Abstract
The genomic effects of circulating glucocorticoids are particularly relevant in cortico-limbic structures, which express a high concentration of steroid hormone receptors. To date, no studies have investigated genomic differences in hippocampal subregions, namely the dorsal (dHPC) and ventral (vHPC) hippocampus, in preclinical models treated with exogenous glucocorticoids. Chronic oral corticosterone (CORT) in mouse is a pharmacological approach that disrupts the activity of the hypothalamic-pituitary-adrenal axis, increases affective behavior, and induces genomic changes after stress in the HPC of wildtype (WT) mice and mice heterozygous for the gene coding for brain-derived neurotrophic factor Val66Met (hMet), a variant associated with genetic susceptibility to stress. Using RNA-sequencing, we investigated the genomic signatures of oral CORT in the dHPC and vHPC of WT and hMet male and female mice, and examined sex and genotype differences in response to oral CORT. Males under CORT showed lower glycemia and increased anxiety- and depression-like behavior compared to females that showed instead opposite affective behavior in response to CORT. Rank–rank-hypergeometric overlap (RRHO) was used to identify genes from a continuous gradient of significancy that were concordant across groups. RRHO showed that CORT-induced differentially expressed genes (DEGs) in WT mice and hMet mice converged in the dHPC of males and females, while in the vHPC, DEGs converged in males and diverged in females. The vHPC showed a higher number of DEGs compared to the dHPC and exhibited sex differences related to glucocorticoid receptor (GR)-binding genes and epigenetic modifiers. Methyl-DNA-immunoprecipitation in the vHPC revealed differential methylation of the exons 1C and 1F of the GR gene (Nr3c1) in hMet females. Together, we report behavioral and endocrinological sex differences in response to CORT, as well as epigenetic signatures that i) differ in the dHPC and vHPC,ii) are distinct in males and females, and iii) implicate differential methylation of Nr3c1 selectively in hMet females.
Similar content being viewed by others
Introduction
Glucocorticoids exert their effects by binding to glucocorticoid receptors (GRs), which regulate up to ~20% of the genome via both direct (by binding to glucocorticoid responsive elements in promoter regions) and indirect mechanisms (by interacting with bound transcription factors and epigenetic modifiers) [1]. GRs play a key role in the feedback regulation of the hypothalamic-pituitary-adrenal (HPA) axis [2]. They are highly expressed in the hippocampus [3, 4] and their distribution is heterogeneous depending on the hippocampal subregion both at baseline [5] and in response to stress [6, 7]. The dorsal (dHPC) and the ventral hippocampus (vHPC) are two functionally distinct subregions that differ in their respective neuroanatomical connectivity and in the biological processes they encode [8, 9]. Regional differences in gene expression also contribute to distinguish the function of the dHPC and vHPC in response to environmental stimuli [10,11,12,13]. These two hippocampal circuits also show sex differences in neuronal proliferation [14, 15], indicating that males and females use distinct networks to modulate the function of the HPA axis. However, little is known on the whole-genome profile that underlies sex differences in the dHPC and vHPC, especially in response to glucocorticoids.
Chronic administration of oral corticosterone (CORT) in mice is a validated pharmacological model for the study of the molecular and behavioral consequences of HPA axis dysregulation [16, 17]. Oral CORT in mice induces hyperphagia, increased locomotion [16], and stress-induced grooming, rearing, and exploratory behavior in males [18, 19]. In females, oral CORT increases stress-induced immobility and decreases social interaction and hyponeophagia [20]. Others have shown that CORT induces decreased prepulse inhibition only in mice heterozygous (hMet) for the gene coding brain-derived neurotrophic factor (BDNF) Val66Met [21], a genetic variant that increases risk for stress-inducible pathologies [22, 23]. Notably, hMet mice show disruption of the HPA axis activity without any applied stressors [24] in association with genomic signatures in the CA3 pyramidal neurons of the hippocampus that differ from wild-type (WT) mice before and after acute stress. Sex differences are also observed in hMet mice, particularly regarding glucocorticoid-dependent networks [25, 26].
Using RNA-sequencing, RT-qPCR, and Methylated DNA Immunopreciptation (MeDIP) we investigated the epigenetic signatures in the dHPC and vHPC induced by chronic low doses of oral CORT as a function of sex and genotype. We report behavioral sex differences in response to CORT, as well as sex- and genotype-specific genomic effects in the dHPC and vHPC that included one cluster of glucocorticoid receptor-binding genes and one cluster of epigenetic modifiers and showed that CORT is associated with differential methylation of the GR gene (Nr3c1).
Methods
Animals
Mice heterozygous for the BDNF allele (hMet) were generated in the Francis Lee laboratory, as previously described [22]. C57/BL6 mice (WT) and BDNF hMet mice were obtained by performing in-house breeding. To control for litter-specific effects, mice were selected from multiple litters. Animals were group housed (n = 4–5) in standard cages (28.5 × 17 × 13 cm), which were changed weekly, and were kept on a 12-h light–dark cycle (lights off 7:00 pm) in a temperature-controlled room maintained at 21 ± 2 °C. A 2lux red light permitted animal maintenance in the dark phase. Food and water were available ad libitum. Based on previous behavioral studies, we found that a sample size of 8–12 mice per group allows us to reliably detect changes of the magnitude we are examining (α = 0.05). Variance was similar among the groups that are being compared. All procedures were performed in accordance with the National Guidelines on the Care and Use of Animals and a protocol approved by The Rockefeller University Animal Care and Use Committee.
