Abstract
Lithium (Li) is a well-established mood disorder treatment and may be neuroprotective. Bi-directional regulation (i.e. affecting manic symptoms and depressive symptoms) by Li has not been demonstrated. This study explored: (1) bidirectional regulation by Li in murine models of depression, mania, and bipolar disorder (BP); and (2) potential Li synergism with antidepressant/anti-mania agents. The chronic unpredictable mild stress (CUMS) and ketamine-induced mania (KM) models were used. These methods were used in series to produce a BP model. In vivo two-photon imaging was used to visualize Ca2+ activity in the dorsolateral prefrontal cortex. Depressiveness, mania, and cognitive function were assessed with the forced swim task (FST), open field activity (OFA) task, and novel object recognition task, respectively. In CUMS mice, Ca2+ activity was increased strongly by Li and weakly by lamotrigine (LTG) or valproate (VPA), and LTG co-administration reduced Li and VPA monotherapy effects; depressive immobility in the FST was attenuated by Li or LTG, and attenuated more strongly by LTG-VPA or LTG-Li; novel object exploration was increased strongly by Li and weakly by LTG-Li, and reduced by LTG, VPA, or LTG-VPA. In KM mice, Li or VPA attenuated OFA mania symptoms and normalized Ca2+ activity partially; Li improved cognitive function while VPA exacerbated the KM alteration. These patterns were replicated in the respective BP model phases. Lithium had bi-directional, albeit weak, mood regulation effects and a cognitive supporting effect. Li co-administration with antidepressant/-manic agents enhanced mood-regulatory efficacy while attenuating their cognitive-impairing effects.
Similar content being viewed by others
Introduction
For nearly a century, lithium (Li) has been widely used to treat acute depression, acute mania, and treatment-resistant depression [1,2,3,4]. A number of randomized controlled trials support the primary use of Li as a mood stabilizer in the maintenance care of patients with bipolar disorder (BP) [5,6,7,8,9,10]. The American Psychiatric Association (APA) [11] recommends Li to prevent manic, hypomanic, and mixed episodes and has described Li as a ‘traditional’ mood stabilizer, as opposed to the ‘new’ mood stabilizers, also known as second-generation antipsychotics, such as olanzapine, risperidone, ziprasidone, quetiapine, and aripiprazole [11]. Continual Li treatment has been shown to prevent relapse of major depression and suicidality better than second-generation antipsychotics [12]. Li has been described as the most effective medication in psychiatry because it affects the disease course as opposed to only symptoms and it has efficacy for diverse mood conditions and possibly dementia [13]. Accordingly, Li remains a gold standard treatment for long-term management of BP type I [14].
There is debate regarding the definition of mood stabilizer. Although some consider Li, the anticonvulsant valproate (VPA; a.k.a., divalproex), and second-generation antipsychotics all to be mood stabilizers [15], the US Food and Drug Administration (FDA) does not acknowledge mood stabilizer as a drug category [16], and all APA-recommended mood stabilizers have anti-manic/hypomanic effects [17]. Stalh has argued that a real mood stabilizer should have bi-directional regulation ability, inclusive of elevating a depressive mood and suppressing a manic mood [16]. However, in a network meta-analysis, Bahji et al. found that Li was ineffective for treating the depressive phase of BP [14]. Although Li is an effective treatment for acute mania and manic episodes with psychotic symptoms, and Li in combination with other agents has been shown to help alleviate unipolar depression/hypomania, Li benefits for BP depression have not been confirmed. However, when used as an adjunct therapy with an antidepressant agent, Li has been reported to enhance depressive symptom alleviation in both unipolar depression and BP depression [18, 19]. Thus, the US FDA and APA have suggested that Li can be used as a synergist in the treatment of unipolar depression or depressiveness in BP.
Recent studies have suggested that Li may have a protective effect on cognitive ability, even for patients with dementia. For example, Xu et al. found that while VPA and antipsychotics can exacerbate cognitive symptoms of BP, Li seems to alleviate them [20]. Nguyen et al. obtained micro-imaging results suggesting that Li normalized cellular and molecular impairments in the prefrontal lobe, parietal lobe, and hippocampus of mouse brains [21]. Furthermore, Li has been reported to improve chronic mild stress-induced depressive and cognitive deficits in rodent models, as evidenced by reduced glycogen synthase kinase-3 beta (GSK3β) overexpression, which otherwise causes cortical neuroinflammation and tauopathy [22]. Burdick et al. observed that Li had neurocognitive improving effects in a clinical study of 262 patients with BP [23]. Meanwhile, in a multi-center neuroimaging study, Hozer et al. found that BP patients treated with Li had reduced gray matter atrophy, especially in key brain regions associated with mood processing [24]. Although these studies provide evidence of biological effects through which Li could improve cognitive impairment, the data consist only of unfluctuating observations. Because BP encompasses switching between depressive and manic phases, dynamic characterization of Li effects on cognitive abilities during both phases should be conducted to elucidate the mechanisms by which Li improves cognitive performance in patients with BP.
