Abstract
Chronic kidney disease (CKD) is a global health issue. Kidney failure patients may undergo a kidney transplantation (KTX) and prescribed an immunosuppressant medication i.e., tacrolimus. Tacrolimus’ efficacy and toxicity varies among patients. This study investigates the cost-utility of pharmacogenomics (PGx) guided tacrolimus treatment compared to the conventional approach in Austrian patients undergone KTX, participating in the PREPARE UPGx study. Treatment’s effectiveness was determined by mean survival, and utility values were based on a Visual Analog Scale score. Incremental Cost-Effectiveness Ratio was also calculated. PGx-guided treatment arm was found to be cost-effective, resulting in reduced cost (3902 euros less), 6% less hospitalization days and lower risk of adverse drug events compared to the control arm. The PGx-guided arm showed a mean 0.900 QALYs (95% CI: 0.862–0.936) versus 0.851 QALYs (95% CI: 0.814–0.885) in the other arm. In conclusion, PGx-guided tacrolimus treatment represents a cost-saving option in the Austrian healthcare setting.
Similar content being viewed by others
Introduction
Nowadays, the prevalence of chronic kidney disease (CKD) is rapidly increasing worldwide, especially the percentage of individuals suffering from kidney failure that can lead to long-term irreversible decline and disability [1]. Based on recent studies, it is believed that the CKD rate will rise during the next years due to population ageing and the existence of important comorbidities such as hypertension and diabetes [2]. For this reason, CKD constitutes one of the major public health issues that put significant pressure on healthcare systems [3].
Kidney transplantation (KTX) is steadily increasing by 1.9% each year and almost 30 individuals per million of population are affected based on European Renal Association Registry. Given that, it is obvious, that CKD exerts an economic burden in healthcare systems [4]. The overall cost of CKD to UK’s healthcare system was estimated as £1.45B in 2009–2010, with more than 50% of expenses to be attributed to renal replacement therapies [1]. This cost is related to high percentage of chronic graft rejections that are estimated to be around 20–50% after the first year of transplantation due to inadequate immunosuppressive scheme or to increased drug toxicity [5].
Kidney failure patients require a renal replacement therapeutic scheme such as hemodialysis, peritoneal dialysis or KTX. Hemodialysis is considered as first-line treatment option besides being a costly and long procedure that reduces patients’ quality of life (QoL) and decreases their life expectancy [6]. Kidney transplantation and subsequent use of immunosuppressant medications such as tacrolimus is a more clinical effective option but it is compromised by shortage of donors, allograft dysfunction and rejection, and by adverse drug events (ADE) related to immunosurppresants [7]. Recent statistical analysis of the European Renal Association Registry has shown an increase in the total number of KTX through the years from 2010 to 2018 due to organ donation from deceased donors and not living ones. This highlights the importance of having a unified and well-designed health policy in terms of KTX, to offer the opportunity for better health outcomes to many more kidney failure patients [4].
Tacrolimus is one of the most widely prescribed calcineurin inhibitors in solid organ transplantation [8, 9]. Being used as a long-term immunosuppressant medication upon transplantation, it is demonstrated to have a slightly narrow therapeutic effectiveness due to its great pharmacokinetics variability observed in the population and it requires close clinical monitoring [10]. Moreover, tacrolimus management frequently needs titration owing to its high toxicity which also varies among individuals. ADEs can occur at concentrations slightly above or even within the recommended dose range causing nephrotoxicity, infections, hypertension, hyperkalemia, hypomagnesemia, hyperglycemia, diabetes, tremor, and other neurotoxic effects, while it was associated with graft rejection in lower or subtherapeutic concentrations [9, 10].
Evidently, the reason behind this response variation is genetic variants in the drug metabolism enzyme. CYP3A5 is an enzyme involved in tacrolimus’ metabolism, with gene’s single nucleotide variants accounting for 40–50% of the inter-individual variability in drug’s pharmacokinetics [11]. People with wild-type CYP3A5 alleles (*1/*1) are considered extensive metabolizers, while individuals with at least one defective allele (*3/*6/*7) are considered as intermediate metabolizers. Poor metabolisers bear the CYP3A5 *3/*3 genotype, and their enzyme activity is significantly decreased [8, 12]. From the aforementioned alleles, there is only evidence that the CYP3A5*3 allele (rs776746) is associated with the pharmacokinetics properties of tacrolimus by inducing alternative mRNA splicing, which can lead to abolished CYP3A5 protein activity, hence requiring lower tacrolimus doses, compared to those bearing at least one CYP3A5*1 allele [12, 13].