Chronic oral CORT treatment
At 2 months of age, male and female WT or hMet mice were randomly assigned to either vehicle or CORT treatment. Drinking water was replaced with either a solution containing 25 µg/ml corticosterone (Sigma, St. Louis, MO) dissolved in 100% ethanol, and then diluted in regular tap water to a final concentration of 1%, or a solution of 1% ethanol in tap water (vehicle). Both corticosterone and vehicle solutions were replaced twice a week. Body weight was measured weekly on a standard scale over the course of CORT administration. Starting at 3 weeks of treatment, mice were tested in a behavioral battery that included the light–dark box, the splash test, and the Y maze test (Fig. 1a) (see Supplementary for detailed protocols). Behavioral assessment and tissue collection were performed in the second half of the light phase to exclude data interpretation biased by an acute effect of exogenous CORT. Plasmatic levels of CORT are similar in both CORT-treated mice and vehicle-treated mice during this phase [16].
a Timeline for CORT treatment experiment. b Body weight recorded at the start and end of CORT treatment. Over the course of treatment, female hMet mice demonstrate a significant increase in body weight compared to WT under the same treatment (three-way ANOVA, treatment: F(1,85) = 7.457, p < 0.01, sex: F(1,85) = 9.093, p < 0.01, genotype: F(1,85) = 194.5, p < 0.0001, genotype × sex: F(1,85) = 14.96, p < 0.001). c Measures of blood glucose concentration after a 24-h fasting period. WT male mice showed a significant decrease in fasting blood glucose after CORT treatment while hMet male mice under vehicle had significantly increased fasting blood glucose levels compared to vehicle-treated hMet females (three-way ANOVA, treatment: F(1,38) = 10.81, p < 0.01, treatment: F(1,38) = 12.37, p < 0.01). d Measures of plasmatic CORT levels after a 24-h fasting period. Females showed an increased concentration of CORT compared to males across genotypes (three-way ANOVA, sex: F(1,38) = 51.85, p < 0.0001). e Adrenal gland weight at the day of sacrifice. After CORT treatment females had a significantly higher adrenal gland mass than males, regardless of genotype (three-way ANOVA, treatment: F(1,45) = 8.365, p < 0.01, sex: F(1,45) = 28.86, p < 0.0001). Columns represent the mean ± SEM of 5–12 determinations per group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. WT wildtype, hMet heterozygous for brain-derived neurotropic factor Val66Met, V vehicle, C/CORT corticosterone, ♀ female, ♂ male.
Blood glucose
Glucose levels were measured from blood samples after 5-weeks of CORT treatment during the dark phase, after a 24-h fasting period, and on the day of sacrifice (n = 5–6 mice/group). Collection occurred at 2:00 a.m. for dark phase testing, 8 days prior to sacrifice, using submandibular bleeding. Food was removed from the cage for 24 h prior to fasting blood collection, 4 days prior to sacrifice, using submandibular bleeding. For this method, we followed the IACUC guidelines for blood collection, namely, of the circulating blood volume, 10% of the total volume was safely removed every 2–4 weeks. On the day of sacrifice mice were rapidly decapitated and trunk blood was immediately collected. At both time points, one drop of blood was used to measure blood glucose with OneTouch UltraMini Blood Glucose Monitor (LifeScan, Zug, Switzerland). The remaining blood was collected for isolating plasma.
Plasma CORT
Corticosterone levels were measured from plasma collected after 5 weeks of CORT treatment during the dark phase, after a 24-h fasting period, and on the day of sacrifice (n = 5–6 mice/group) using a Corticosterone Double Antibody RIA kit (MP Biomedicals Inc., Santa Ana, CA, USA). Briefly, blood was collected in K3 EDTA (K3E) 12 mg Blood Collection Tubes (BD Vacutainer, Franklin Lakes, NJ, USA). Samples were then centrifuged at 1000 × g for 10 min to collect plasma, which was rapidly frozen at −80 °C. Five microliter of plasma [diluted 1:200 in phosphosaline gelatin buffer (pH 7.0 ± 0.1)] and 100 μL of standard calibrators were incubated for 2 h with radioactive corticosterone I125 (7 μCi per vial) and then centrifuged at 1000 × g for 15 min. Radioactivity in the resulting precipitant was measured using a Hidex Automatic Gamma Counter (Turku, Finland). Corticosterone concentration was calculated using the count per minute (CPM) as a function of the logarithmic equation generated from the calibrators.
Tissue collection and RNA-seq
After 6 weeks of treatment, mice were cervical dislocated, and brains were dissected for tissue collection. The dorsal (dHPC) and ventral hippocampi (vHPC) were isolated, immediately flash frozen, and stored at −80 °C. Four biological replicates were used per experimental group, comprising of RNA pooled from two animals each (see Supplementary for details). Quality control was performed on the reads obtained from the core and reads with a score of <15 were discarded [27, 28]. The reads were then aligned to the GRCm38 genome using the STAR aligner [29] with Ensemble annotation [30] and quantified to the gene-level using featureCounts [31]. These counts were analyzed using the R/Bioconductor framework [32] (www.R-project.org). Differentially expressed gene (DEG) analysis was conducted using the limma-voom package [33]. Overlaps between the differential expression of two independent RNA-seq comparisons were visualized and measured with “stratified” rank–rank hypergeometric overlap (RRHO) analysis [34] (see Supplementary for details). RNA-seq data have been deposited to GEO (GSE194059). DEGs are included in Supplementary Data 1. All other data are available upon request.
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Total mRNA (n = 5–7 mice/group) was extracted from the vHPC using Qiagen Lipid Tissue Mini Kit (Qiagen, Germantown, MD, USA) and then reverse transcribed using mixed random primers and MuLV reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed using SYBR Green master mix and a CFX Connect qPCR system (Bio-Rad) using primers specifically designed for Nr3c1 exons (Supplementary Table 1). Gapdh was used as a reference gene. Data are represented as 2−ΔCt.
Methyl-DNA-immunoprecipitation assay
DNA from the vHPC (n = 5–7 mice/group) was extracted using Qiagen Lipid Tissue Mini Kit (Qiagen, Germantown, MD, USA) according to manufacturer’s instructions. DNA methylation levels were assessed by methyl-DNA-immunoprecipitation (meDIP) using the MagMeDIP kit (Diagenode, Denville, NJ) as previously described [35]. Primers were designed in the Nr3c1 exons (Supplementary Table 1). MeDIP assay efficiency was assessed by RT-qPCR using internal positive and negative DNA controls (methylated/hydroxymethylated and unmethylated DNA) as well as control primers for testis specific H2B histone gene (which is methylated in all somatic cells but not in testis), and Gapdh promoter (which is poorly methylated), following manufacturer’s instructions. Data are represented as 2−ΔCt.