The aforementioned literature raises two questions. Firstly, it remains to be determined whether Li may have bi-directional regulation effects, but with inadequately strong antidepressant effects. If so, such an effect may be enhanced by combining Li with another drug. Alternatively, it is possible that Li acts only a synergist, boosting the antidepressant effects of other antidepressant agents. Secondly, it is not known whether Li has different effects on cognitive ability in the depressive phase versus in the manic phase. Recently, using DNAzyme-based Li-selective imaging techniques, McGhee et al. found that Li accumulates more in the differentiated neurons of BP patients than in those from healthy controls [25].
The aim of the present study was to use in vivo two-photon imaging in depressive model, mania model, and BP model mice to characterize the relationship between Li-related alterations in functional brain activity and behavior across the bipolar phases. The information provided by this study may be useful for answering the above two questions. In this study, we tested the following three hypotheses: (1) Li has a weak antidepressant effect and a robust anti-manic effect, making it a bi-directional mood regulator; (2) Li can attenuate cognitive impairments in both depressive and manic phases; and (3) the cognitive protective effects of Li may be influenced by other antidepressant/anti-mania agents.
Materials and methods
Animals
A total of 115 male C57BL/6 mice from multiple litters purchased from the medical animal center of Jinan University (Guangzhou, China) were used in this study. Groups of mice were housed five per cage in a total of 23 polycarbonate cages (18 × 30 × 17 cm) designed for 24-h activity and social behavior monitoring (Ohara Co. Ltd., Tokyo, Japan) and bedded with Palsoft paper (Oriental Yeast, Tokyo, Japan). The animal room was maintained at 23 ± 2 °C and 50 ± 10% relative humidity under a 12-h light/12-h dark cycle (lights on at 06:00). The animals were allowed free access to CE-2 food (CLEA Japan, Tokyo, Japan) and water.
An adeno-associated viral vector expressing GCaMP6s, a fluorescent calcium indicator, was injected stereotaxically into the bilateral dorsal lateral prefrontal cortex (dlPFC) of all mice. After recovery, the mice were allocated randomly to 23 groups (5/group). The investigator was not blinded to the treatment. Mouse maintenance and all experiments were performed in accordance with institutional ethical standards, and all procedures were approved by the ethics committee for Animal Care and Use of Tianjin Medical University Affiliated Tianjin Fourth Center Hospital and Wenzhou Seventh Peoples Hospital approved this study (Institutional Review Board no., TW-joint-project-2020-1).
CUMS model
In accordance with published CUMS protocols [26, 27], mice were subjected to eight kinds of stress-inducing stimuli over 21 days, with the order and onset time of the exposures being determined by a random number table. The eight stress-inducing stimuli were: food deprivation for 24 h; water deprivation for 24 h; restraint stress for 3 h; swimming in cold water (10 °C) for 5 min; heat stress for 20 min; electric stress for 20 min; wet and soiled cage for 24 h; and crowded cage for 24 h. A single stress-inducing exposure was administered per day.
KM model
As described previously [28], mice received daily single intraperitoneal injections of 25 mg/kg ketamine for 10 consecutive days. This protocol provokes the expression of manic-like behavior.
BP murine model
The BP model was designed to mimic a protocol for mania prevention [26,27,28]. Because BP patients usually experience a depression episode before their first mania episode, we established a depressive phase prior to establishing a manic phase. We established the depressive phase by exposing mice to a standard CUMS protocol; 1 day later, we initiated the above KM protocol. Assessments were performed after the establishment of each phase.
Multiple-arm animal-model arrangement
Depression model arm
In this arm, there were six depression groups and one naive group. The six depression groups were differentiated as follows: no treatment (comparison group); Li monotherapy; lamotrigine (LTG)-Li dual therapy; LTG monotherapy; LTG-VPA dual therapy; VPA monotherapy. We included VPA to observe potential antidepressant and cognitive effects. The treatment period was 2 weeks.
Mania model arm
In this arm, there were four groups, including one naive group and the following three mania groups: no treatment (comparison group); Li monotherapy; and VPA monotherapy. This arm was used to compare brain functional alterations and behavioral expression between the two different monotherapy strategies.
BP model arm
In this arm, there were a total of ten BP model groups and two naive groups. Six of the groups were assessed in the CUMS-induced depression phase and four were assessed during the KM phase; the former was switched to the latter by initiating the KM protocol. In the depressive phase, the following groups were compared: untreated; Li monotherapy; LTG-Li dual therapy; LTG monotherapy; LTG-VPA dual therapy; and VPA monotherapy. In the manic phase, the following groups were compared: untreated; VPA monotherapy; and Li monotherapy. The untreated model mice were used as a reference for characterization of brain activity alterations and behavioral expression. A naive group was included in each phase analysis for comparison (considering a time influence).
