Abstract
Hyperthyroidism and clinical depression are common, and there is preliminary evidence of substantial comorbidity. The extent of the association in the general population, however, has not yet been estimated meta-analytically. Therefore we conducted this systematic review and meta-analysis (registered in PROSPERO: CRD42020164791). Until May 2020, Medline (via PubMed), PsycINFO, and Embase databases were systematically searched for studies on the association of hyperthyroidism and clinical depression, without language or date restrictions. Two reviewers independently selected epidemiological studies providing laboratory or ICD-based diagnoses of hyperthyroidism and diagnoses of depression according to operationalized criteria (e.g. DSM) or to cut-offs in established rating scales. All data, including study quality based on the Newcastle-Ottawa Scale, were independently extracted by two authors. Odds ratios for the association of clinical depression and hyperthyroidism were calculated in a DerSimonian-Laird random-effects meta-analysis. Out of 3372 papers screened we selected 15 studies on 239 608 subjects, with 61% women and a mean age of 50. Relative to euthyroid individuals, patients with hyperthyroidism had a higher chance of being diagnosed with clinical depression: OR 1.67 ([95% CI: 1.49; 1.87], I2: 6%; prediction interval: 1.40 to 1.99), a result supported in a number of sensitivity and subgroup analyses. The OR was slightly less pronounced for subclinical as opposed to overt hyperthyroidism (1.36 [1.06; 1.74] vs. 1.70 [1.49; 1.93]). This comorbidity calls for clinical awareness and its reasons need investigation and may include neurobiological mechanisms, common genetic vulnerability and a generally heightened risk for clinical depression in patients with chronic somatic disorders.
Similar content being viewed by others
Introduction
A link between thyroid disorders and depression has been investigated for decades [1]. Researchers uncovered several possible interactions between thyroid metabolism, the HPT-Axis and mood regulation [2,3,4,5,6], and the association of hypothyroidism with depression has been the focus of various meta-analyses [7,8,9]. They yielded positive results, but the extent of the comorbidity may have been overestimated [10]: In the most recent meta-analysis, we estimated an odds ratio for hypothyroidism and clinical depression of 1.30 [1.08–1.57] in population-based studies [11].
The association of depression with the other end of the thyroid disorder spectrum, hyperthyroidism, has been much less investigated, although it consists of a common group of conditions [12]. For example, NHANES III found general population prevalences of 0.7% and 0.5% for subclinical and overt hyperthyroidism, respectively [13]. A Danish register-based study [14] found that subjects with hyperthyroidism had a higher likelihood of developing depression than euthyroid controls (Hazard Ratio (HR): 1.54 [1.36–1.74]). Similarly, Williams et al. [15] found serum T4 to be positively associated with depression. In contrast, an individual patient data meta-analysis of six studies by Wildisen et al. [16] yielded no relevant association of subclinical hyperthyroid states and depression.
So far, no clear picture of a link between hyperthyroidism and depression emerged. Therefore, we conducted a systematic review and meta-analysis of studies presenting data on established hyperthyroidism—either subclinical or overt—and clinically relevant depression. To reduce bias, we restricted this meta-analysis to epidemiological and population-based studies and did not include studies based on samples from outpatient departments for thyroid or mood disorders.
Methods
This is a systematic review and meta-analysis registered in PROSPERO (CRD42020164791). Its reporting is based on the PRISMA 2020 and MOOSE guidelines [17, 18].
Data sources and searches
We conducted a systematic search in MEDLINE and PubMed Central via PubMed, in PsycINFO via EBSCOhost, and in Embase to identify epidemiological studies on the association of hyperthyroidism and depression (last update on May 4, 2020). We combined generic terms for depression, hyperthyroidism, and population-based study designs (Supplementary Methods).
Study selection
Inclusion criteria
-
1.
Study design: Cohort and cross-sectional studies.
-
2.
Study population: Studies needed to be representative of the general population. If study groups were not randomly sampled from the general population, studies were only eligible if they (1) drew on very broad and diverse populations, such as civil servants or the totality of hospitalized patients in one country, and (2) study reports were not suggestive of biases, i.e. included a complete report of the recruitment and selection process.
-
3.
Exposure: Hyperthyroid thyroid disorders, either subclinical, overt or, if nothing else was stated, of autoimmune origin (i.e. Graves’ Disease, but not Hashimoto thyroiditis). Disorders needed to be diagnosed by established laboratory methods or had to be drawn from registers employing data with documented reliability.
-
4.