Sequencing analysis and statistics
Behavioral, endocrine, mRNA levels, and DNA methylation (see Supplementary for details) data were analyzed using GraphPad Prism (GraphPad Software, Inc., USA) to perform a three-way ANOVA followed by Neumann–Keuls, post-hoc analysis. The z-score was calculated using the following formula: z = [(x−x̅CONT)/σCONT]*(±1)], where x = behavioral variable and σ = standard deviation. Microsoft Excel (Microsoft, USA) was used to obtain gene expression profiles by sorting genes based on fold change.
Results
Endocrinological parameters in males and females after chronic oral CORT
Previous reports show that low dose oral CORT in males do not significantly change body weight but induce atrophy of the adrenal glands and increase levels of leptin, insulin, and plasmatic CORT at night [16]. We found that oral CORT did not affect body weight in either sex or genotype, however hMet females showed lower body weight than WT females regardless of treatment (p < 0.01) (Fig. 1b). Glycemia, measured after a 24-h fasting, was reduced in WT males under CORT (p < 0.05) but not in females, and no sex differences were observed at baseline. Yet, hMet females showed lower glycemia than hMet males with no effect of treatment (p < 0.05) (Fig. 1c). Levels of plasma CORT were also measured after 24-h fasting and showed that females displayed higher plasma CORT than males regardless of genotype, with no major changes after treatment (p < 0.001) (Fig. 1d). Glycemia and plasma CORT were also measured during the dark phase and at the end of the treatment, with no significant effect found at either timepoint (Supplementary Fig. 1a, b, e). Yet, during the dark phase, hMet male mice under CORT showed higher CORT than vehicle-treated hMet males (p < 0.001), an effect that was not observed in females of either genotype (Supplementary Fig. 1d). At the end of treatment, adrenal gland, uteri, and testes were dissected and weighed. Oral CORT increased the difference in adrenal gland weight between sexes, with females showing higher adrenal gland weight than males (p < 0.05) (Fig. 1e). However, no effect was observed after CORT on uterine and testes weight (Supplementary Fig. 1c, f). Therefore, while oral CORT did not affect body weight, it induced sex- and genotype-dependent effects on endocrinological parameters.
Males and females exhibit opposite affective behavior after chronic oral CORT
To validate the behavioral phenotype in males and compare it with females, mice were assessed for anxiety- and depression-like behavior using the light-dark box test and the splash test. CORT increased the latency to enter the light box in WT males compared to vehicle-treated WT males (p < 0.05) but not in females of either genotype (Fig. 2b). No differences in latency to the dark box or percent time in light were found between groups (Fig. 2a, c). In the splash test, while no differences were found in grooming time across groups (Fig. 2e), only hMet males under CORT showed increased grooming latency (p < 0.01) and a decreased number of grooming sessions (p < 0.05) compared to vehicle-treated hMet males, and we found no differences in females of either genotype (Fig. 2d, f). However, when a z-normalization was applied across complementary variables of behavioral measurements [36], hMet females under CORT attained a negative behavioral score compared to hMet females under vehicle (p < 0.0001), indicating not only that CORT did not induce increased anxiety- or depression-like behavior in hMet females, but also that it had an opposite behavioral effect than in males (Fig. 2g). Thus, oral CORT was able to increase affective behavior in males (p < 0.05), but not in females, with hMet females displaying a behavioral phenotype that differed from all other groups. Cognitive performance was measured using the Y maze test. No differences in the discrimination ratio (preference for the novel arm), were found as function of sex, treatment, or genotype (Supplementary Fig. 2a–c).
a–c Light–dark box test reveals increased anxiety-like behavior in male mice treated with CORT (three-way ANOVA, Latency to Light: treatment × genotype, F(1,72) = 10.31, p < 0.01; % Time in Light: sex F(1,72) = 10.31, p < 0.01). d–f Splash test reveals increased anxiety-like behavior in hMet mice treated with CORT (three-way ANOVA, grooming latency: genotype × sex: F(1,72) = 4.116, p < 0.05, treatment × genotype: F(1,72) = 7.351, p < 0.01, treatment × genotype × sex: F(1,72) = 6.163, p < 0.05; grooming time: genotype × sex: F(1,72) = 5.66, p < 0.05; # of grooming sessions: genotype: F(1,72) = 6.527, p < 0.05, treatment × genotype: F(1,72) = 6.125, p < 0.05). g Complementary variables of behavior across sex and genotype were compiled to calculate a z-score, which show that CORT-treated male mice displayed higher emotionality scores compared to vehicle-treated males, regardless of genotype, whereas CORT-treated hMet females displayed lower emotionality scores compared to vehicle-treated hMet females (three-way ANOVA, genotype: F(1,68) = 6.748, p < 0.05, sex: F(1,68) = 27.20, p < 0.0001, treatment × sex: F(1,68) = 36.72, p < 0.0001, treatment × sex: F(1,68) = 4.431, p < 0.05). Columns represent the mean ± SEM of 10 determinations per group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. PND post-natal day, WT wildtype, hMet heterozygous for brain-derived neurotropic factor Val66Met, V vehicle, C/CORT corticosterone, ♀ female, ♂ male.
CORT induces a higher number of DEGs in the vHPC than the dHPC
We then investigated the genomic effects of CORT in the dHPC and vHPC, moving from recent evidence demonstrating that behavioral susceptibility in males correlates with genes implicated in stress coping [37]. DEGs (p < 0.05, FC > 1.3) in the dHPC and vHPC were identified using RNA-seq, and compared based on treatment, sex, and genotype (Supplementary Data 1). CORT induced similar levels of DEGs in the dHPC of WT males (119) and females (151), whereas hMet males exhibited a higher number of DEGs (178) than hMet females (36) (Fig. 3a). In the vHPC, CORT induced similar levels of DEGs in WT (54) and hMet females (80), albeit lower than both WT (184) and hMet males (493) (Fig. 3b). Notably, CORT induced a greater amount of DEGs in the vHPC (811) than in the dHPC (484).