Behavioral assays
The mice were subjected to a series of behavioral assays [sucrose preference test, forced swim test (FST), and prepulse inhibition (PPI)], performed at ≥24-h intervals beginning 1 day after the completion of the treatment interventions. Sucrose preference tests and FSTs were performed as described previously [26, 27]. The PPI paradigm was adapted to enable quantitation of sensory gating function [29]. Briefly, the mice were acclimated to 65-dB background noise in a sound-isolating chamber; then, a 75-dB prepulse (PP) was applied for 20 ms, followed 100 ms later by a 120-dB startle stimulus (PA) for 40 ms. Each mouse underwent three such trials with an intertrial interval of 30 s. Trial scores were averaged, and the PPI ratio was calculated as (PA – PP)/PA × 100%.
Two-photon imaging
Calcium activity in the PFC was visualized as described previously [30], with adaptation. Briefly, the mice were anesthetized by isoflurane inhalation and then given intra-PFC (~2.8 mm anterior to bregma and 0.5 mm, lateral) injections of 150 nl AAV2/9-syn-GCaMP6 virus (1013 genome copies/ml; University of Pennsylvania Vector Core). Then, 24 h before imaging, a transcranial window was created by microdrill superior to the PFC. A circular coverslip was fixed to the cranium with dental cement to cover the exposed dura. To ensure head fixation during imaging, a customized steel bar was also fixed to the cranium. After recovering from this procedure, the mice were acclimated to being placed under a two-photon microscope (LSM780, Zeiss, Germany) to minimize motion artifacts during imaging. Twenty-four hours postoperatively, awake mice were fixed under this microscope. Time-lapse two-photon imaging was performed continuously for 300 s with a 16 × 0.8-N.A. water-immersed objective, an excitation wavelength of 950 nm, and a 1.9-Hz frame rate. ImageJ software (National Institutes of Health, USA) with a FIJI plug-in package was used for image analysis as described previously [30]. Calcium signal strengths were quantified on raw images, normalized (∆F/F0), and plotted against time.
Statistical analysis
We first used Shapiro–Wilk tests to check the normality of our data distributions. For normally distributed data, we employed analyses of variance (ANOVAs) to detect factor effects on mean values. Significant ANOVA results were followed up with Tukey-Kramer multiple comparison testing to find inter-group differences. For non-normally distributed data, we employed Kruskal-Wallis tests to detect significant factor effects on median values. Significant Kruskal-Wallis test results were followed up with Dunn’s multiple comparisons tests to find inter-group differences. All statistical testing was completed in Matlab software; and GraphPad Prism software (version 8.0) was used to visualize the data. Reported P values reflect multiple comparison adjustments; the sample size was chosen based on previous experience with the aim of detecting at least a P < 0.05 that was considered to be significant. F values are reported with degrees of freedom (df).
Results
Depression model
Depression model validation
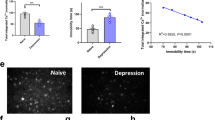
As shown in Fig. 1A–D, compared to naive mice, CUMS-induced depression model mice (‘depressed’ mice from here forward) showed less dlPFC Ca2+ activity, greater immobility times in the FST, and shorter novel object exploration times in the NOR task (all P < 0.001; data in Table 1). These results provide evidence of depressed brain activity, depressive emotionality, and impaired cognition, respectively, affirming successful establishment of a depressive model.
Treatment effects in depressed mice
The mean (±SD) values obtained for the CUMS model experiments are reported in Table 1. Compared to the untreated control group, the Li monotherapy-treated depressed mice exhibited increased dlPFC Ca2+ activity (P = 0.0003, Fa,b = 1.210), decreased immobility time in the FST (P = 0.0004, Fa,b = 2.981), and increased novel object exploration time in the NOR task (P = 0.012, Fa,b = 1.569). These data (see Table 1) show that Li improved depressive symptoms and cognitive impairment in depressed mice (Fig. 1A–D).
Unexpectedly, compared to the Li monotherapy group, the LTG-Li–treated depressed group had reduced dlPFC Ca2+ activity, reduced immobility time in the FST, and a reduced novel object exploration time percentage in the NOR task (all P < 0.05); Table 1. Compared to the LTG-Li dual therapy group, depressed mice treated with LTG monotherapy had decreased dlPFC Ca2+ activity (P = 0.028, Fa,b = 2.592), increased immobility time in the FST (P = 0.039, Fa,b = 2.111), and a reduced percentage of novel object time in the NOR task (P = 0.0003, Fa,b = 1.989). Compared to the LTG monotherapy group, depressed mice treated with LTG-VPA dual therapy had similar dlPFC Ca2+ activity (P = 0.063, Fa,b = 2.730), but spent more time immobile in the FST (P = 0.00024, Fa,b = 1.114) and spent less time with the novel object in the NOR task (P = 0.019, Fa,b = 3.020). Compared to the LTG-VPA group, depressed mice treated with VPA monotherapy had less dlPFC Ca2+ activity (P = 0.0002, Fa,b = 2.000); greater FST immobility time (P = 0.0009, Fa,b = 1.954), and a greater percentage of time with the novel object in the NOR task (P = 0.0378, Fa,b = 2.679) (Fig. 1A–D).