Outcome: Clinically significant depression, either defined as major depressive disorder (MDD) diagnosis according to established diagnostic systems, e.g. DSM or ICD, or an above-threshold score in established psychopathology rating scales for depression [19], as specified by study authors. Diagnoses could originate with assessment rating scales, standardized interviews (e.g. WHO-CIDI) or from registers including hospital data with documented reliability. To err on the conservative side we did not associate hyperthyroidism with any change in depression scores, because, in the general population, variations in depression scale scores below a predefined cut-off point for caseness are not indicative of clinical depression and may create pseudo-effects.
Exclusion criteria
-
1.
Study design: Case-control studies
Data extraction and quality assessment
Titles and abstracts retrieved in the literature search were independently screened by two authors (HB, BI). “Grey” literature was included and no language or date restrictions applied. We searched bibliographies of every article eventually included. All articles potentially eligible were read independently by two authors (HB, BI), and data of included studies were extracted independently by two authors (HB, BI) using an Excel-based standardized data extraction form in accordance with the Cochrane Collaboration Handbook [20]. Disagreements were solved by discussion with the senior author (CB). If no effect sizes or sufficient data for calculation were reported, we contacted authors by e-mail.
All studies included were rated independently by two authors (HB, BI) for their risk of bias, using the Newcastle-Ottawa Scale (NOS) adaptation for cohort [21] and cross-sectional studies [22]. To be rated as “low risk of bias”, studies needed to be categorized in the highest NOS-category, i.e. they needed to receive all or all but one star in the rating system.
Data synthesis and analysis
To account for differences in study settings and methodology we used random effects analyses (DerSimonian & Laird) [23]. Statistical heterogeneity is reported as I2 statistic and Tau (τ). We assessed publication bias in funnel plots and Egger’s test [24] and estimated the role of missing studies in trim-and-fill-analyses [25]. Prediction intervals were calculated to account for the heterogeneity between studies [26]. We conducted leave-one-out analyses if forest plots indicated a disproportionate effect of single studies. All calculations have been carried out in Comprehensive Meta-Analysis (CMA) Version 3 [27] and R [28], using the packages meta [29] and metafor [30].
Primary outcome analysis
The primary outcome is the association of hyperthyroidism and clinical depression. We compared depression prevalence of patients with versus without hyperthyroidism and expressed the results as odds ratio (OR) ± 95% confidence interval (CI). If studies reported effects as risk ratio (RR) or hazard ratio (HR), we transformed these effects into ORs (Supplementary Methods). If studies reported multiple differently adjusted effect sizes, we included those with minimum adjustment to be as coherent as possible with unadjusted or self-calculated effect sizes. The analysis includes all studies reporting results for overt and/or subclinical hyperthyroidism. If a study reported effects for both, only results for overt hyperthyroidism were included to maintain independence. Calculations and formulae are listed in the supplementary information (Supplementary Methods).
Subgroup analyses
We subdivided hyperthyroidism into both its overt and subclinical form, as defined in the studies included, and stratified our primary analyses by gender, risk of bias, intake of thyroid medication, a core group of strictly population-based studies, and assessment of depression. To minimize bias, the subgroup analysis investigating gender-specific effects was conducted only on studies that reported effects for both genders. All of the above stratifications were also repeated in the subgroup analyses of overt and subclinical hyperthyroidism.
Post hoc analyses
We compared studies on older populations (age ≥ 60 years) with studies on subjects of all ages to detect age-specific trends. To compare the effects of hyperthyroidism with those of hypothyroidism, we selected studies reporting an effect for both thyroid disorders and analysed them separately.
Results
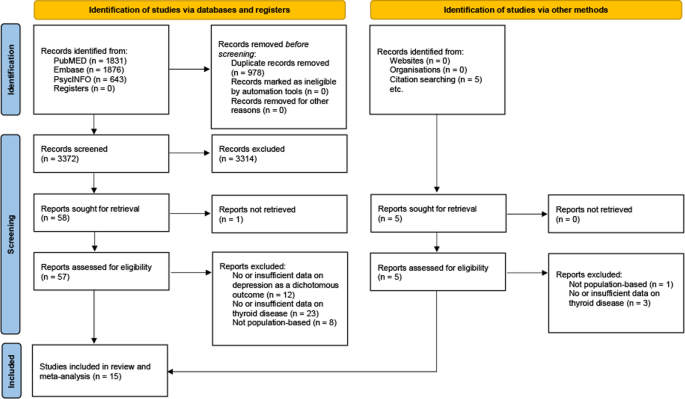
Our search yielded 4350 articles. After exclusion of duplicates, 3372 were screened and out of those, 62 were assessed for eligibility in full text. Fifteen studies [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] are included in this meta-analysis (PRISMA flowchart, Fig. 1). Three studies reported effects for overt, 7 for subclinical and 4 for both types of hyperthyroidism. The study of Chen et al. [33] reported effects for Grave’s disease, which we considered a plausible proxy of overt hyperthyroidism.