The number of DEGs induced by CORT treatment within the a dorsal hippocampus and b ventral hippocampus. c Threshold free rank-rank hypergeometric overlap comparisons of DEGs. The lower left quadrant designates co-downregulated genes by CORT, upper right quadrant designates co-upregulated genes by CORT, and the upper left and lower right quadrants include oppositely regulated genes. Genes along each axis are listed from greatest to least significantly regulated from the outer to middle corners. Pixels represent the overlap between the transcriptome of each comparison as noted, with the significance of overlap [−log10(p-value) of a hypergeometric test] color coded. The quadrants insignificant to the analysis are shaded in gray. Comparison of WT and hMet DEGs in the dHPC in d females (max-log10(p-value) = 183) and e males (max-log10(p-value) = 184). Comparison of WT and hMet DEGs in the vHPC in f females (max-log10(p-value) = 241) and g males (max-log10(p-value) = 652). Comparison of WT female and WT male DEGs in the h dHPC (max-log10(p-value) = 120) and i vHPC (max-log10(p-value) = 179). Comparison of hMet female and hMet male DEGs in the j dHPC (max-log10(p-value) = 223) and k vHPC (max-log10(p-value) = 232). CORT corticosterone, WT wildtype, hMet heterozygous for brain-derived neurotropic factor Val66Met, ♀ female, ♂ male, DEG differentially expressed gene, dHPC dorsal hippocampus, vHPC ventral hippocampus.
Stratified RRHO test [34], a threshold-free approach that ranks genes by their p-value and effect size direction, was used to identify genes differentially regulated by CORT from a continuous gradient of significancy that were concordant across treatment, sex, and genotype in each brain region (Fig. 3c). Significant overlaps were identified using the point of highest −log10(p-value) from each quadrant as described in Plaiser et al. [38] (see Supplementary for details). Genes from WT and hMet mice converged in the dHPC in both sexes (Fig. 3d, e). In the vHPC, genes were discordant between WT females and hMet females (Fig. 3f). However, males showed concordance in the vHPC between genes in both WT and hMet mice (Fig. 3g). The patterns of overlap indicated that gene expression differences between WT and hMet mice were more prominent in the vHPC of female mice compared to males. An additional set of RRHOs was generated to compare the gene set of males and females in the dHPC and vHPC. Gene expression in WT mice converged across sexes in both the dHPC and vHPC (Fig. 3h, i). However, gene expression in hMet mice diverged across sexes in both the dHPC and vHPC (Fig. 3j, k). This indicates that the hMet variant drives sex differences in gene expression after CORT in both regions of the hippocampus.
Together, we found that CORT affects gene expression change predominantly in the vHPC, and that sex differences are more marked in the vHPC than in the dHPC, especially in hMet mice.
CORT increases methylation of GR exons 1C and 1F in the vHPC of hMet females
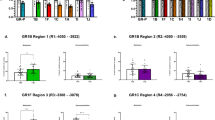
Genomic sex differences in the dHPC and vHPC prompted the investigation on CORT-associated genes in males and females, based on evidence that CORT has genomic and epigenomic effects through the glucocorticoid receptor (GR) [2]. The analysis on genes that were concordant in the RRHOs was narrowed to investigate glucocorticoid receptor-binding genes (GRBGs) [39] and epigenetic modifiers [40]. Genes were clustered into separate heatmaps whose cladograms indicated concordance in CORT-induced gene expression across groups in the dHPC and vHPC (Fig. 4a, b). Both GRBGs and epigenetic modifiers clustered together in the vHPC of WT and hMet males, and they were weakly or not associated with WT females and hMet females, respectively. Thus, sex differences mainly drove the clustering of GRBGs and epigenetic modifiers. The same pattern of sex differences was not observed in the dHPC, where WT males and hMet females clustered instead, indicating that, in the dHPC, similarity in gene expression change after CORT was independent of sex and genotype. We then focused the investigation of sex differences in the vHPC by measuring the expression of the GR (encoded by Nr3c1) and its epigenetic regulation. Total Nr3c1 mRNA was reduced in WT females under CORT (64%; p < 0.0001), as well as hMet females (p < 0.0001), but not in WT males or hMet mice of either genotype (Fig. 4c). Epigenetic regulation of Nr3c1 was then assessed using RT-qPCR and meDIP on exons 1A, 1C, 1D, 1F, and 1H, whose methylation status is altered in several affective disorders in both animal models and clinical studies [41,42,43,44,45,46]. The mRNA levels of exon 1C were increased in hMet females under CORT compared to CORT-treated WT females (p < 0.001), while levels in hMet females exceeded hMet males regardless of treatment (p < 0.0001) (Fig. 4d). The mRNA levels of exon 1F were reduced in WT females under CORT (p < 0.05), but not in males (Fig. 4e). We then assessed the levels of 5-methylcytosine (5mC), a de novo enzymatic modification of the 5-position of the cytosine catalyzed by DNA methyltransferase proteins, which indicates epigenetic modification of DNA [47]. Levels of 5mC for exon 1C were increased in hMet females under CORT (p < 0.0001), but not in males of either genotype (Fig. 4f). In addition, CORT increased 5mC levels of exon 1F in hMet females (p < 0.01), but not in males of either genotype (Fig. 4g). No differences were found in levels of exons 1A, 1D, or 1H as a function of sex, treatment, or genotype (Supplementary Fig. 3a–d). Therefore, we found that only females showed changes in methylation and mRNA levels of Nr3c1 exons 1C and 1F in the vHPC in response to CORT, and that there is an epigenetic response to CORT in the vHPC that is unique to females carrying the BDNF Met variant.