Summary of data obtained with depressed mice
The above-reported data together demonstrated that the presently employed CUMS depression model animals exhibited cognitive impairment simultaneous with depressive symptom onset. Li monotherapy was beneficial for improving cognitive impairments as well as for improving depressive symptoms although the latter effect was relatively weak. Although Li improved cognitive impairment, the animals retained an evident impairment compared to naive mice not subjected to the CUMS protocol.
The antidepressant LTG improved depressive symptoms while having a deteriorating effect on cognitive function. Combining Li with LTG resulted in more pronounced improvement of depressive behavior than LTG alone, but resulted in a worsening of cognitive impairment compared to Li alone. VPA, alone or with LTG, exacerbated cognitive impairment without having a substantial effect on depressive behavior expression.
Mania model
Mania model validation
As shown in Fig. 2A–D, compared to naive mice, KM model mice (referred to as manic mice from here forward) showed greater dlPFC Ca2+ activity, had longer pathlengths in the OFT, and spent lower percentages of time with the novel object in the NOR task (all P < 0.001). These data (Table 2) confirm the establishment of our mania murine model and suggest that cognitive activities may be altered simultaneously with the onset of mania symptoms.
Treatment effects in manic mice
The mean (±SD) values obtained for the KM model experiments are reported in Table 2. Compared to untreated manic mice, the Li monotherapy-treated manic mice had reduced dlPFC Ca2+ activity (P = 0.0427, Fa,b = 2.335), covered less distance in the OFT (P = 0.0302, Fa,b = 3.046), and spent a greater portion of their time with the novel object in the NOR task (P < 0.0001, Fa,b = 1.259). These data are consistent with an attenuation of mania-associated symptoms, including effects on cognitive function (Fig. 2A–D). Compared to the Li monotherapy-treated group, VPA monotherapy-treated manic mice had lower levels of dlPFC Ca2+ activity (P = 0.0284, Fa,b = 2.369), covered less distance in the OFT (P = 0.0321, Fa,b = 3.589), and spent a smaller percentage of time with the novel object in the NOR task (P = 0.0001, Fa,b = 1.258). Notably, the VPA monotherapy-treated group also spent a smaller percentage of their time with the novel object in the NOR task than untreated manic mice, despite having a shorter pathlength in the OFT (both P < 0.001, Fig. 2A–D).
Summary of data obtained with manic mice
The above-reported data together demonstrated that the KM model animals exhibited altered cognitive function simultaneous with mania symptom onset. Although VPA had a mania symptom-alleviating effect, it had a concomitant exacerbating effect on altered cognitive function. Meanwhile, Li alleviated mania symptoms less strongly than VPA, but improved the associated cognitive impairment, albeit not to the same level observed in naive mice (Fig. 2A–D).
BP model
BP model validation
Brain Ca2+ activity and behavioral data obtained for BP murine model mice (BP mice from here forward) in the depressive (i.e. CUMS) and manic (i.e. KM) phases were consistent with the findings obtained for the depressive mice and manic mice above as well as with findings from our previous studies, thus validating the two-phase BP model.
Treatment effects in BP mice
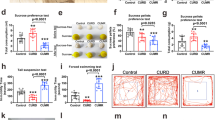
The mean (±SD) values obtained for the BP model experiments are reported in Table 3. As shown in Fig. 3A–D, compared with untreated BP mice in the depression phase, BP mice in the depression phase treated with Li monotherapy showed greater dlPFC Ca2+ activity (P = 0.0036, Fa,b = 2.997), less immobility time in the FST (P = 0.0015, Fa,b = 1.459), and greater time with the novel object in the NOR task (P = 0.0039, Fa,b = 2.852), but spent less time with the novel object in the NOR task than naive mice (P = 0.0027, Fa,b = 3.586). BP mice in the depression phase given LTG-Li dual therapy had less dlPFC Ca2+ activity than the Li monotherapy group (P = 0.0004, Fa,b = 2.000), but more than the untreated group (P = 0.0021, Fa,b = 0.113). Compared with the Li monotherapy group, BP mice in the depression phase given LTG-Li dual therapy spent less time immobile in the FST (P < 0.0001, Fa,b = 1.003) as well as less time with the novel object in the NOR task (P = 0.0010, Fa,b = 1.254). Compared with the LTG-Li dual therapy group, BP mice in the depression phase treated with only LTG had less dlPFC Ca2+ activity (P < 0.0001, Fa,b = 2.987), spent more time immobile in the FST (P < 0.0001, Fa,b = 3.213), and spent less time with the novel object in the NOR task (P < 0.0001, Fa,b = 1.147). Compared to the LTG monotherapy-treated group, the LTG-VPA–treated group exhibited less dlPFC Ca2+ activity (P < 0.0001, Fa,b = 1.396), spent a similar amount of time immobile in the FST (P = 0.1945, Fa,b = 4.259), and spent less time with the novel object in the NOR task (P = 0.1697, Fa,b = 3.293). Compared to the LTG-VPA group, the VPA monotherapy group had greater dlPFC Ca2+ activity (P < 0.0001, Fa,b = 1.951), spent more time immobile in the FST (P < 0.0001, Fa,b = 1.753), and spent more time with the novel object in the NOR task (P < 0.0001; Fa,b = 3.951; Fig. 3A–D).