According to Nguyen and coworkers (2020), 60% of patients are carriers of three or four actionable phenotypes, for which PGx guidelines are available, and can affect pharmacokinetics, pharmacodynamics and drug toxicity [11]. Furthermore, the prevalence of CYP3A5 metabolizer status varies among different races; most individuals of Caucasian origin are mainly carriers of two loss-of-function alleles, while African Americans have an estimated frequency of 0.33 [14]. PGx testing can optimize and tailor individual’s tacrolimus treatment scheme.
Besides the fact that there is a strong evidence of the association of genomic biomarkers with KTX and especially in terms of immunosuppressant treatment, PGx testing isn’t yet widely adopted in clinical routine by nephrologists [10, 11]. Nephrologists seem to prefer medication titration or the use of other non-genomic biomarkers to determine individual’s dosage upon transplantation and during maintenance therapy [10].
Here, we report our findings from a cost-utility analysis (CUA) with the aim to estimate if PGx-guided tacrolimus treatment is cost-effective compared to its conventional scheme among Austrian patients (n = 269) who had undergone KTX and participated in the PREemptive Pharmacogenomic testing for preventing Adverse drug REactions (PREPARE) study [15].
Material and methods
Data collection
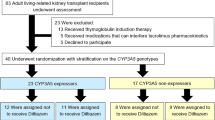
All available data both clinical and economic, derived from the PREPARE, which is a multinational and multisite, prospective, open label, randomized controlled clinical study investigating the impact of PGx testing of a panel of actionable PGx variants, on adverse event occurrence. The study took place at the Medical University of Vienna, in Austria starting from May 2017 until June 2020 [16]. PREPARE is the first and largest multinational, open-label, controlled, cluster-randomized, crossover implementation study that had included important secondary outcome measures including the healthcare expenditures related to the ADEs and quality of life with the objective to estimate the cost-effectiveness of implementing preemptive PGx testing in the population [15, 16]. The PREPARE protocol is reported elsewhere [15, 16]. The study analysis was undertaken based on 269 participants of the Austrian site for both arms, 145 subjects in the PGx group and 124 in the control group, for whom detailed medical records were documented in source documents and in study’s electronic case report system (eCRF).
Study design
All inclusion and exclusion criteria of the study are briefly described below. Subjects of any ethnicity, ≥18 years of age with a history of recent KTX that were primer naïve to tacrolimus, hadn’t undertaken any genetic testing in the past for CYP3A5, consented to be followed up for at least 12 weeks and could give blood or saliva sample were eligible to participate in the study. Patients were excluded in case that (a) they were reluctant to give signed informed consent, (b) were pregnant or breastfeeding, (c) were suffering from advanced liver failure (stage Child-Pugh C), (d) their estimated life expectancy was less than 3 months and (e) had no fixed address or an assigned general practitioner. Physicians participating in the study confirmed the diagnosis of kidney failure, the life expectancy, and the medical history of each patient relying on all available clinical data [16].
The control group run from May 2017 until October 2018 and the PGx-guided group from November 2018 until June 2020 at the Austrian site of PREPARE. Based on the protocol, participants should be followed-up for a minimum of 12 weeks and no more than 18 months. The control group followed an initial non-PGx tailored tacrolimus treatment based on the common local clinical routine using therapeutic drug monitoring, while PGx-guided group received initiallly adjusted tacrolimus prescription with respect to their genotyping results and were also subject to therapeutic drug monitoring. During the study, subjects were asked to complete two online questionnaires at week 2 and at week 8 and to perform four interviews called “nurse assessments” on baseline visit, week 4, week 12 and upon 18 months. Nurse assessments were conducted either remotely via phone calls or on-site interviews by trained research personnel. Assessments’ goal was to follow up on participants about updates on their disease status, QoL, the existence of any ADE, the change in their concomitant medication and the occurrence of any hospitalization event.
Basic participants’ demographic information including gender, age, body-mass index (BMI), smoking and alcohol consumption status along with clinical data such as comorbidities and co-medication use were recorded at the baseline visit by well-trained physicians upon getting participants’ signed informed consent (Table 1). Data related to ADE, utilities, visits to emergency units, hospital admissions were collected via the nurse assessments as mentioned above.