Table 1 displays characteristics of all studies: Three cohort (72.5% of participants) and 12 cross-sectional studies (27.5%) accounted for a total of 239,608 participants, ranging from 60 [41] in the smallest to 150,960 [44] in the largest study. Study size weighted mean age of participants was 50 years. The overall proportion of women was 60.8%.
Six studies reported depression in DSM- or ICD-conforming diagnoses of major depressive disorder. Cut-off points in depression scores were employed in 9 studies.
In 13 studies, authors provided diagnoses of thyroid disorders based upon established laboratory methods, 2 used register data on ICD-based diagnoses. Intake of thyroid or anti-thyroid medication was allowed in 10 studies.
Applying the Newcastle-Ottawa Scale, 3 cohort and 2 cross-sectional studies were rated as carrying a “low risk of bias”.
Full data describing all included studies are presented in the supplementary information (Supplementary Table 1).
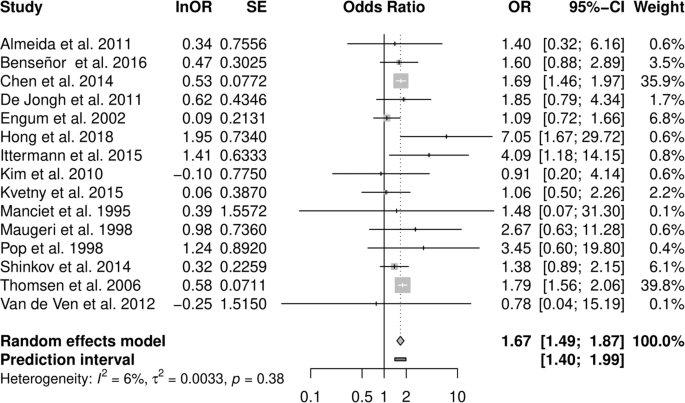
Pooled analysis of all studies resulted in an OR estimate of 1.67 [1.49–1.87] of clinical depression in all types of hyperthyroidism relative to euthyroidism (Fig. 2 and Table 2), no relevant heterogeneity was observed (I² = 6.4%, τ = 0.058; prediction interval: 1.40 to 1.99).
Subgroup analysis of studies restricted to overt hyperthyroidism resulted in a similar OR of 1.70 [1.49–1.93], and subclinical hyperthyroidism was also associated with depression (1.36 [1.06–1.74], Table 2). Stratification by gender, based on results from three studies, revealed an OR of 1.37 [0.91–2.05] among women and 1.84 [1.34–2.54] in men (p-value between effects: 0.257). Strictly population-based studies as well as studies that allowed for thyroid or anti-thyroid medication reported slightly weaker summary associations with depression. A cohort study design and a DSM- or ICD-conforming diagnosis of depression resulted in moderately stronger effects (Supplementary Table 2.1). Stratifying subgroup analyses in overt and subclinical hyperthyroidism yielded similar effects (Supplementary Table 2.2).
The funnel plot of the primary analysis indicated an asymmetric reporting of effects, with studies missing in the lower right quadrant, indicating the possibility of biased reporting in favour of weaker effects. However, Egger’s test was not positive.
Analysing studies with low risk of bias confirmed the results of the primary subgroup analyses on overt hyperthyroidism, while the association of subclinical hyperthyroidism with depression became negligible (1.08 [0.8–1.46]).
In a leave-one-out analysis, study weight was distributed unevenly with two studies carrying almost 80% of the weight. Exclusion of these large register studies by Chen et al. [33] and Thomsen et al. [44] slightly lowered the association (1.61 [1.34–1.93] and 1.58 [1.35–1.85] respectively). In subclinical hyperthyroidism, removal of the study by Engum et al. [35] increased ORs to 1.52 [1.16–1.99].
Similar to the primary analysis, heterogeneity was low or moderate in subgroup and sensitivity analyses, with I² generally not exceeding 50% (Supplementary Table 2).
Post-hoc analyses on studies on older populations reported effects similar to those of studies on all ages (Supplementary Table 2).
In fourteen studies effects for both, hyper- and hypothyroidism were reported [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Thomsen et al. published corresponding data on hypothyroidism in a separate study [46]. Combining studies on overt and subclinical disease resulted in a nominally weaker association of depression with hypothyroidism (1.36 [1.02–1.82]]) than with hyperthyroidism (1.61 [1.34–1.93]). In overt hyperthyroidism (1.66 [1.22–2.26]) associations were similar to those in overt hypothyroidism (1.69 [0.83–3.45]). Subclinical disease showed equal ORs in hyper- (1.36 [1.06–1.74]) and in hypothyroidism (1.35 [1.05–1.73]). After correction for potentially missing studies (Egger’s test p-value = 0.003) however, the association of hypothyroidism with depression decreased to an OR of 1.14 [0.88–1.47].