Brain Ca2+ activity revealed by in vivo two-photon imaging and behavior in BD mice (A). Depressive phase assessments of Ca2+ activity (B), forced swim task (FST) immobility time (C), and novel object recognition (NOR) behavior (D). Representative imaging micrographs for each group in the depressive phase are shown in (A). Manic phase assessments of Ca2+ activity (F), open field activity (OFA) pathlength (G), and NOR behavior (H). Representative imaging micrographs for each group in the manic phase are shown above the graphs (E). Li lithium, LTG lamotrigine, VPA valproate.
As shown in Fig. 3E–H, compared with untreated controls, BP mice in the manic phase treated with only Li showed less dlPFC Ca2+ activity (P = 0.0102, Fa,b = 1.837), had shorter pathlengths in the OFA task (P = 0.0132, Fa,b = 3.248), and spent more time with the novel object in the NOR task (P < 0.0001, Fa,b = 3.257). The manic BP mice spent less time with the novel object in the NOR task than naive mice (P < 0.0001, Fa,b = 1.761).
Summary of data obtained with BP mice
Depressive behavior in BP mice was best alleviated with LTG-Li dual therapy, whereas manic behavior was similarly alleviated with either VPA alone or Li alone. Being subjected to two model-induction methods did not appear to cause any further cognitive deterioration over either modeling method alone. In the depression phase, Li alleviated cognitive impairment in BP mice, although not to the level of naive mice, and showed a weak antidepressant effect. LTG alone or VPA alone were cognitive function impairing. When either was combined with Li, the cognitive enhancing effect of Li was attenuated. When LTG and VPA were combined, impairment of cognitive function was worse than with either alone. Unexpectedly, NOR performance values were similar across the depressive and mania phases of the BP model (P = 0.05217, Fa,b = 2.749; see Table 3).
Discussion
The present study demonstrated several notable phenomena. Firstly, Li showed bi-directional regulation effects, and thus may be considered a true mood stabilizer, although each directional effect was weaker than that observed with the respective pure antidepressant/anti-mania agent. Secondly, we observed cognitive and dlPFC Ca2+ activity alterations concomitantly with the onset of depressive or manic symptoms in CUMS and KM model mice, respectively. Importantly, the BP model mice did not show worse cognitive changes than the singular models. Thirdly, Li partially reversed brain activity and cognitive alterations produced by each model and attenuated the cognitive alteration-exacerbating effects of antidepressant/anti-mania agents. Lastly, Li monotherapy did not disrupt cognitive function, whereas the LTG and VPA monotherapies did.
These findings support the use of Li as a mood stabilizer itself as well as its use as a synergist, consistent with clinical study observations of Li treatment reducing suicidality in depressed patients, especially within 5 years of unipolar depression onset, and alleviating manic episodes [3, 31, 32]. Our findings also support the precept that adjunct Li may augment the effectiveness of antidepressant medication and/or electroconvulsive therapy, particularly in recalcitrant unipolar depression [33].
In the last decade, there has been a convergence of findings indicating that Li can regulate GSK3β activity. GSK-3β is a serine/threonine kinase that serves as a molecular hub in the crosstalk among numerous signaling pathways. Findings indicating that it plays a crucial role hippocampal neuron development and survival suggest that regulation of GSK3β could be a clinical target for neuropsychiatric disorders, such as depression and anxiety disorders [1, 4]. Indeed GSK-3β-actuated molecular cascades have been reported to have modulatory influences on depression and anxiety disorders [5,6,7]. Notably, regulation of GSK3β has been implicated in antidepressant mechanisms of action and in the pathogenesis of depression [8, 9]. GSK-3β/β-catenin cascades have been reported that play a crucial role in the onset of depressive symptoms in animal models and thus have become a target of interest for depressive symptom alleviation and elucidation of the pathogenesis of depression [10,11,12,13,14]. β-catenin has been reported to induce de novo synthesis of brain-derived neurotrophic factor, an important regulator of adult hippocampal neurogenesis and behavioral effects of antidepressants [15, 16].
Many studies have confirmed that Li has an inhibitory effect on GSK3β [17, 18], and Li inhibition of GSK3β has been reported to normalize stress-induced-behavioral changes, reduce microglial activation, and enhance expression of Wnt/β-catenin signaling pathway proteins in the hippocampus [19]. Wnt/β-catenin signaling has been suggested to be a potentially ideal therapeutic target for depression treatment [20].