Heat map representing the normalized read densities of epigenetic and GR-binding genes across all groups in the a dHPC and the b vHPC. The cladogram in the right indicates the similarity in gene expression profiles, with average hierarchical linkage clustering based on Pearson correlation. Measurement of Nr3c1 mRNA levels from the vHPC are expressed as 2−ΔCt for c total Nr3c1 mRNA as well as Nr3c1 mRNA for d exon 1C and e exon 1F. Total Nr3c1 mRNA was significantly decreased in WT females after treatment with CORT. Baseline hMet females had lower total Nr3c1 mRNA levels than matched WT females (three-way ANOVA, genotype: F(1,46) = 5.301, p < 0.05, treatment: F(1,46) = 5.928, p < 0.05, sex: F(1,46) = 37.66, p < 0.0001, sex × genotype: F(1,46) = 4.947, p < 0.05, treatment × genotype: F(1,46) = 12.71, p < 0.001, treatment × genotype × sex: F(1,46) = 8.730, p < 0.01). Levels of mRNA for Nr3c1 exon 1C in hMet females under CORT was significantly higher than hMet females at baseline, while hMet females had higher levels of exon 1C mRNA than males regardless of treatment (three-way ANOVA, genotype: F(1,46) = 4.304, p < 0.05 sex: F(1,46) = 75.38, p < 0.0001, sex × genotype: F(1,46) = 13.64, p < 0.001). Levels of Nr3c1 exon 1F mRNA was significantly decreased in WT females after treatment with CORT (3-way ANOVA, treatment: F(1,46) = 5.384, p < 0.05, treatment × sex: F(1,46) = 9.458, p < 0.01). Measurement of Nr3c1 f exon 1C and g exon 1F methylation levels from the vHPC are expressed as 2−ΔCt. CORT significantly increased levels of 5mC for Nr3c1 exon 1C and 1F for hMet females (three-way ANOVA, exon 1C: three-way ANOVA, sex: F(1,40) = 4.875, p < 0.05, treatment: F(1,40) = 27.04, p < 0.0001, treatment × genotype: F(1,40) = 4.166, p < 0.05, treatment × sex: F(1,40) = 15.11, p < 0.001; exon 1F: sex: F(1,40) = 11.19, p < 0.05, treatment: F(1,40) = 11.19, p < 0.01, treatment × sex: F(1,40) = 5.394, p < 0.05). Columns represent the mean ± SEM of 5–7 determinations per group. *p < 0.05, **p < 0.01, ***p < 0.001. PND post-natal day, WT wildtype, hMet heterozygous for brain-derived neurotropic factor Val66Met, V vehicle, C/CORT corticosterone, dHPC dorsal hippocampus, vHPC ventral hippocampus.
Discussion
We report, for the first time, behavioral sex differences in mice treated with non-invasive oral CORT, a paradigm that induces impaired activity of the HPA axis [16, 17]. Not only is this the first study investigating sex differences in anxiety- and depression-like behavior in the oral CORT model and in BDNF Val66Met mice, but the novelty also arises from the investigation of genomic signatures that depended on the hippocampal region analyzed, with the dHPC and vHPC expressing distinct pattern of gene networks, particularly GRBGs and epigenetic modifiers. We found that one epigenomic consequence of oral CORT was the methylation levels of the Nr3c1 gene that differed in males and females as a function of the BDNF Met genotype.
As previously reported in males [16], we demonstrated that low-dose oral CORT did not induce changes in female body weight compared to controls, indicating that major metabolic consequences are indeed limited in both sexes. A trend in the atrophy of adrenal glands was only observed in males, consistent with studies showing that chronic treatment with CORT leads to smaller adrenal glands compared to controls [16], a phenomenon that is explained by adaptation of the adrenal gland at the cellular level [48], and that correlates with the blunted response to stress observed in this model [18]. Circulating plasma CORT levels measured after 24-h fasting were lower in males than in females, but treatment did not affect plasmatic CORT. This is consistent with evidence showing that mRNA and serum levels of corticosteroid-binding globulin are regulated by gonadal hormones, leading to sex differences in CORT levels [49,50,51]. High levels of glycemia reported in hMet males at baseline are consistent with previous findings showing insulin resistance in hMet mice [52]. Impairment in glucose regulation have also been observed in humans carrying BDNF Met variant that show insulin resistance and increased risk for metabolic disorders [53].
As reported in our findings, previous studies have also shown that oral CORT increases affective behavior in male mice of both genotypes [18, 54, 55]. Here we showed that WT females display no behavioral changes, consistent with previous findings on negative valence behaviors in WT females [56, 57], and that hMet females show instead an opposite effect on affective behavior compared to males. Other studies have investigated these behavioral phenotypes using exogenous CORT in males and found that prolonged CORT administration, independent of the administration route, produce both anxiety [58] and depression-like behavior [59]. In fact, a single acute injection of corticosterone was found to induce anxiety-like behavior 12 days later [60]. Females also display increased depression-like behavior in response to CORT injection, and effect that is not observed when CORT is delivered in drinking water [61].
We found that gene expression change after CORT was greater in the vHPC than in the dHPC in males of both genotypes and larger in the vHPC of males compared to females regardless of genotype. Curiously, in the dHPC, WT males and females treated with CORT showed comparable gene expression change, while hMet females under CORT displayed fewer DEGs compared to WT females. Accordingly, RRHOs showed genotypic differences in the vHPC of females, and discordant CORT-induced DEGs in both brain regions only in hMet males and females. This pattern of gene expression after CORT mirrored behavioral sex differences as well as the unique behavioral phenotype found in hMet females. Dorsoventral differences in gene expression have also been observed in other models of stress. For example, the immediate early gene Fos is primed for activation one month after traumatic stress in the vHPC but not the dHPC [62]. Six-week exposure to chronic mild stress increases the expression of FKBP5, a co-chaperone of GR, in the vHPC but not in the dHPC [6]. Others have shown that when chronic stress is combined with prenatal alcohol exposure, the expression of MR, GR, and type 1 CRH receptor is altered in a region-specific manner in the hippocampus and differs in males and females [63]. Interestingly, this evidence relating to GR gene expression compliments the sensitivity of the vHPC to glucocorticoid exposure [64, 65], and the importance of the GR in regulating neurotransmission in this brain region [66]. Collectively, previous data support our observations on the criticality of the vHPC in the response to exogenous CORT or other types of stressors.