All subjects provided DNA samples before starting tacrolimus, either by blood or by saliva samples. Genotyping of the isolated DNA for a panel of 50 genetic variants, identified in 12 pharmacogenes, was performed using the standardized SNPline platform [15]. The variants of interest for this specific analysis were CYP3A5*3 (rs776746), CYP3A5*6 (rs10264272) and CYP3A5*7 (rs41303343). Genotyping results were released within less than seven days upon patient enrolment. As far as PGx-guided group is concerned, participants’ results were recorded in their (electronic) medical record and were also provided to them in the form of a plastic safety-code card. DNA samples of patients participating in the other group were genotyped at the end of the corresponding arm.
All available data were collected by trained clinical staff in study’s protocol and systems. Data were reviewed and reconciliated by two of the main authors of the paper for any typos or discrepancies between source documents and eCRF. The PREPARE study was performed in compliance with the 1964 Helsinki declaration and all patients provided written informed consent. The study was approved by the ethics committee of the Medical University of Vienna (unique ethics committee identifier: 2091/2016), and it is registered on clinicaltrials.gov (NCT03093818).
Perspective of analysis
The perspective of this study was that of Austrian healthcare system. Direct medical costs (hospitalization costs and genetic testing cost) were included. Those costs were reimbursed by the payers in Vienna. Other direct costs borne and paid by the patients (diet costs, travel expenses, home nurse aide, etc.) or indirect costs such as loss of productivity due to presentism, absentism, etc were not taken into consideration [17].
Utility values
Participants’ QoL was also documented during the study and it was associated with different health states. The utility valuation method applied was that of Visual Analog Scale (VAS). In particular, the quality of life was estimated by means of participants’ VAS score given at baseline visit, week 4, week 12 and 18 months from baseline. Quality-Adjusted Life-Years (QALYs) were measured by calculating the integral of the product of individual’s life expectancy multiplied by weighted VAS score and adjusting the baseline measures of utility in accordance with the literature [18].
Costs
Treatment’s effectiveness was determined by mean survival and it was estimated based on the official prescription start date of tacrolimus to (a) death related to the disease, and (b) death from any cause. Total cost included: hospitalization’s costs, ADEs costs and the cost of PGx testing applicable only for PGx-guided group. Cost of index drug itself was not taken into account in the analysis, since both groups represented a pool of patients with different health status and comorbidities and tacrolimus cost won’t impact the overall analysis. Reimbursement tariffs used were obtained from official sources [19] and were applicable to all public hospitals and public payers of Vienna. Hospitalization cost per day was estimated at €1544 based on official tariff. In the present analysis discount rate was not applied. Moreover, PGx testing price was calculated at €147 based on PREPARE’s lab flowchart. Incremental Cost-Effectiveness Ratio (ICER) was determined as the ratio of the difference in costs between PGx-guided group vs control group divided by the difference in QALYs.
Results
The main findings of our study are shown in Tables 2 and 3 and Figs. 1 and 2. In particular, Table 2 illustrates the total cost and its components as well as main statistics for each treatment group, while Table 3 shows the percentage of ADEs by type and grade for each comparator. Probabilistic sensitivity analysis revealed no statistically significant difference between the costs of the two groups in the 95% level of significance. Indeed, the mean bootstrapped cost of the PGx-guided group was €21,685 (95% CI: 17,163–26,842) compared to €25,587 (95% CI: 20,009–31,653) for control group, showing a difference of €3902 (95% CI: −3544–11,619) in favor of the PGx-guided group.
Moreover, hospitalization cost was shown to be the main factor driving of total treatment cost in both groups because it accounts for 99.31% (95% CI: 99.14–99.45%) of the total cost. The cost of PGx testing was estimated at €147/patient and accounted for 0.7% of the total cost for PGx-guided group. In addition, participants in PGx-guided arm had a slightly lower possibility to experience more severe (over grade 3) ADE. In particular, PGx-guided group had a combined probability to experience any grade 2 adverse event at 54.8% (95% CI: 39.3–71.0%) as opposed to 41.4% (95% CI: 25.8–59.7%) for the alternative option indicating a difference of 13.4% (95% CI: −10.8–36.6%) in favor of the other group. For all grade 3 ADE (combinational) the percentages were 2.1% (95% CI: 0.0–4.8%) and 4.8% (95% CI: 0.8–9.7%) for PGx-guided and control group, respectively. The difference between the two groups was estimated at 2.7% (95% CI: −2.0–8.3%) in favor of PGx-guided one. Concerning the combinational grade 5 ADEs, the related percentages were estimated as follows: 1.4% (95% CI: 0.0–3.4%) and 0.8% (95% CI: 0.0–2.4%) for PGx-guided and control group, respectively. The difference between the arms were not significant; 0.6% (95% CI: −1.7–2.8%). Finally, in the PGx-guided group three death cases were reported against two in the control group.