Discussion
To our knowledge, this is the first meta-analysis investigating both subclinical and overt hyperthyroidism and its association with clinical depression in the general population. We found a statistically significant association of hyperthyroidism and depression, with small confidence as well as prediction intervals indicating a robust effect. The results are supported by a variety of subgroup as well as sensitivity analyses and by low heterogeneity. Therefore, even though fewer studies have been carried out on hyper- than on hypothyroidism (OR: 1.30 [1.08–1.57]) [11], we consider the evidence of an association with clinical depression and the effect itself marginally stronger in hyperthyroidism (OR 1.67 [1.49–1.87]).
These findings challenge earlier research on hyperthyroidism and depression: Wildisen et al. [16], for example, reported no relevant effect of subclinical hyperthyroidism on BDI-scores. However, they did not analyse patients with overt hyperthyroidism. Of note, more in line with Wildisen and co-authors’ study, our results point to a weaker, if any, association of subclinical hyperthyroidism with clinical depression than in overt hyperthyroidism.
This gradient in effect size may support the notion that it is not primarily autoimmunity that drives the association but possibly the increase in thyroid hormones. Further, in an earlier meta-analysis we could not support an association of TPO-antibody positivity with clinical depression [11]. At the pathophysiological level, the findings are consistent with several hypotheses of neuroendocrine causes of depression: Dysbalanced, thyroid hormones as key regulators of metabolism can contribute to typical symptoms of depression such as sleep disturbance, weight change, fatigue or psychomotor agitation [47]. They also stimulate cortical 5-HT secretion [48] and might act as co-transmitters in the noradrenergic system [49], influencing monoaminergic transmission in the brain. Several animal models showed that induction of hypo- and hyperthyroid states in rats significantly altered cortical monoamine levels: While in hypothyroidism 5-HT levels decreased in multiple brain regions [50,51,52], in hyperthyroidism, norepinephrine concentration as well as the number of 5-HT2-receptors were downregulated simultaneously with an increase in 5-HT levels [50, 52,53,54]. One study reported depression-like behaviour in rats with induced states of both hypo- and hyperthyroidism [55]. Interestingly, hyperthyroid rats also showed anxiety-like behaviour. Further, both hypo- as well as hyperthyroid disturbances of the HPT-axis can lead to hypercortisolism [56,57,58,59,60], which is often found in patients suffering from depression [6, 61,62,63]. Possibly, inflammation provides a link between thyroid disorders and depression, because with Hashimoto and Graves‘ disease, two of the leading causes of thyroid dysfunction result from autoantibodies against thyroid tissue, are characterized by lymphocytic infiltrates and increased levels of proinflammatory cytokines (such as IL-6 and TNF-alpha), and may have their origins in viral infections [64, 65]. Recently, similar factors have been discussed in the etiology of depression [66], and common pathways may explain the comorbidity described in primary studies and in this meta-analysis.
In principle, the association between thyroid disorders and depression may also be based on a common genetic vulnerability. Some structures involved in brain thyroid hormone metabolism, such as the deiodinase enzymes (DIO) type 1,2 and 3 or the thyroid hormone transporter OATP1C1, may lead to a local state of hormone deficiency when functionally impaired or overactive, facilitating the development of depression potentially regardless of serum thyroid hormone levels [4, 5, 67, 68]. Studies showed that some DIO2 polymorphisms were indeed associated with worse psychological well-being [69] and DIO2 expression was reduced in patients suffering from recurrent depressive disorders (rDD) [70]. DIO1 variants were associated with lifetime MDD in Caucasian female individuals [71] and DIO1 expression was also found to be decreased in subjects with rDD [72]. However, other studies could not link DIO expression or certain polymorphisms to depression or impaired well-being [73, 74]. Variation of the OATP1C1 gene was connected to fatigue and depression in hypothyroid individuals [75] and to depression in subjects who suffered from ischemic stroke [76]. We are not aware, however, of studies investigating the association of depression with genetic variations leading to a brain-specific local state of hyperthyroidism.
In an entirely different approach, the association of hyperthyroidism and clinical depression may be explained by the observation that chronic conditions as such are often related to a greater risk of being depressed [77,78,79,80]. In this framework, a chronic condition, for example, hyperthyroidism, acts as a stressor and may, particularly in vulnerable people, contribute to the development of clinical depression.