It is worth noting that the distribution of GR in the hippocampus differs across subregions and subfields [5], a neuroanatomical difference that is also observed after exposure to exogenous CORT. Robertson et al. reported that GR expression is twice as high in the dHPC as in the vHPC in rats [67], a pattern that relates to differential GR expression in distinct hippocampal subfields in the presence of high levels of CORT [68]. Curiously, treatment with exogenous CORT in males has not been associated with changes in GR expression in the vHPC [69], in line with our measurements of mRNA levels in the vHPC of CORT-treated males.
These findings prompted us to investigate whether GR had different genomic effects in the dHPC and vHPC that intersected with sex and genotype. Notably, after translocating to the nucleus the GR interacts with number of epigenetic modifiers [70]. Thus, we also investigated whether changes in GR could be associated with differences in epigenetic regulation. Differential expression of GRBGs and several epigenetic modifiers (e.g., chromatin remodelers and histone acetyltransferases, demethylases, and methyltransferases) across groups showed that sex differences were observed in the vHPC but not in the dHPC where clustering was independent of sex and genotype. Interestingly, CORT-induced changes in the expression of GRBGs and epigenetic modifiers in hMet females differed substantially from the other groups, again reflecting the unique behavioral and genomic profile of hMet females. We have previously reported that the expression of GRBGs in CA3 pyramidal neurons of the hippocampus differs between WT and hMet male mice after stress [26]. Yet, DEGs from this hippocampal subregion in unstressed hMet mice largely overlap with gene expression changes observed in WT mice after stress [25]. Previous work from our group highlighted changes in GRBGs and a significant cluster of epigenetic modifiers in CA3 pyramidal neurons of adult offspring with a history of early life stress exposed to a second hit of stress in adulthood [71], suggesting an important role for the GR in the epigenetic signatures mediated by stress. While others also showed that exposure to exogenous CORT alter genome-wide epigenetic networks [72] as well as the expression of GRBGs [73] in the hippocampus, this is the first study demonstrating that these changes critically depend on the sex, genotype, and the hippocampal region that is investigated.
The relevance of exogenous CORT and its transcriptomic consequences is emphasized when reminded that up to 1/5 of the human genome is regulated by the GR [1]. Additionally, epigenetic modifications and DNA methylation of GR exons have been shown to reflect the stress status of an individual [45, 74]. These changes are often a consequence of adverse stimuli or overexposure to CORT. Variations in maternal licking and grooming in rats are associated with epigenetic modifications of the GR exon 17 (corresponding to 1F in humans and mice) that persist through adulthood [75]. In humans, methylation levels of Nr3c1 are affected in offspring of prenatal depressive mothers [46] as well as subjects suffering from psychiatric disorders, including individuals with alcohol use disorder [35], victims of suicide [41, 44], combat veterans of PTSD [42], and women with bulimia nervosa [43]. Interestingly, these epigenetic modifications often occur on the exons 1C and 1F of the NR3C1 gene. Here, we found that CORT increased DNA methylation of exons 1C and 1F of the Nr3c1 gene selectively in the vHPC of hMet females under CORT, while the respective mRNA levels were unchanged (1C) or oppositely (1F) regulated in hMet females compared to WT females. In males, total mRNA levels and methylation of Nr3c11C and Nr3c11F were unaffected regardless of genotype. Interestingly, we found a negative correlation between levels of Nr3c11C methylation and emotionality score in hMet females under CORT (r = −0.966, p < 0.05) that did not exist in other groups (Table 1). This suggests that (i) a mechanism different than methylation controls the total expression of Nr3c1 in CORT-treated WT females and males of either genotype; (ii) methylation in response to CORT at specific exons depends on genotype; (iii) the BDNF Met variant interacts with CORT to modulate methylation at discrete exons of Nr3c1.
Glucocorticoids and BDNF share a complementary role in synaptic plasticity. Previous work showed that overexposure to CORT affects the signaling of BDNF in the hippocampus [76, 77], and that methylation changes of Nr3c1 are linked to BDNF expression [78]. BDNF signaling not only modulates the excitability of the hippocampus but is directly impacted by fluctuations in the estrous cycle [79, 80]. In addition, CORT signaling is inhibited by estrogens, a mechanism that impairs the negative feedback on the HPA axis [2, 81]. Curiously, ovariectomy rescues impairment in object memory location in hMet females [25], which also show increased affective behavior compared to controls at discrete stages of the estrus cycle [82, 83] or in the presence of exogenous estradiol [84]. This suggests that the BDNF Met variant orchestrates complex gene pathways and behaviors that are unique to hMet females due to the distinctive intersection of CORT, BDNF, and estrogen signaling. Gomes de Assis and Gasanov (2019) recently discussed the implication of the BDNF and CORT integrative system in the BDNF Val66Met polymorphism [85], however further studies are needed to validate the mechanistic function of the BDNF Met variant and its distinct adaptive role in males and females.
Together, we showed that, in mouse models that recapitulate stress-related disorders, similar constructive validity (chronic oral CORT) is associated with opposed face validity (affective behavior) in males and females and suggests that distinct brain circuits and genomic regulation underlies behavioral outcomes in response to exogenous CORT. This opens a window for the investigation of biomarkers of stress that are both unique to genetically susceptible individuals and altered regardless of the behavioral phenotype that one may manifest.
References
Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286:3177–84.
Gray JD, Kogan JF, Marrocco J, McEwen BS. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat Rev Endocrinol. 2017;13:661–73.
Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Brain Res. 1985;350:159–64.
Chao HM, Choo PH, McEwen BS. Glucocorticoid and mineralocorticoid receptor mRNA expression in rat brain. Neuroendocrinology 1989;50:365–71.
Sarabdjitsingh RA, Meijer OC, de Kloet ER. Specificity of glucocorticoid receptor primary antibodies for analysis of receptor localization patterns in cultured cells and rat hippocampus. Brain Res. 2010;1331:1–11.
Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology 2013;38:616–27.
Kvichansky AA, Volobueva MN, Manolova AO, Bolshakov AP, Gulyaeva NV. Neonatal proinflammatory stress alters the expression of genes of corticosteroid receptors in the rat hippocampus: septo-temporal differences. Neurochem J. 2017;11:255–8.
Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99:10825–30.
Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010;65:7–19.
