Abstract
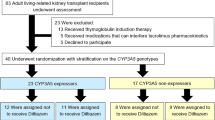
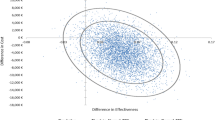
The objective of this study was to estimate the cost-effectiveness of CYP3A5 genotype-guided tacrolimus dosing in kidney, liver, heart, and lung transplant recipients relative to standard of care (SOC) tacrolimus dosing, from a US healthcare payer perspective. We developed decision-tree models to compare economic and clinical outcomes between CYP3A5 genotype-guided and SOC tacrolimus therapy in the first six months post-transplant. We derived inputs for CYP3A5 phenotype frequencies and physician use of genotype test results to inform clinical care from literature; tacrolimus exposure [high vs low tacrolimus time in therapeutic range using the Rosendaal algorithm (TAC TTR-Rosendaal)] and outcomes (incidences of acute tacrolimus nephrotoxicity, acute cellular rejection, and death) from real-world data; and costs from the Medicare Fee Schedule and literature. We calculated cost per avoided event and performed sensitivity analyses to evaluate the robustness of the results to changes in inputs. Incremental costs per avoided event for CYP3A5 genotype-guided vs SOC tacrolimus dosing were $176,667 for kidney recipients, $364,000 for liver recipients, $12,982 for heart recipients, and $93,333 for lung recipients. The likelihood of CYP3A5 genotype-guided tacrolimus dosing leading to cost-savings was 19.8% in kidney, 32.3% in liver, 51.8% in heart, and 54.1% in lung transplant recipients. Physician use of genotype results to guide clinical care and the proportion of patients with a high TAC TTR-Rosendaal were key parameters driving the cost-effectiveness of CYP3A5 genotype-guided tacrolimus therapy. Relative to SOC, CYP3A5 genotype-guided tacrolimus dosing resulted in a slightly greater benefit at a higher cost. Further economic evaluations examining intermediary outcomes (e.g., dose modifications) are needed, particularly in populations with higher frequencies of CYP3A5 expressers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Brunet M, van Gelder T, Asberg A, Haufroid V, Hesselink DA, Langman L, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41:261–307.
Kang JS, Lee MH. Overview of therapeutic drug monitoring. Korean J Intern Med. 2009;24:1–10.
Dasari BVM, Hodson J, Nassir A, Widmer J, Isaac J, Mergentel H, et al. Variations in practice to therapeutic monitoring of tacrolimus following primary adult liver transplantation. Int J Organ Transplant Med. 2016;7:1–8.
Chang DH, Youn J-C, Dilibero D, Patel JK, Kobashigawa JA. Heart transplant immunosuppression strategies at cedars-sinai medical center. Int J Heart Fail. 2021;3:15–30.
Kamdem LK. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clinical Chemistry. 2005;51:1374–81.
Rojas LE, Herrero MJ, Boso V, Garcia-Eliz M, Poveda JL, Librero J, et al. Meta-analysis and systematic review of the effect of the donor and recipient CYP3A5 6986A>G genotype on tacrolimus dose requirements in liver transplantation. Pharmacogenet Genomics. 2013;23:509–17.
Rojas L, Neumann I, Herrero MJ, Boso V, Reig J, Poveda JL, et al. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: a systematic review and meta-analysis of observational studies. Pharmacogenomics J. 2015;15:38–48.
Deininger KM, Vu A, Page RL 2nd, Ambardekar AV, Lindenfeld J, Aquilante CL. CYP3A pharmacogenetics and tacrolimus disposition in adult heart transplant recipients. Clin Transplant. 2016;30:1074–81.
Calabrese DR, Florez R, Dewey K, Hui C, Torgerson D, Chong T, et al. Genotypes associated with tacrolimus pharmacokinetics impact clinical outcomes in lung transplant recipients. Clin Transplant. 2018;32:e13332.
Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. 2015;98:19–24.
Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87:721–6.