Reverse causation also needs consideration: Subsequent to the elevation of cortisol caused by depression, TRH production might be stimulated and lead to an overproduction of T4 [2, 5]. Normalization or, rather, a decrease of T4 levels after successful treatment of depressive disorder has also been observed [81, 82]. In general, however, the common paradigm in depression research is one of interdependence, not of one factor causing the other [83, 84], for example, with regard to the heightened cardiovascular risk of patients with depression.
A hint towards the predominant direction of the effect responsible for the observed association might be found in our subgroup analyses. Here, studies were stratified by study design, with cohort studies that assured absence of depression at baseline showing stronger effects than cross-sectional studies. While this can be understood as a sign for a stronger effect of hyperthyroidism on depression than vice versa, the analysis was only based on three cohort studies and therefore has to be regarded as preliminary.
A key feature of the present study is its focus on clinical depression. As a result, we may have missed subtle changes in psychopathology. However, not only was there no indication of such an effect in Wildisen and co-authors’ study [16], but in searching for differences in low and subclinical score ranges lies the risk of inflating small findings of doubtful clinical relevance. The problem of employing subclinical phenomena works also in thyroid parameters: Williams et al. [15], in their early meta-analysis, took into account the full range of thyroid hormones, physiological and pathological alike. Interestingly, however, even with their very broad approach they arrived at no stronger association than the one in the present study.
At the same time, relying on clinical data derived only from routine examinations in outpatient clinics for mood or metabolism disorders introduces selection bias [10], hence our restriction to epidemiological studies. In our view, therefore, the OR presented in this study represents a conservative estimate of the association. Since the effect we estimated is moderate it is worth noting that relatively small effect sizes are common in medicine, even in established biomarkers [85].
With regard to clinical practice, our results suggest that heightened awareness of depression is justified in patients with hyperthyroidism, as is TSH screening among patients with depression. In distinction to our analysis of hypothyroidism and depression—where the observed ORs were 0.71 [0.40–1.25] and 1.48 [1.18–1.85] for men and women respectively [11]—we did not find a clear-cut gender differential, although men with hyperthyroidism were slightly more affected by clinical depression than women. In fact, for women, our results are inconclusive, as the confidence interval includes a null effect. This applies all the more to the findings regarding women with subclinical hyperthyroidism where no association is apparent. Of note, the data were not sufficient for a subgroup analysis on ethnic differences indicating a need for future research.
In regard to therapy, both conditions, hyperthyroidism and depression, demand guideline-oriented treatment. We are not aware of an established treatment that would target the two diseases in one approach.
Hyperthyroidism is not as prevalent as hypothyroidism in the general population. Assuming a population of 332.5 million people in the US [86], a hyperthyroidism prevalence of 1.3% [13], and a 12-months depression prevalence of 6.7% [87], an OR of 1.67 translates into about 484 thousand people with the comorbidity, 194 thousand of those presumably due to hyperthyroidism. Provided that there are 22.3 million people with depression each year, in a model assuming that hyperthyroidism causes depression, hyperthyroidism contributes about 0.9% to the pandemic of depression.
We focussed on population-based studies, but the term leaves some room for debate: For example, a sample of civil servants, such as the one investigated by Benseñor et al. [32], is not population-based in the strict sense. However, while it is likely that prevalence differs contingent on the sample, it seems implausible that the association between hyperthyroidism and depression differs meaningfully in such a large and diverse group of people. Reassuringly, sensitivity analysis restricted to strictly population-based studies yielded no substantially different results (Supplementary Table 2). In the same vein, exclusion of register studies did not substantially change the summary estimate.
In conclusion, there is an association of hyperthyroidism with clinical depression (1.67 [95% CI: 1.49–1.87]), that is stronger in overt than in subclinical hyperthyroidism, pointing to a possibly biological association of both conditions. This should raise awareness in clinicians and researchers alike: Not only hypothyroid but also, and especially, hyperthyroid patients are at higher risk for depressive disorders and should be monitored for signs of clinical depression. How a hyperthyroid metabolism influences mood is not yet explained and, particularly regarding sex, deserves greater attention in the future research of thyroid–brain interactions.
References
Whybrow PC, Prange AJ Jr, Treadway CR. Mental changes accompanying thyroid gland dysfunction. a reappraisal using objective psychological measurement. Arch Gen Psychiatry. 1969;20:48–63.
Bahls SC, de Carvalho GA. The relation between thyroid function and depression: a review. Braz J Psychiatry. 2004;26:41–49.
Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20:1101–14.
Feldman AZ, Shrestha RT, Hennessey JV. Neuropsychiatric manifestations of thyroid disease. Endocrinol Metab Clin North Am. 2013;42:453–76.
Hage MP, Azar ST. The link between thyroid function and depression. J Thyroid Res. 2012;2012:590648.
Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model - are we there yet? Behav Brain Res. 2018;341:79–90.
Loh HH, Lim LL, Yee A, Loh HS. Association between subclinical hypothyroidism and depression: an updated systematic review and meta-analysis. BMC Psychiatry. 2019;19:12.
Siegmann EM, Müller HHO, Luecke C, Philipsen A, Kornhuber J, Grömer TW. Association of depression and anxiety disorders with autoimmune thyroiditis: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:577–84.
Tang R, Wang J, Yang L, Ding X, Zhong Y, Pan J, et al. Subclinical hypothyroidism and depression: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2019;10:340.
Baethge C. Autoimmune thyroiditis and depression. JAMA Psychiatry. 2018;75:1204–1204.
Bode H, Ivens B, Bschor T, Schwarzer G, Henssler J, Baethge C. Association of hypothyroidism and clinical depression: a systematic review and meta-analysis. JAMA Psychiatry 2021.
Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301–16.
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–99.
Brandt F, Thvilum M, Almind D, Christensen K, Green A, Hegedüs L, et al. Hyperthyroidism and psychiatric morbidity: evidence from a Danish nationwide register study. Eur J Endocrinol. 2014;170:341–8.
Williams MD, Harris R, Dayan CM, Evans J, Gallacher J, Ben-Shlomo Y. Thyroid function and the natural history of depression: findings from the Caerphilly Prospective Study (CaPS) and a meta-analysis. Clin Endocrinol (Oxf). 2009;70:484–92.
Wildisen L, Del Giovane C, Moutzouri E, Beglinger S, Syrogiannouli L, Collet TH, et al. An individual participant data analysis of prospective cohort studies on the association between subclinical thyroid dysfunction and depressive symptoms. Sci Rep. 2020;10:19111.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–12.
Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken). 2011;63:S454–66.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, Accessed April, 2021.
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0147601.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. Bmj. 2011;342:d549.
Borenstein M, Hedges, L, Higgins, J, Rothstein, H Comprehensive Meta-Analysis Version 3. Biostat: Englewood, NJ, 2013.
Team RCR: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2020.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Viechtbauer W. Conducting meta-analyses in R with the metafor Package. 2010; 36:48.
Almeida OP, Alfonso H, Flicker L, Hankey G, Chubb SA, Yeap BB. Thyroid hormones and depression: the Health in Men study. Am J Geriatr Psychiatry. 2011;19:763–70.
Benseñor IM, Nunes MA, Sander Diniz MF, Santos IS, Brunoni AR, Lotufo PA. Subclinical thyroid dysfunction and psychiatric disorders: cross-sectional results from the Brazilian Study of Adult Health (ELSA-Brasil). Clin Endocrinol (Oxf). 2016;84:250–6.
Chen HH, Yeh SY, Lin CL, Chang SN, Kao CH. Increased depression, diabetes and diabetic complications in Graves’ disease patients in Asia. Qjm. 2014;107:727–33.
de Jongh RT, Lips P, van Schoor NM, Rijs KJ, Deeg DJ, Comijs HC, et al. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur J Endocrinol. 2011;165:545–54.
Engum A, Bjøro T, Mykletun A, Dahl AA. An association between depression, anxiety and thyroid function-a clinical fact or an artefact? Acta Psychiatr Scand. 2002;106:27–34.
Hong JW, Noh JH, Kim DJ. Association between subclinical thyroid dysfunction and depressive symptoms in the Korean adult population: the 2014 Korea National Health and Nutrition Examination Survey. PLoS ONE. 2018;13:e0202258.
Ittermann T, Völzke H, Baumeister SE, Appel K, Grabe HJ. Diagnosed thyroid disorders are associated with depression and anxiety. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1417–25.
Kim JM, Stewart R, Kim SY, Bae KY, Yang SJ, Kim SW, et al. Thyroid stimulating hormone, cognitive impairment and depression in an older korean population. Psychiatry Investig. 2010;7:264–9.
Kvetny J, Ellervik C, Bech P. Is suppressed thyroid-stimulating hormone (TSH) associated with subclinical depression in the Danish General Suburban Population Study? Nord J Psychiatry. 2015;69:282–6.
Manciet G, Dartigues JF, Decamps A, Barberger-Gateau P, Letenneur L, Latapie MJ, et al. The PAQUID survey and correlates of subclinical hypothyroidism in elderly community residents in the southwest of France. Age Ageing. 1995;24:235–41.
Maugeri D, Motta M, Salerno G, Rosso D, Mazzarella R, Salomone S, et al. Cognitive and affective disorders in hyper- and hypothyreotic elderly patients. Arch Gerontol Geriatrics. 1998;26:305–12.
