent depression: a valuable bio-readout? Transl Psychiatry. 2022;12:1–12.
Holsboer F, Doerr HG, Sippell WG. Increased sensitivity of the dexamethasone suppression test in depressed female patients based on multisteroid analysis. Psychiatry Res. 1983;8:49–57.
Ancelin ML, Norton J, Ritchie K, Chaudieu I, Ryan J. 11β-Hydroxylase (CYP11B1) gene variants and new-onset depression in later life. J Psychiatry Neurosci. 2021;46:E147–53.
Oldehinkel AJ, Bouma EMC. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: a review of gender differences. Neurosci Biobehav Rev. 2011;35:1757–70.
McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–33.
Wang L, Ahn YJ, Asmis R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox Biol. 2020;31:101410.
Massafra C, Gioia D, De Felice C, Muscettola M, Longini M, Buonocore G. Gender-related differences in erythrocyte glutathione peroxidase activity in healthy subjects. Clin Endocrinol. 2002;57:663–7.
Rush JWE, Sandiford SD. Plasma glutathione peroxidase in healthy young adults: influence of gender and physical activity. Clin Biochem. 2003;36:345–51.
Rapoport R, Sklan D, Hanukoglu I. Electron leakage from the adrenal-cortex mitochondrial P450Scc and P450C11 systems: NADPH and steroid dependence. Arch Biochem Biophys. 1995;317:412–6.
Agarwal AK, Auchus RJ. Minireview: Cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology. 2005;146:2531–8.
Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, Kamper M, Dalla C, Pitychoutis PΜ, et al. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav. 2009;98:215–22.
Kuloglu M, Atmaca M, Tezcan E, Ustundag B, Bulut S. Antioxidant enzyme and malondialdehyde levels in patients with panic disorder. Neuropsychobiology. 2002;46:186–9.
Kodydková J, Vávrová L, Zeman M, Jirák R, Macášek J, Staňková B, et al. Antioxidative enzymes and increased oxidative stress in depressive women. Clin Biochem. 2009;42:1368–74.
Coloigner J, Batail JM, Commowick O, Corouge I, Robert G, Barillot C, et al. White matter abnormalities in depression: a categorical and phenotypic diffusion MRI study. NeuroImage Clin. 2019;22:101710.
Madsen KS, Jernigan TL, Vestergaard M, Mortensen EL, Baaré WFC. Neuroticism is linked to microstructural left-right asymmetry of fronto-limbic fibre tracts in adolescents with opposite effects in boys and girls. Neuropsychologia. 2018;114:1–10.
Aggarwal N, Williams LE, Tromp DPM, Pine DS, Kalin NH. A dynamic relation between whole-brain white matter microstructural integrity and anxiety symptoms in preadolescent females with pathological anxiety. Transl Psychiatry. 2022;12:1–11.
Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. https://pubmed.ncbi.nlm.nih.gov/19847447/.
Ravera S, Bartolucci M, Cuccarolo P, Litamè E, Illarcio M, Calzia D, et al. Oxidative stress in myelin sheath: the other face of the extramitochondrial oxidative phosphorylation ability. Free Radic Res. 2015;49:1156–64. https://www.tandfonline.com/doi/abs/10.3109/10715762.2015.1050962.
Corcoba A, Steullet P, Duarte JMN, Van De Looij Y, Monin A, Cuenod M, et al. Glutathione deficit affects the integrity and function of the fimbria/fornix and anterior commissure in mice: relevance for schizophrenia. Int J Neuropsychopharmacol. 2016;19:1–11. https://doi.org/10.1093/ijnp/pyv110.
Noble M, Smith J, Power J, Mayer-Pröschel M. Redox state as a central modulator of precursor cell function. Ann N Y Acad Sci. 2003;991:251–71.
Noble M, Mayer-Pröschel M, Pröschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7:1456–67. https://home.liebertpub.com/ars.
Callaghan BL, Tottenham N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 2016;7:76–81.
Tooley UA, Bassett DS, Mackey AP. Environmental influences on the pace of brain development. Nat Rev Neurosci. 2021;22:372–84.
Ellis BJ. Timing of pubertal maturation in girls: an integrated life history approach. Psychol Bull. 2004;130:920–58.
Conley CS, Rudolph KD, Bryant FB. Explaining the longitudinal association between puberty and depression: sex differences in the mediating effects of peer stress. Dev Psychopathol. 2012;24:691.
Ge X, Conger RD, Elder GH Jr. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–17.
Acknowledgements
We are grateful to Gloria Reuteler, Adeline Cottier and Morgane Baumgartner for expert technical assistance, Carole Grasset-Salomon, Maria Isabel Rodriguez and Fabienne Marechal-Rouiller at the Paediatric Research Platform at Geneva University Hospitals for their involvement in the blood collection and Eleonore Pham, Sondes Jouabli and Valdimira Ivanova for their implication in the clinical assessment. We would like to acknowledge the Brain and Behavior Laboratory (BBL) at University of Geneva, Bruno Bonet, Frédéric Grouiller Damien Marie and Céline Gaignot for MRI recording. We would like to thank all the subjects for their participation. This work was supported by a fund granted by the Leenaards Foundation, the Schmidheiny foundation and National Center of Competence in Research (NCCR) “SYNAPSY—The Synaptic Bases of Mental Diseases” financed by the Swiss National Science Foundation (no. 51NF40-158776). PK and DD are funded by a fellowship from the Adrian and Simone Frutiger Foundation.
Author information
Authors and Affiliations
Contributions
ZS wrote the manuscript, processed the blood samplings, and performed the statistical analysis. YA-G performed MRI images pre-processing and gFA computation and participated in writing the manuscript. MMS participated in subject recruitment and ZC in data acquisition. BM participated in the statistical analysis. P-AB supervised the steroid measurements. PS performed quality control check and validated the GPx and Gred data. CP, AM, DD and PK conceived the study and contributed to the manuscript editing. PK and DD directly supervised the analysis and contributed to writing the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schilliger, Z., Alemán-Gómez, Y., Magnus Smith, M. et al. Sex-specific interactions between stress axis and redox balance are associated with internalizing symptoms and brain white matter microstructure in adolescents. Transl Psychiatry 14, 30 (2024). https://doi.org/10.1038/s41398-023-02728-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02728-4