Shuker N, Bouamar R, van Schaik RH, Clahsen-van Groningen MC, Damman J, Baan CC, et al. A randomized controlled trial comparing the efficacy of Cyp3a5 Genotype-Based With Body-Weight-Based Tacrolimus Dosing After Living Donor Kidney Transplantation. Am J Transplant. 2016;16:2085–96.
Anutrakulchai S, Pongskul C, Kritmetapak K, Limwattananon C, Vannaprasaht S. Therapeutic concentration achievement and allograft survival comparing usage of conventional tacrolimus doses and CYP3A5 genotype-guided doses in renal transplantation patients. Br J Clin Pharmacol. 2019;85:1964–73.
Reininger KA, Onyeaghala G, Anderson-Haag T, Schladt DS, Wu B, Guan W, et al. Higher number of tacrolimus dose adjustments in kidney transplant recipients who are extensive and intermediate CYP3A5 metabolizers. Clin Transplant. 2023;37:e14893.
Natasha P, Baye J, Aifaoui A, Wilke RA, Lupu RA, Savageau J, et al. Implementation of wide-scale pharmacogenetic testing in primary care. Pharmacogenomics. 2019;20:903–13.
Bielinski SJ, St Sauver JL, Olson JE, Larson NB, Black JL, Scherer SE, et al. Cohort Profile: the right drug, right dose, right time: using genomic data to individualize treatment protocol (RIGHT Protocol). Int J Epidemiol. 2020;49:23–24.
Liu M, Vnencak-Jones CL, Roland BP, Gatto CL, Mathe JL, Just SL, et al. A tutorial for pharmacogenomics implementation through end-to-end clinical decision support based on ten years of experience from PREDICT. Clin Pharmacol Ther. 2021;109:101–15.
Pasternak AL, Marshall VD, Gersch CL, Rae JM, Englesbe M, Park JM. Evaluating the Impact of CYP3A5 genotype on post-transplant healthcare resource utilization in pediatric renal and heart transplant recipients receiving tacrolimus. Pharmacogenomics Personalized Med. 2021;14:319–26.
Wang L, Scherer SE, Bielinski SJ, Muzny DM, Jones LA, Black JL 3rd, et al. Implementation of preemptive DNA sequence-based pharmacogenomics testing across a large academic medical center: The Mayo-Baylor RIGHT 10K Study. Genet Med. 2022;24:1062–72.
Tillman E, Nikirk MG, Chen J, Skaar TC, Shugg T, Maddatu JP, et al. Implementation of Clinical Cytochrome P450 3A Genotyping for Tacrolimus Dosing in a Large Kidney Transplant Program. J Clin Pharmacol. 2023;63:961–7.
Prograf prescribing information: http://www.astellas.us/docs/prograf.pdf.
Phan L, Jin Y, Zhang H, Qiang W, Shekhtman E, Shao D, et al. “ALFA: Allele Frequency Aggregator” (2022). National Center for Biotechnology Information, U.S. National Library of Medicine; Available from: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/.
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43.
Khan BA, Robinson R, Fohner AE, Muzquiz LI, Schilling BD, Beans JA, et al. Cytochrome P450 genetic variation associated with tamoxifen biotransformation in american indian and alaska native people. Clin Transl Sci. 2018;11:312–21.
Mohamed ME, Schladt DP, Guan W, Wu B, van Setten J, Keating BJ, et al. Tacrolimus troughs and genetic determinants of metabolism in kidney transplant recipients: A comparison of four ancestry groups. Am J Transplant. 2019;19:2795–804.
Fohner AE, Dalton R, Skagen K, Jackson K, Claw KG, Hopkins SE, et al. Characterization of CYP3A pharmacogenetic variation in American Indian and Alaska Native communities, targeting CYP3A4*1G allele function. Clin Transl Sci. 2021;14:1292–302.
Deininger KM, Tran JN, Tsunoda SM, Young GK, Lee YM, Anderson HD, et al. Stakeholder perspectives of the clinical utility of pharmacogenomic testing in solid organ transplantation. Pharmacogenomics. 2019;20:1291–302.