Pop VJ, Maartens LH, Leusink G, van Son MJ, Knottnerus AA, Ward AM, et al. Are autoimmune thyroid dysfunction and depression related? J Clin Endocrinol Metab. 1998;83:3194–7.
Shinkov AD, Borisova AM, Kovacheva RD, Vlahov YD, Dakovska LN, Atanassova ID, et al. Influence of serum levels of thyroid-stimulating hormone and anti-thyroid peroxidase antibodies, age and gender on depression as measured by the Zung Self-Rating Depression Scale. Folia Med (Plovdiv). 2014;56:24–31.
Thomsen AF, Kvist TK, Andersen PK, Kessing LV. Increased risk of affective disorder following hospitalisation with hyperthyroidism - a register-based study. Eur J Endocrinol. 2005;152:535–43.
van de Ven AC, Muntjewerff JW, Netea-Maier RT, de Vegt F, Ross HA, Sweep FC, et al. Association between thyroid function, thyroid autoimmunity, and state and trait factors of depression. Acta Psychiatr Scand. 2012;126:377–84.
Thomsen AF, Kvist TK, Andersen PK, Kessing LV. Increased risk of developing affective disorder in patients with hypothyroidism: a register-based study. Thyroid. 2005;15:700–7.
Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–82.
Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry. 2002;7:140–56.
Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3′,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience. 1999;93:943–54.
Beley A, Beley P, Bralet J. Influence of hypo- and hyperthyroidism on the turnover rate of noradrenaline, dopamine and serotonin in various rat cerebral structures. Arch Int Physiol Biochim. 1975;83:471–80.
Hassan WA, Aly MS, Rahman TA, Shahat AS. Impact of experimental hypothyroidism on monoamines level in discrete brain regions and other peripheral tissues of young and adult male rats. Int J Dev Neurosci. 2013;31:225–33.
Ito JM, Valcana T, Timiras PS. Effect of hypo- and hyperthyroidism on regional monoamine metabolism in the adult rat brain. Neuroendocrinology. 1977;24:55–64.
Hassan WA, Rahman TA, Aly MS, Shahat AS. Alterations in monoamines level in discrete brain regions and other peripheral tissues in young and adult male rats during experimental hyperthyroidism. Int J Dev Neurosci. 2013;31:311–8.
Sandrini M, Vitale G, Vergoni AV, Ottani A, Bertolini A. Effect of acute and chronic treatment with triiodothyronine on serotonin levels and serotonergic receptor subtypes in the rat brain. Life Sci. 1996;58:1551–9.
Yu D, Zhou H, Yang Y, Jiang Y, Wang T, Lv L, et al. The bidirectional effects of hypothyroidism and hyperthyroidism on anxiety- and depression-like behaviors in rats. Horm Behav. 2015;69:106–15.
Agbaht K, Gullu S. Adrenocortical reserves in hyperthyroidism. Endocrine. 2014;45:136–43.
Gallagher TF, Hellman L, Finkelstein J, Yoshida K, Weitzman ED, Roffwarg HD, et al. Hyperthyroidism and cortisol secretion in man. J Clin Endocrinol Metab. 1972;34:919–27.
Iranmanesh A, Lizarralde G, Johnson ML, Veldhuis JD. Dynamics of 24-hour endogenous cortisol secretion and clearance in primary hypothyroidism assessed before and after partial thyroid hormone replacement. J Clin Endocrinol Metab. 1990;70:155–61.
Seck G, Ndoye O, Mbodj M, Akala A, Cisse F, Niang M, et al. Serum cortisol level variations in thyroid diseases. Dakar Med. 2000;45:30–3.
Walter KN, Corwin EJ, Ulbrecht J, Demers LM, Bennett JM, Whetzel CA, et al. Elevated thyroid stimulating hormone is associated with elevated cortisol in healthy young men and women. Thyroid Res. 2012;5:13.
Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: is HPA axis function a biomarker? A systematic review. J Affect Disord. 2018;233:45–67.
Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–6.
Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68.
Dong YH, Fu DG. Autoimmune thyroid disease: mechanism, genetics and current knowledge. Eur Rev Med Pharm Sci. 2014;18:3611–8.
Li Q, Wang B, Mu K, Zhang J-A. The pathogenesis of thyroid autoimmune diseases: new T lymphocytes – Cytokines circuits beyond the Th1−Th2 paradigm. J Cell Physiol. 2019;234:2204–16.
Drevets WC, Wittenberg GM, Bullmore ET, Manji HK. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Disco. 2022;21:224–44.
Verloop H, Dekkers OM, Peeters RP, Schoones JW, Smit JW. Genetics in endocrinology: genetic variation in deiodinases: a systematic review of potential clinical effects in humans. Eur J Endocrinol. 2014;171:R123–35.