Based on the aforementioned evidence, we have postulated that Li may have an inhibitory influence on the GSK3β cascade through multiple signaling pathways (the mechanisms of which remain to be clarified), thereby resulting in an alleviation of the depressive phase in animal models of BP. Though Li treatment has been shown to increase levels of activated (i.e. phosphorylated) GSK3β in patients with BP, in one study, GSK3β levels (total and phosphorylated) in drug-free BP subjects in the depressive phase were found to be similar to levels in healthy controls, while being higher than levels in drug-free BP subjects in the manic phase [21]. It has been suggested that the mood stabilizing effects of Li may be consequent to Li inhibition of GSK3β activity augmenting neuronal activity during depressive phases and Li inhibition of K+ channel activation suppressing neuronal excitability during manic phases [22]. We plan to explore this possibility in future studies examining Li’s bi-directional regulatory effects on BP symptoms.
Our hypothesis asserting that Li monotherapy may have some antidepressant effect was supported. Our hypothesis predicting that Li would have positive effects on cognitive function was also supported. Likewise, our hypothesis predicting that Li would act synergistically with antidepressant/anti-mania drugs was also supported. The mechanisms underlying these synergistic effects and dose-response interactions have yet to be elucidated. In our mouse experiments, VPA had cognitive-impairing effects. These data contrast with some prior studies suggesting that VPA may have cognitive function-protective effects [34]. However, VPA has been reported to be cognitive-impairing in patients with epilepsy [35] and to have the potential to be disruptive to fetal brain development when taken by pregnant women [33,34,35,36,37,38,39]. Contrary to the apparent cognitive-impairing effects of VPA and LTG, Li may be neuroprotective and may help to reverse cognitive impairments, leading to recommendation of its use in patients with dementia [39,40,41,42,43,44,45,46].
Although Li improved cognitive impairments in our mouse experiments, it did not fully reverse the CUMS or KM-induced cognitive disrupting effects. The extent to which Li can alleviate extant cognitive impairments is unclear. Notwithstanding, it is notable that the use of adjunct Li reduced the impairing effects of LTG and VPA, compared to the LTG and VPA monotherapy effects, while augmenting the positive anti-depressive and anti-manic effects of these drugs, respectively. Clinical studies in human patients are needed to optimize these drug interactions.
This study had several limitations. Firstly, our data cannot explain why our CUMS-KM BP model mice did not exhibit more disrupted brain Ca2+ activity or NOR behavior than our CUMS depression model mice. It is possible that broader imaging of more brain regions, including subcortical regions, could reveal additional changes. More advanced techniques, such as multiphoton focusing technology approaches, may provide more complete information regarding the effects of these models and psychiatric drugs on brain activity. Secondly, the present data do not enable us to judge whether antidepressant exposure during the depressive phase had a cognitive effect in the subsequent mania phase. Thirdly, second-generation antipsychotic agents were not tested.
Conclusion
In conclusion, functional brain alterations were confirmed in a BP murine model for the first time to our knowledge. Li was shown to have bi-directional regulation effects as well as to be beneficial to brain functioning. Although the antidepressant and anti-manic effects of Li were weaker than those seen with antidepressant and anti-mania agents, when Li was combined with these agents, it exerted synergistic effects. Moreover, combining Li with antidepressant/anti-manic agents lessened the cognitive-impairing effects of those drugs.
Data availability
The data that supports the findings of this study are available from the corresponding author upon request.
References
Katz IR, Rogers MP, Lew R, Thwin SS, Doros G, Ahearn E, et al. Lithium treatment in the prevention of repeat suicide-related outcomes in veterans with major depression or bipolar disorder: a Randomized Clinical Trial. JAMA Psychiatry. 2022;79:24–32. https://doi.org/10.1001/jamapsychiatry.2021.3170
Hsu CW, Carvalho AF, Tsai SY, Wang LJ, Tseng PT, Lin PY, et al. Lithium concentration and recurrence risk during maintenance treatment of bipolar disorder: Multicenter cohort and meta-analysis. Acta Psychiatr Scand. 2021;144:368–78. https://doi.org/10.1111/acps.13346
Lambrichts S, Detraux J, Vansteelandt K, Nordenskjöld A, Obbels J, Schrijvers D, et al. Does lithium prevent relapse following successful electroconvulsive therapy for major depression? A systematic review and meta-analysis. Acta Psychiatr Scand. 2021;143:294–306. https://doi.org/10.1111/acps.13277
Del Matto L, Muscas M, Murru A, Verdolini N, Anmella G, Fico G, et al. Lithium and suicide prevention in mood disorders and in the general population: a systematic review. Neurosci Biobehav Rev. 2020;116:142–53. https://doi.org/10.1016/j.neubiorev.2020.06.017
Kuperberg M, Köhler-Forsberg O, Shannon AP, George N, Greenebaum S, Bowden CL, et al. Cardiometabolic risk markers during mood-stabilizing treatment: correlation with drug-specific effects, depressive symptoms and treatment response. J Affect Disord. 2021;300:41–49. https://doi.org/10.1016/j.jad.2021.12.047
Federoff M, McCarthy MJ, Anand A, Berrettini WH, Bertram H, Bhattacharjee A, et al. Correction of depression-associated circadian rhythm abnormalities is associated with lithium response in bipolar disorder. Bipolar Disord. 2021. https://doi.org/10.1111/bdi.13162.