Peterson JF, Field JR, Unertl KM, Schildcrout JS, Johnson DC, Shi Y, et al. Physician response to implementation of genotype-tailored antiplatelet therapy. Clin Pharmacol Ther. 2016;100:67–74.
Deininger KM, Tsunoda SM, Hirsch JD, Anderson H, Lee YM, McIlvennan CK, et al. National survey of physicians’ perspectives on pharmacogenetic testing in solid organ transplantation. Clin Transpl. 2020;34:e14037.
Salah LM, Oreschak K, Ambardekar AV, Page RL II, Lindenfeld J, Aquilante CL. Effect of CYP3A Genetic Variants on Different Measures of Tacrolimus Variability in Heart Transplant Recipients. J Heart Lung Transpl. 2020;39:S212.
Davis S, Gralla J, Klem P, Tong S, Wedermyer G, Freed B, et al. Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor-specific antibodies in the first year of kidney transplantation. Am J Transplant. 2018;18:907–15.
Ensor CR, Iasella CJ, Harrigan KM, Morrell MR, Moore CA, Shigemura N, et al. Increasing tacrolimus time-in-therapeutic range is associated with superior one-year outcomes in lung transplant recipients. Am J Transplant. 2018;18:1527–33.
Song T, Yin S, Jiang Y, Huang Z, Liu J, Wang Z, et al. Increasing time in therapeutic range of tacrolimus in the first year predicts better outcomes in living-donor kidney transplantation. Front Immunol. 2019;10:2912.
Davis S, Gralla J, Klem P, Stites E, Wiseman A, Cooper JE. Tacrolimus intrapatient variability, time in therapeutic range, and risk of de novo donor-specific antibodies. Transplantation. 2020;104:881–7.
Adie SK, Bitar A, Konerman MC, Dorsch MP, Andrews CA, Pogue K, et al. Tacrolimus time in therapeutic range and long-term outcomes in heart transplant recipients. Pharmacotherapy. 2021;42:106–11.
Leino AD, Park JM, Pasternak AL. Impact of CYP3A5 phenotype on tacrolimus time in therapeutic range and clinical outcomes in pediatric renal and heart transplant recipients. Pharmacotherapy. 2021;41:649–57.
Yin S, Huang Z, Wang Z, Fan Y, Wang X, Song T, et al. Early monitoring and subsequent gain of tacrolimus time-in-therapeutic range may improve clinical outcomes after living kidney transplantation. Ther Drug Monit. 2021;43:728–35.
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–9.
Pierce DR, West-Thielke P, Hajjiri Z, Gaitonde S, Tzvetanov I, Benedetti E, et al. Clinical implications of tacrolimus time in therapeutic range and intrapatient variability in urban renal transplant recipients undergoing early corticosteroid withdrawal. Transplant Direct. 2021;7:e698.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–184.
Oetting WS, Wu B, Schladt DP, Guan W, van Setten J, Keating BJ, et al. Genetic variants associated with immunosuppressant pharmacokinetics and adverse effects in the DeKAF genomics genome-wide association studies. Transplantation. 2019;103:1131–9.
Broce JC, Price LL, Liangos O, Uhlig K, Jaber BL. Hospital-acquired acute kidney injury: an analysis of nadir-to-peak serum creatinine increments stratified by baseline estimated GFR. Clin J Am Soc Nephrol. 2011;6:1556–65.
Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-63.
Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20.
Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–42.
Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation. 2018;102:1795–814.
Quest Diagnostics. Pain Management, CYP450 3A5 Genotype, Qualitative. https://testdirectory.questdiagnostics.com/test/test-detail/91618/pain-management-cyp450-3a5-genotype-qualitative?p=h&cc=MASTER.
Laboratory Corporation of America. Cytochrome P450 3A4/3A5 Genotyping. https://www.labcorp.com/tests/504155/cytochrome-p450-3a4-3a5-genotyping.