Control group showed to have greater risk of getting hospitalized for any reason; 56.8% (95% CI: 48.4–66.1%) compared to 51.4% (95% CI: 43.4–59.3%), with a difference of 5.4% between groups. The average of hospitalization days for each group were 13.9 days (95% CI: 11.0–17.2) for PGx-guided group compared to 16.6 days (95% CI: 12.9–20.5) in the control group, with a mean difference in hospitalization days at 2.7 days (95% CI: −2.4–7.8) in favor of PGx-guided arm.
Finally, as far as utilities are concerned, it was shown that the PGx-guided arm shared better results expressed in QALYs. Indeed, the PGx-guided group had 0.900 QALYs (95% CI: 0.862–0.936) in comparison with 0.851 QALYs (95% CI: 0.814–0.885) for the control group, presenting a difference of 0.049 QALYs (95% CI: −0.003–0.100). The estimated ICER for QALYs was calculated by comparing the incremental cost to incremental gain between treatment arms. ICER was €-80,992 (95% CI: −444.209–233,248) in favor of the PGx-guided group, indicating that preemptive PGx might be a dominant option. The cost-effectiveness plane reported the plotted differences in costs and effects showing the relative distributions for both arms (see Fig. 1). The ellipse was calculated based on the assumption that cost and effects followed the bivariate normal distribution, and its contours represented the 95% and the 99% confidence intervals.
Based on probabilistic results, PGx-guided treatment may represent a dominant option (less costly and more effective) with a probability of 82.22%, while for the conventional approach the probability was limited to 1.36%. In the first quadrant (more costly/more effective) lied the 14.48% of the experiments and in the third quadrant the 1.94% of all experiments. A cost-effectiveness acceptability curve, showing the probability that the intervention arm is a cost-effective alternative to the comparator arm, versus different values for the willingness-to-pay for a QALY was also reported in Fig. 2. Even in a case of very strict threshold such as €10,000 per QALY, the probability of PGx treatment arm being cost-effective is as high as 86.6% while in the €50,000 per QALY threshold, the probability was 91.8%.
Discussion
CKD constitutes one of the biggest health challenges of the next decades due to its detrimental consequences to patients’ life. As it was previously stated, KTX is the most efficient and productive treatment option for kidney failure patients to have a longer and simultaneously better life since it is introduced with strong immunosuppressant drugs such as tacrolimus. In an effort to decrease tacrolimus disadvantages, PGx-guided treatment was demonstrated to slightly improve tacrolimus’ clinical effectiveness and reducing higher grade of ADEs that can provoke a series of different repercussions to patient’s health.
Based on our CUA results, the PGx-guided treatment strategy costs 3902 euros less compared to the conventional strategy and is slightly better in terms of utilities indicating that PGx may be a favorable alternative. More precisely, it was demonstrated that patients in the PGx-guided group had a reduced risk of being hospitalized by almost 6%, shared less days of hospitalization, suffered less but also milder ADEs compared to their counterparts and had better reported utility. PGx-guided treatment approach had 10% less total cost compared to the most conventional approach, while most costs were related to hospitalizations in both arms. Evidently, a PGx-guided therapeutic approach brings an overall improvement in CKD management, improves patients’ experience and subsequently decreases related costs. To the best of our knowledge, this is the first CUA of PGx testing in KTX patients in Austria and elsewhere. This fact commonly highlighted in the field of KTX [7, 20]. Hence, no direct comparisons can be made regarding these findings.
Nevertheless, Vannaprasaht and coworkers (2019), recently investigated the association of CYP3A5 genotypes and use of healthcare resources in different transplant cohorts, and concluded that individuals CYP3A5*1/*1 or CYP3A5*1/*3 shared higher costs for drug management and hospitalizations compared to CYP3A5*3/*3 [21]. This fact was also highlighted by Pasternak and coworkers (2019) [22], that agrees with our findings and implies the effectiveness and need for preemptive PGx in KTX.