Schoretsanitis G, de Filippis R, Brady BM, Homan P, Suppes T, Kane JM. Prevalence of impaired kidney function in patients with long-term lithium treatment: a systematic review and meta-analysis. Bipolar Disord. 2021. https://doi.org/10.1111/bdi.13154.
Nayak R, Rosh I, Kustanovich I, Stern S. Mood stabilizers in psychiatric disorders and mechanisms learnt from in vitro model systems. Int J Mol Sci 2021;22:9315. https://doi.org/10.3390/ijms22179315
Khayachi A, Schorova L, Alda M, Rouleau GA, Milnerwood AJ. Posttranslational modifications & lithium’s therapeutic effect—Potential biomarkers for clinical responses in psychiatric & neurodegenerative disorders. Neurosci Biobehav Rev. 2021;127:424–45. https://doi.org/10.1016/j.neubiorev.2021.05.002
Sakrajda K, Szczepankiewicz A. Inflammation-related changes in mood disorders and the immunomodulatory role of lithium. Int J Mol Sci. 2021;22:1532. https://doi.org/10.3390/ijms22041532
Guideline: practice guideline for the treatment of patients with bipolar disorder, second edition. 2002. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf
Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19:575–86. https://doi.org/10.1111/bdi.12543
Barroilhet SA, Ghaemi SN. When and how to use lithium. Acta Psychiatr Scand. 2020;142:161–72. https://doi.org/10.1111/acps.13202
Bahji A, Ermacora D, Stephenson C, Hawken ER, Vazquez G. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta-analysis. J Affect Disord. 2020;269:154–84. https://doi.org/10.1016/j.jad.2020.03.030
Nath M, Gupta V Mood Stabilizers. 2021. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical application. Cambridge University Press; 2021. https://doi.org/10.1017/9781108975292
Podawiltz A. A review of current bipolar disorder treatment guidelines. J Clin Psychiatry. 2012;73:e12. https://doi.org/10.4088/JCP.10060tx2cc
Verdolini N, Hidalgo-Mazzei D, Del Matto L, Muscas M, Pacchiarotti I, Murru A, et al. Long-term treatment of bipolar disorder type I: A systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23:324–40. https://doi.org/10.1111/bdi.13040
Fountoulakis KN. An update of evidence-based treatment of bipolar depression: where do we stand? Curr Opin Psychiatry. 2010;23:19–24. https://doi.org/10.1097/YCO.0b013e328333e132
Xu N, Huggon B, Saunders KEA. Cognitive impairment in patients with bipolar disorder: impact of pharmacological treatment. CNS Drugs. 2020;34:29–46. https://doi.org/10.1007/s40263-019-00688-2
Nguyen LD, Fischer TT, Ehrlich BE. Pharmacological rescue of cognitive function in a mouse model of chemobrain. Mol Neurodegener. 2021;16:41. https://doi.org/10.1186/s13024-021-00463-2
Ebeid MA, Habib MZ, Mohamed AM, Faramawy YE, Saad SST, El-Kharashi OA, et al. Cognitive effects of the GSK-3 inhibitor “lithium” in LPS/chronic mild stress rat model of depression: Hippocampal and cortical neuroinflammation and tauopathy. Neurotoxicology. 2021;83:77–88. https://doi.org/10.1016/j.neuro.2020.12.016
Burdick KE, Millett CE, Russo M, Alda M, Alliey-Rodriguez N, Anand A, et al. The association between lithium use and neurocognitive performance in patients with bipolar disorder. Neuropsychopharmacology. 2020;45:1743–9. https://doi.org/10.1038/s41386-020-0683-2
Hozer F, Sarrazin S, Laidi C, Favre P, Pauling M, Cannon D, et al. Lithium prevents grey matter atrophy in patients with bipolar disorder: An international multicenter study. Psychol Med. 2021;51:1201–10. https://doi.org/10.1017/S0033291719004112
McGhee CE, Yang Z, Guo W, Wu Y, Lyu M, DeLong CJ, et al. DNAzyme-based lithium-selective imaging reveals higher lithium accumulation in bipolar disorder patient-derived neurons. ACS Cent Sci. 2021;7:1809–20. https://doi.org/10.1021/acscentsci.1c00843
Liu W, Xue X, Xia J, Liu J, Qi Z. Swimming exercise reverses CUMS-induced changes in depression-like behaviors and hippocampal plasticity-related proteins. J Affect Disord. 2018;227:126–35.
Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012:e3638.
Chen M, Tian H, Huang G, Fang T, Lin X, Shan J, et al. Calcium imaging reveals depressive- and manic-phase-specific brain neural activity patterns in a murine model of bipolar disorder: a pilot study. Transl Psychiatry. 2021;11:619. https://doi.org/10.1038/s41398-021-01750-8
Feldcamp LA, Boutros PC, Raymond R, Fletcher PJ, Nobrega JN, Wong AHC. Pdxdc1 modulates prepulse inhibition of acoustic startle in the mouse. Transl Psychiatry. 2017;7:e1125.