ARUP Laboratories. CYP3A4 and CYP3A5. https://ltd.aruplab.com/Tests/Pub/3001518.
Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP). https://datatools.ahrq.gov/hcupnet/.
Centers for Medicare & Medicaid Services. 2022 ASP Drug Pricing Files. https://www.cms.gov/medicare/payment/part-b-drugs/asp-pricing-files.
Centers for Medicare & Medicaid. Physician Fee Schedule. https://www.cms.gov/medicare/physician-fee-schedule/search.
Vannaprasaht S, Limwattananon C, Anutrakulchai S, Chan-On C. Effect of CYP3A5 genotype on hospitalization cost for kidney transplantation. Int J Clin Pharm. 2019;41:88–95.
Nguyen TT, Pearson RA, Mohamed ME, Schladt DP, Berglund D, Rivers Z, et al. Pharmacogenomics in kidney transplant recipients and potential for integration into practice. J Clin Pharm Ther. 2020;45:1457–65.
Plumpton CO, Pirmohamed M, Hughes DA. Cost-effectiveness of panel tests for multiple pharmacogenes associated with adverse drug reactions: an evaluation framework. Clin Pharmacol Ther. 2019;105:1429–38.
McWilliam A, Lutter R, Nardinelli C. Healthcare impact of personalized medicine using genetic testing: an exploratory analysis for warfarin. Per Med. 2008;5:279–84.
You JH, Chan FW, Wong RS, Cheng G. The potential clinical and economic outcomes of pharmacogenetics-oriented management of warfarin therapy - a decision analysis. Thromb Haemost. 2004;92:590–7.
You JH, Tsui KK, Wong RS, Cheng G. Potential clinical and economic outcomes of CYP2C9 and VKORC1 genotype-guided dosing in patients starting warfarin therapy. Clin Pharmacol Ther. 2009;86:540–7.
Mizzi C, Dalabira E, Kumuthini J, Dzimiri N, Balogh I, Başak N, et al. A european spectrum of pharmacogenomic biomarkers: implications for clinical pharmacogenomics. PLoS One. 2016;11:e0162866.
Al-Mahayri ZN, Patrinos GP, Wattanapokayakit S, Iemwimangsa N, Fukunaga K, Mushiroda T, et al. Variation in 100 relevant pharmacogenes among emiratis with insights from understudied populations. Sci Rep. 2020;10:21310.
Runcharoen C, Fukunaga K, Sensorn I, Iemwimangsa N, Klumsathian S, Tong H, et al. Prevalence of pharmacogenomic variants in 100 pharmacogenes among Southeast Asian populations under the collaboration of the Southeast Asian Pharmacogenomics Research Network (SEAPharm). Hum Genome Var. 2021;8:7.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103.
Acknowledgements
This study was funded by a PhRMA Foundation Health Outcomes Research Pre-Doctoral Fellowship (to KMD) and supported by the Health Data Compass Data Warehouse project (healthdatacompass.org).
Funding
This study was funded by a PhRMA Foundation Health Outcomes Research Pre-Doctoral Fellowship (to KMD).
Author information
Authors and Affiliations
Contributions
KMD and CLA conceived of and designed the study. KMD acquired and analyzed the study data. KMD, HDA, GPP, CM, and CLA played an important role in interpreting the results. KMD drafted the manuscript. CLA, HDA, GPP, and CM critically revised the manuscript and approved the final version. CLA had full access to the study data and final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
KMD was an employee of the University of Colorado at the time of this work and is currently an employee of Amgen Pharmaceuticals. None of the other authors have any competing financial interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deininger, K.M., Anderson, H.D., Patrinos, G.P. et al. Cost-effectiveness analysis of CYP3A5 genotype-guided tacrolimus dosing in solid organ transplantation using real-world data. Pharmacogenomics J 24, 14 (2024). https://doi.org/10.1038/s41397-024-00334-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-024-00334-1