Moreover, the superiority of PGx-guided treatment was also demonstrated in terms of less hospital admissions, less emergency visits and 50% reduction in ADRs occurrence and especially in those of high grade in other health indications. All these features imply that PGx-testing can offer a more optimal disease management and constitutes a promising treatment strategy for kidney failure patients. Based on Koufaki and coworkers (2023), PGx-guided treatment of cardiovascular patients under clopidogrel treatment was a cost-saving option with almost half cases of patients hospitalizations, and a 13% less ADEs occurrence [23]. They also showed that PGx-guided group had 50% less total cost and an insignificantly higher QALY compared to conventional treatment [23].
In the Fragoulakis and coworkers (2023) study, genome-guided treatment results in less hospitalization days, better clinical effectiveness due to personalization of treatment scheme and a significant decrease in healthcare expenses [24]. Researchers also performed a cost-effectiveness analysis for patients suffering from colorectal cancer and the PGx-guided arm was found less costly by 33% and had slightly better QALYs [24]. Moreover, Skokou and coworkers (2024), stated that economic benefit of PGx testing was clear for patients suffering from major depression [25]. It was pinpointed that the lifelong value of preemptive PGx testing is bigger than estimated based on the fact that one pharmacogene like CYP3A5 can metabolize more than one medications so its benefit outcomes its cost in the long run [25].
Finally, in their systematic review, Verbelen and coworkers (2020), found that, in general, most economic evaluations shared a positive attitude towards PGx-guided treatment [26]. PGx-guided strategy was presented as dominant in 27% of published economic analysis while 30% of the studies concluded that the PGx option is cost-effective [26]. Rancic and coworkers (2016), also indicated that preemptive PGx testing before immunosuppressants prescription to KTX patients can be a cost-saving option but this study didn’t state any conclusive results due to lack of available CUA studies in the field [7].
This study has a few limitations. PREPARE didn’t follow the strict structure of a randomized controlled trial, but used cluster randomization. Only two types of direct costs were taken into account due to lack of available data. Including more types of costs such as laboratory assessments costs, medications costs, other health assessments could bring more comprehensive results. Finally, there is limited literature on the field, making it hard to confirm some findings.
In conclusion, kidney failure is an important worldwide problem with high mortality and disability risk. PGx testing provides valuable information and may improve severe CKD drug management to great extent, while it can reduce healthcare expenses. Our study is one of the few studies that aims to compare the cost-utility of PGx-guided treatment of this immunosuppressant agent versus control approach in such cohort and the first one in this drug/gene combination that is based on real-life clinical data derived from a prospective clinical study. Given that it included 269 participants that were followed up for 18 months, it provides an insight and thus has a meaningful contribution to the literature.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Jones-Hughes T, Snowsill T, Haasova M, Coelho H, Crathorne L, Cooper C, et al. Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model. NIHR J Libr. 2016;20:1–594.
Mudiayi D, Shojai S, Okpechi I, Christie EA, Wen K, Kamaleldin M, et al. Global estimates of capacity for kidney transplantation in world countries and regions. Transplantation. 2022;106:1113–22.
Chemello C, Aguilera M, Cañadas Garre M, Calleja MA. Pharmacogenetics and pharmacogenomics of chronic kidney disease comorbidities and kidney transplantation. In: Barh D et al., editors. Omics for personalized medicine. 1st ed. India: Springer eBooks; 2013. p.801–18.
Boenink R, Kramer A, Tuinhout RE, Savoye E, Åsberg A, Idrizi A, et al. Trends in kidney transplantation rate across Europe: study from the ERA Registry. Nephrol Dial Transpl. 2023;38:1528–39.
McEwan P, Dixon S, Baboolal K, Conway P, Currie CJ. Evaluation of the cost effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the UK. Pharmacoeconomics. 2006;24:67–79.
Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13:104–14.
Rancic N, Dragojevic-Simic V, Vavic N, Kovacevic A, Segrt Z, Djordjevic N. Economic evaluation of pharmacogenetic tests in patients subjected to renal transplantation: a review of literature. Front Public Health. 2016;4:189–97.
Provenzani A, Santeusanio A, Mathis E, Notarbartolo M, Labbozzetta M, Poma P, et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol. 2013;19:9156–73.
Turolo S, Edefonti A, Syren ML, Montini G. Pharmacogenomics of old and new immunosuppressive drugs for precision medicine in kidney transplantation. J Clin Med. 2023;12:4454–70.