Ardi Z, Albrecht A, Richter-Levin A, Saha R, Richter-Levin G. Behavioral profiling as a translational approach in an animal model of posttraumatic stress disorder. Neurobiol Dis. 2016;88:139–47.
Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N. Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. Elife 2016;5:e14997.
Zhang T-Y, Keown CL, Wen X, Li J, Vousden DA, Anacker C, et al. Environmental enrichment increases transcriptional and epigenetic differentiation between mouse dorsal and ventral dentate gyrus. Nat Commun. 2018;9:298.
Chandramohan Y, Droste SK, Reul JMHM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-d-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochemistry. 2007;101:815–28.
Yagi S, Splinter JEJ, Tai D, Wong S, Wen Y, Galea LAM. Sex differences in maturation and attrition of adult neurogenesis in the hippocampus. eNeuro. 2020;7:ENEURO.0468-19.2020.
Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–9.
Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 2010;151:2117–27.
David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009;62:479–93.
Kinlein SA, Wilson CD, Karatsoreos IN. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry. 2015;6:31.
Kinlein SA, Phillips DJ, Keller CR, Karatsoreos IN. Role of corticosterone in altered neurobehavioral responses to acute stress in a model of compromised hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology 2019;102:248–55.
Berger S, Gureczny S, Reisinger SN, Horvath O, Pollak DD. Effect of chronic corticosterone treatment on depression-like behavior and sociability in female and male C57BL/6N Mice. Cells 2019;8:1018.
Notaras M, Hill R, Gogos JA, van den Buuse M. BDNF Val66Met genotype determines hippocampus-dependent behavior via sensitivity to glucocorticoid signaling. Mol Psychiatry. 2016;21:730–2.
Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006;314:140–3.
Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25:2251–74.
Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–64.
Marrocco J, Petty GH, Ríos MB, Gray JD, Kogan JF, Waters EM, et al. A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nat Commun2017;8:808.
Gray JD, Rubin TG, Kogan JF, Marrocco J, Weidmann J, Lindkvist S, et al. Translational profiling of stress-induced neuroplasticity in the CA3 pyramidal neurons of BDNF Val66Met mice. Mol Psychiatry. 2018;23:904–13.
Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018;34:i884–i90.
Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016;32:3047–8.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21.
Cunningham F, Achuthan P, Akanni W, Allen J, Amode MR, Armean IM, et al. Ensembl 2019. Nucleic Acids Res. 2019;47(D1):D745–d51.
Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41:e108.
Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12:115–21.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Cahill KM, Huo Z, Tseng GC, Logan RW, Seney ML. Improved identification of concordant and discordant gene expression signatures using an updated rank–rank hypergeometric overlap approach. Sci Rep. 2018;8:9588.
Gatta E, Grayson DR, Auta J, Saudagar V, Dong E, Chen Y, et al. Genome-wide methylation in alcohol use disorder subjects: implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (NR3C1). Mol Psychiatry. 2021;26:1029–41.
Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods. 2011;197:21–31.
Caradonna SG, Zhang T-Y, O’Toole N, Shen M-J, Khalil H, Einhorn NR, et al. Genomic modules and intramodular network concordance in susceptible and resilient male mice across models of stress. Neuropsychopharmacology. 2021. https://doi.org/10.1038/s41386-021-01219-8.
Plaisier SB, Taschereau R, Wong JA, Graeber TG. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38:e169.
Polman JAE, Welten JE, Bosch DS, de Jonge RT, Balog J, van der Maarel SM, et al. A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci. 2012;13:118-.
Zhu X, Girardo D, Govek E-E, John K, Mellén M, Tamayo P, et al. Role of Tet1/3 genes and chromatin remodeling genes in cerebellar circuit formation. Neuron 2016;89:100–12.
McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8.
Yehuda R, Flory JD, Bierer LM, Henn-Haase C, Lehrner A, Desarnaud F, et al. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:356–64.
Steiger H, Labonté B, Groleau P, Turecki G, Israel M. Methylation of the glucocorticoid receptor gene promoter in bulimic women: Associations with borderline personality disorder, suicidality, and exposure to childhood abuse. Int J Eat Disord. 2013;46:246–55.
Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, et al. Differential glucocorticoid receptor exon 1B, 1C, and 1H expression and methylation in suicide completers with a history of childhood abuse. Biol Psychiatry 2012;72:41–8.
Turecki G, Meaney MJ. Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol Psychiatry. 2016;79:87–96.
Braithwaite EC, Kundakovic M, Ramchandani PG, Murphy SE, Champagne FA. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics 2015;10:408–17.
Jin S-G, Kadam S, Pfeifer GP. Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic Acids Res. 2010;38:e125-e.
Berger I, Werdermann M, Bornstein SR, Steenblock C. The adrenal gland in stress—adaptation on a cellular level. J Steroid Biochem Mol Biol. 2019;190:198–206.
Misao R, Nakanishi Y, Fujimoto J, Iwagaki S, Tamaya T. Levels of sex hormone-binding globulin and corticosteroid-binding globulin mRNAs in corpus luteum of human subjects: correlation with serum steroid hormone levels. Gynecol Endocrinol. 1999;13:82–8.
Moisan MP. Sexual dimorphism in glucocorticoid stress response. Int J Mol Sci. 2021;22:3139 https://doi.org/10.3390/ijms22063139.
Kokras N, Krokida S, Varoudaki TZ, Dalla C. Do corticosterone levels predict female depressive-like behavior in rodents? J Neurosci Res. 2021;99:324–31.
Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 2003;144:2446–53.
Bonaccorso S, Sodhi M, Li J, Bobo WV, Chen Y, Tumuklu M, et al. The brain-derived neurotrophic factor (BDNF) Val66Met polymorphism is associated with increased body mass index and insulin resistance measures in bipolar disorder and schizophrenia. Bipolar Disord. 2015;17:528–35.
Ding H, Cui X-Y, Cui S-Y, Ye H, Hu X, Zhao H-L, et al. Depression-like behaviors induced by chronic corticosterone exposure via drinking water: time-course analysis. Neurosci Lett. 2018;687:202–6.