Dayan CM, Panicker V. Novel insights into thyroid hormones from the study of common genetic variation. Nat Rev Endocrinol. 2009;5:211–8.
Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94:1623–9.
Gałecka E, Talarowska M, Maes M, Su KP, Górski P, Kumor-Kisielewska A, et al. Expression levels of interferon-ɣ and type 2 deiodinase in patients diagnosed with recurrent depressive disorders. Pharm Rep. 2018;70:133–8.
Philibert RA, Beach SR, Gunter TD, Todorov AA, Brody GH, Vijayendran M, et al. The relationship of deiodinase 1 genotype and thyroid function to lifetime history of major depression in three independent populations. Am J Med Genet B Neuropsychiatr Genet. 2011;156b:593–9.
Gałecka E, Kumor-Kisielewska A, Orzechowska A, Maes M, Górski P, Szemraj J. Assessment of type 1 and type 3 deiodinase expression levels in depressive disorders. Acta Neurobiol Exp (Wars). 2017;77:225–35.
Gałecka E, Talarowska M, Maes M, Su KP, Górski P, Szemraj J. Polymorphisms of iodothyronine deiodinases (DIO1, DIO3) genes are not associated with recurrent depressive disorder. Pharm Rep. 2016;68:913–7.
Appelhof BC, Peeters RP, Wiersinga WM, Visser TJ, Wekking EM, Huyser J, et al. Polymorphisms in type 2 deiodinase are not associated with well-being, neurocognitive functioning, and preference for combined thyroxine/3,5,3′-triiodothyronine therapy. J Clin Endocrinol Metab. 2005;90:6296–9.
van der Deure WM, Appelhof BC, Peeters RP, Wiersinga WM, Wekking EM, Huyser J, et al. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin Endocrinol (Oxf). 2008;69:804–11.
Taroza S, Rastenytė D, Burkauskas J, Podlipskytė A, Kažukauskienė N, Patamsytė V, et al. Deiodinases, organic anion transporter polypeptide polymorphisms and symptoms of anxiety and depression after ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29:105040.
Moldin SO, Scheftner WA, Rice JP, Nelson E, Knesevich MA, Akiskal H. Association between major depressive disorder and physical illness. Psychol Med. 1993;23:755–61.
Patten SB, Williams JVA, Lavorato DH, Wang JL, Jetté N, Sajobi TT, et al. Patterns of association of chronic medical conditions and major depression. Epidemiol Psychiatr Sci. 2018;27:42–50.
Scott KM, Bruffaerts R, Tsang A, Ormel J, Alonso J, Angermeyer MC, et al. Depression-anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys. J Affect Disord. 2007;103:113–20.
Wells KB, Golding JM, Burnam MA. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry. 1988;145:976–81.
Eker SS, Akkaya C, Sarandol A, Cangur S, Sarandol E, Kirli S. Effects of various antidepressants on serum thyroid hormone levels in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:955–61.
Gitlin M, Altshuler LL, Frye MA, Suri R, Huynh EL, Fairbanks L, et al. Peripheral thyroid hormones and response to selective serotonin reuptake inhibitors. J Psychiatry Neurosci. 2004;29:383–6.
Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35:1365–72.
Khawaja IS, Westermeyer JJ, Gajwani P, Feinstein RE. Depression and coronary artery disease: the association, mechanisms, and therapeutic implications. Psychiatry (Edgmont). 2009;6:38–51.
Ioannidis JP, Panagiotou OA. Comparison of effect sizes associated with biomarkers reported in highly cited individual articles and in subsequent meta-analyses. Jama. 2011;305:2200–10.
United States Census Bureau, U.S. and World Population Clock. https://www.census.gov/popclock/, Accessed July, 2021.
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27.
Acknowledgements
HB and BI were supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne, Germany, grant numbers 388/2020 and 389/2020.
Author contributors
All authors had full access to all of the data in the study and had final responsibility to submit this work for publication. HB and CB verified the data and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: HB, TB, CB. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: HB, CB. Critical revision of the manuscript for important intellectual content: HB, BI, TB, GS, JH. Statistical analysis: HB, BI, TB, GS, CB. Obtained funding: HB, BI. Administrative, technical, or material support: TB, JH. Supervision: TB, JH, CB.
Funding
Supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne, Germany. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Schwarzer reported personal fees from Roche Pharma as external statistical consultant outside the submitted work. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bode, H., Ivens, B., Bschor, T. et al. Hyperthyroidism and clinical depression: a systematic review and meta-analysis. Transl Psychiatry 12, 362 (2022). https://doi.org/10.1038/s41398-022-02121-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02121-7