Adams SM, Crisamore KR, Empey PE. Clinical pharmacogenomics: applications in nephrology. Clin J Am Soc Nephrol. 2018;13:1561–71.
Nguyen TT, Pearson RA, Mohamed ME, Schladt DP, Berglund D, Rivers Z, et al. Pharmacogenomics in kidney transplant recipients and potential for integration into practice. J Clin Pharm Ther. 2020;45:1457–65.
Crowley LE, Mekki M, Chand S. Biomarkers and pharmacogenomics in kidney transplantation. Mol Diagn Ther. 2018;22:537–50.
Chen L, Prasad GVR. CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment. Pharmgenomics Pers Med. 2018;11:23–33.
Lamba J, Hebert JM, Schuetz EG, Klein TE, Altman RB. PharmGKB summary: very important pharmacogene information for CYP3A5. Pharmacogenet Genomics. 2012;22:555–8.
Swen JJ, van der Wouden CH, Manson LE, Abdullah-Koolmees H, Blagec K, Blagus T, et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401:347–56.
van der Wouden CH, Cambon-Thomsen A, Cecchin E, Cheung KC, Dávila-Fajardo CL, Deneer VH, et al. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharm Ther. 2017;101:341–58.
Gordois AL, Toth PP, Quek RG, Proudfoot EM, Paoli CJ, Gandra SR. Productivity losses associated with cardiovascular disease: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16:759–69.
Willan AR, Briggs A. Statistical analysis of cost‐effectiveness data. 1st ed. Canada: John Wiley & Sons Ltd; 2006.
General Hospital of Vienna, Clinical Insitute of Medical Analysis. Catalog of Parameters. 2023. https://www.akhwien.at/default.aspx?pid=3982.
Chung R, Howard K, Craig JC, Chapman JR, Turner R, Wong G. Economic evaluations in kidney transplantation: frequency, characteristics, and quality-a systematic review. Transplantation. 2014;97:1027–33.
Vannaprasaht S, Limwattananon C, Anutrakulchai S, Chan-On C. Effect of CYP3A5 genotype on hospitalization cost for kidney transplantation. Int J Clin Pharm. 2019;41:88–95.
Pasternak AL, Kidwell KM, Dempsey JM, Gersch CL, Pesch A, Sun Y, et al. Impact of CYP3A5 phenotype on tacrolimus concentrations after sublingual and oral administration in lung transplant. Pharmacogenomics. 2019;20:421–32.
Koufaki MI, Fragoulakis V, Díaz-Villamarín X, Karamperis K, Vozikis A, Swen JJ, et al. Economic evaluation of pharmacogenomic-guided antiplatelet treatment in Spanish patients suffering from acute coronary syndrome participating in the U-PGx PREPARE study. Hum Genomics. 2023;17:51–66.
Fragoulakis V, Roncato R, Bignucolo A, Patrinos GP, Toffoli G, Cecchin E, et al. Cost-utility analysis and cross-country comparison of pharmacogenomics-guided treatment in colorectal cancer patients participating in the U-PGx PREPARE study. Pharm Res. 2023;197:106949–58.
Skokou M, Karamperis K, Koufaki MI, Tsermpini EE, Pandi MT, Siamoglou S, et al. Clinical implementation of preemptive pharmacogenomics in psychiatry. eBioMedicine. 2024;101:105009–23.
Verbelen M, Weale ME, Lewis CM. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J. 2017;17:395–402.
Funding
The project was funded by the European Commission Horizon 2020 Program via Grant Agreement 668353 (U-PGx; www.upgx.eu) to CM.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception, design and drafting of the paper. CJR and GSP performed data collection and documentation in eCRF and were part of the Austrian clinical team working on the PREPARE study. CM was one of the delegated persons, responsible for the design and the conduction of PREPARE project. VF and MIK reviewed and purified the study’s database. VF and MIK conducted data analysis. VF and MIK drafted the first and all subsequent versions of this paper. All authors reviewed and approved the final version of paper. VF and MIK are the guarantor for the overall content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by ethics committee of the Medical University of Vienna, Vienna, Austria.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fragoulakis, V., Koufaki, MI., Joefield-Roka, C. et al. Cost-utility analysis of pharmacogenomics-guided tacrolimus treatment in Austrian kidney transplant recipients participating in the U-PGx PREPARE study. Pharmacogenomics J 24, 10 (2024). https://doi.org/10.1038/s41397-024-00330-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-024-00330-5