Klug M, Hill RA, Choy KH, Kyrios M, Hannan AJ, van den Buuse M. Long-term behavioral and NMDA receptor effects of young-adult corticosterone treatment in BDNF heterozygous mice. Neurobiol Dis. 2012;46:722–31.
Yohn CN, Ashamalla SA, Bokka L, Gergues MM, Garino A, Samuels BA. Social instability is an effective chronic stress paradigm for both male and female mice. Neuropharmacology 2019;160:107780.
Mekiri M, Gardier AM, David DJ, Guilloux J-P. Chronic corticosterone administration effects on behavioral emotionality in female c57bl6 mice. Exp Clin Psychopharmacol. 2017;25:94–104.
Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol. 2008;583:115–27.
Lussier AL, Romay-Tallón R, Kalynchuk LE, Caruncho HJ. Reelin as a putative vulnerability factor for depression: examining the depressogenic effects of repeated corticosterone in heterozygous reeler mice. Neuropharmacology 2011;60:1064–74.
Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA 2008;105:5573–8.
Kott JM, Mooney-Leber SM, Shoubah FA, Brummelte S. Effectiveness of different corticosterone administration methods to elevate corticosterone serum levels, induce depressive-like behavior, and affect neurogenesis levels in female rats. Neuroscience 2016;312:201–14.
Ritov G, Boltyansky B, Richter-Levin G. A novel approach to PTSD modeling in rats reveals alternating patterns of limbic activity in different types of stress reaction. Mol Psychiatry. 2016;21:630–41.
Raineki C, Ellis L, Weinberg J. Impact of adolescent stress on the expression of stress-related receptors in the hippocampus of animals exposed to alcohol prenatally. Hippocampus 2018;28:201–16.
Marrocco J, Mairesse J, Ngomba RT, Silletti V, Van Camp G, Bouwalerh H, et al. Anxiety-like behavior of prenatally stressed rats is associated with a selective reduction of glutamate release in the ventral hippocampus. J Neurosci. 2012;32:17143–54.
Marrocco J, Reynaert M-L, Gatta E, Gabriel C, Mocaër E, Di Prisco S, et al. The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders. J Neurosci. 2014;34:2015–24.
Mairesse J, Gatta E, Reynaert M-L, Marrocco J, Morley-Fletcher S, Soichot M, et al. Activation of presynaptic oxytocin receptors enhances glutamate release in the ventral hippocampus of prenatally restraint stressed rats. Psychoneuroendocrinology 2015;62:36–46.
Robertson DAF, Beattie JE, Reid IC, Balfour DJK. Regulation of corticosteroid receptors in the rat brain: the role of serotonin and stress. Eur J Neurosci. 2005;21:1511–20.
De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301.
Buret L, van den Buuse M. Corticosterone treatment during adolescence induces down-regulation of reelin and NMDA receptor subunit GLUN2C expression only in male mice: implications for schizophrenia. Int J Neuropsychopharmacol. 2014;17:1221–32.
Bartlett AA, Lapp HE, Hunter RG. Epigenetic mechanisms of the glucocorticoid receptor. Trends Endocrinol Metab. 2019;30:807–18.
Marrocco J, Gray JD, Kogan JF, Einhorn NR, O’Cinneide EM, Rubin TG, et al. Early life stress restricts translational reactivity in CA3 neurons associated with altered stress responses in adulthood. Front Behav Neurosci. 2019;13:157.
Seifuddin F, Wand G, Cox O, Pirooznia M, Moody L, Yang X, et al. Genome-wide Methyl-Seq analysis of blood-brain targets of glucocorticoid exposure. Epigenetics 2017;12:637–52.
Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology 2010;151:4332–43.
Thomassin H, Flavin M, Espinás ML, Grange T. Glucocorticoid-induced DNA demethylation and gene memory during development. EMBO J 2001;20:1974–83.
Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54.
Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience 2013;239:173–95.
Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress 2000;3:201–8.
Kundakovic M, Lim S, Gudsnuk K, Champagne F. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry. 2013;4:78 https://doi.org/10.3389/fpsyt.2013.00078.
Harte-Hargrove LC, MacLusky NJ, Scharfman HE. Brain-derived neurotrophic factor–estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience 2013;239:46–66.
Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641.
Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 2009;159:883–95.
Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci USA 2010;107:4395–400.
Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, et al. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 2012;72:499–504.
Marrocco J, Einhorn NR, Petty GH, Li H, Dubey N, Hoffman J, et al. Epigenetic intersection of BDNF Val66Met genotype with premenstrual dysphoric disorder transcriptome in a cross-species model of estradiol add-back. Mol Psychiatry. 2020;25:572–83.
de Assis GG, Gasanov EV. BDNF and cortisol integrative system—plasticity vs. degeneration: implications of the Val66Met polymorphism. Front Neuroendocrinol. 2019;55:100784.
Acknowledgements
This work was supported by the Hope for Depression Research Foundation and the Rockefeller Gary Helman Fellowship. E.G. was supported by a NIH-NIAAA grant K99AA028817, A.G. was supported by a NIH-NIAAA P50AA022538 (Center for Alcohol Research in Epigenetics). We thank the Rockefeller Genomics Resource Center and the Rockefeller Bioinformatics Resource Center for the RNA-sequencing procedure and data mining. We are immensely grateful to Dr. Bruce McEwen in wake of his passing, for believing in this project.
Author information
Authors and Affiliations
Contributions
J.M. and B.S.M. designed research; J.M., S.G.C., N.R.E., V.S., G.H.P., A.L., C.L., and E.G. performed research; J.M., S.G.C., and H.K. analyzed data; J.M., S.G.C., and E.G. wrote the paper; J.M., F.S.L., H.A., B.S.M., A.G., and E.G. supervised research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caradonna, S.G., Einhorn, N.R., Saudagar, V. et al. Corticosterone induces discrete epigenetic signatures in the dorsal and ventral hippocampus that depend upon sex and genotype: focus on methylated Nr3c1 gene. Transl Psychiatry 12, 109 (2022). https://doi.org/10.1038/s41398-022-01864-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-01864-7