Koukouli F, Rooy M, Tziotis D, Sailor KA, O’Neill HC, Levenga J, et al. Nicotine reverses hypofrontality in animal models of addiction and schizophrenia. Nat Med. 2017;23:347–54. https://doi.org/10.1038/nm.4274
De Fazio P, Gaetano R, Caroleo M, Pavia M, De Sarro G, Fagiolini A, et al. Lithium in late-life mania: a systematic review. Neuropsychiatr Dis Treat. 2017;13:755–66. https://doi.org/10.2147/NDT.S126708
McKnight RF, de La Motte de Broöns de Vauvert SJGN, Chesney E, Amit BH, Geddes J, Cipriani A. Lithium for acute mania. Cochrane Database Syst Rev. 2019;6:CD004048. https://doi.org/10.1002/14651858.CD004048.pub4
Masmoudi K, Gras-Champel V, Masson H, Andréjak M. Parkinsonism and/or cognitive impairment with valproic acid therapy: a report of ten cases. Pharmacopsychiatry. 2006;39:9–12. https://doi.org/10.1055/s-2006-931471
Ito M, Kinjo T, Seki T, Horie J, Suzuki T. The long-term prognosis of hippocampal neurogenesis and behavioral changes of offspring from rats exposed to valproic acid during pregnancy. Neuropsychopharmacol Rep. 2021;41:260–4. https://doi.org/10.1002/npr2.12181
El Sabaa RM, Hamdi E, Hamdy NA, Sarhan HA. Effects of levetiracetam compared to valproate on cognitive functions of patients with epilepsy. Neuropsychiatr Dis Treat. 2020;16:1945–53. https://doi.org/10.2147/NDT.S256117
Song A, Cho GW, Vijayakumar KA, Moon C, Ang MJ, Kim J, et al. Neuroprotective effect of valproic acid on salicylate-induced tinnitus. Int J Mol Sci. 2021;23:23. https://doi.org/10.3390/ijms23010023
Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–605. https://doi.org/10.1056/NEJMoa0803531
Duman B, Can KC, Ağtaş-Ertan E, Erdoğan S, İlhan RS, Doğan Ö, et al. Risk factors for valproic acid induced hyperammonemia and its association with cognitive functions. Gen Hosp Psychiatry. 2019;59:67–72. https://doi.org/10.1016/j.genhosppsych.2019.05.005
Taleb A, Lin W, Xu X, Zhang G, Zhou QG, Naveed M, et al. Emerging mechanisms of valproic acid-induced neurotoxic events in autism and its implications for pharmacological treatment. Biomed Pharmacother. 2021;137:111322. https://doi.org/10.1016/j.biopha.2021.111322
Nadebaum C, Anderson VA, Vajda F, Reutens DC, Barton S, Wood AG. Language skills of school-aged children prenatally exposed to antiepileptic drugs. Neurology. 2011;76:719–26. https://doi.org/10.1212/WNL.0b013e31820d62c7
Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;27:346. https://doi.org/10.1136/bmj.f3646
Souza FG, Goodwin GM. Lithium treatment and prophylaxis in unipolar depression: a meta-analysis. Br J Psychiatry. 1991;158:666–75.
Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519–30.
Nilsson A. Mortality in recurrent mood disorders during periods on and off lithium—a complete population study in 362 patients. Pharmacopsychiatry. 1995;28:8–13.
Devanand DP, Crocco E, Forester BP, Husain MM, Lee S, Vahia IV, et al. Low dose lithium treatment of behavioral complications in Alzheimer’s disease: Lit-AD randomized clinical trial. Am J Geriatr Psychiatry. 2022;30:32–42. https://doi.org/10.1016/j.jagp.2021.04.014
Baethge C. Low-dose lithium against dementia. Int J Bipolar Disord. 2020;8:25. https://doi.org/10.1186/s40345-020-00188-z
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81871052, 82171503 to CJZ). Jiangsu Enhua Pharmaceutical Co., Ltd; Jingdong Group Co., Ltd; Jiangsu Haosen Pharmaceutical Co., Ltd; Beijing Yimin Pharmaceutical Co., Ltd all sponsored this work, but they all did not participate any processing of this work.
Author information
Authors and Affiliations
Contributions
CJZ, XS, HT, GC, and CHZ conceived and designed the experiments. XS, HT, QL, JC, LY, QZ, RL, XM, ZC, YX, GC, CHZ performed the experiments and analyzed the data, CJZ analyzed the data and wrote the manuscript. All authors reviewed and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhuo, C., Zhou, C., Tian, H. et al. Lithium produces bi-directionally regulation of mood disturbance, acts synergistically with anti-depressive/-manic agents, and did not deteriorate the cognitive impairment in murine model of bipolar disorder. Transl Psychiatry 12, 359 (2022). https://doi.org/10.1038/s41398-022-02087-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02087-6