Abstract
Objective
To investigate factors affecting the efficacy and tolerability of verapamil for migraine prevention using individual pharmacogenomic phenotypes.
Background
Verapamil has a wide range of dosing in headache disorders without reliable tools to predict the optimal doses for an individual.
Methods
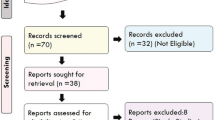
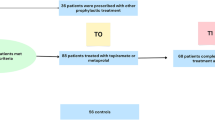
This is a retrospective chart review examining adults with existing pharmacogenomic reports at Mayo Clinic who had used verapamil for migraine. Effects of six cytochrome P450 phenotypes on the doses of verapamil for migraine prevention were assessed.
Results
Our final analysis included 33 migraine patients (82% with aura). The mean minimum effective and maximum tolerable doses of verapamil were 178.2(20-320) mg and 227.9(20-480) mg. A variety of CYP2C9, CYP2D6, and CYP3A5 phenotypes were found, without significant association with the verapamil doses after adjusting for age, sex, body mass index, and smoking status.
Conclusions
We demonstrated a wide range of effective and tolerable verapamil doses used for migraine in a cohort with various pharmacogenomic phenotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: Updated age, sex, and socioeconomic-specific estimates from government health surveys. Headache. 2021;61:60–8.
Collaborators. GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–602.
Burch RC, Buse DC, Lipton RB. Migraine: Epidemiology, burden, and comorbidity. Neurol Clin. 2019;37:631–49.
Blumenfeld AM, Bloudek LM, Becker WJ, Buse DC, Varon SF, Maglinte GA, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53:644–55.
Matharu MS, Boes CJ, Goadsby PJ. Management of trigeminal autonomic cephalgias and hemicrania continua. Drugs. 2003;63:1637–77.
Robbins MS, Starling AJ, Pringsheim TM, Becker WJ, Schwedt TJ. Treatment of Cluster Headache: The American Headache Society Evidence-Based Guidelines. Headache. 2016;56:1093–106.
Burch R. Preventive migraine treatment. Continuum. 2021;27:613–32.
Wu JW, Yang CP. 2022 Taiwan Guidelines for Preventive Treatment of Migraine. Acta Neurol Taiwan. 2022;31:164–202.
Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of. Neurol Am Headache Soc Neurol 2012;78:1337–45.
Kowalska M, Prendecki M, Piekut T, Kozubski W, Dorszewska J. Migraine: Calcium Channels and Glia. Int J Mol Sci. 2021;22:2688.
Di Stefano V, Rispoli MG, Pellegrino N, Graziosi A, Rotondo E, Napoli C, et al. Diagnostic and therapeutic aspects of hemiplegic migraine. J Neurol Neurosurg Psychiatry. 2020;91:764–71.
Solomon GD, Steel JG, Spaccavento LJ. Verapamil prophylaxis of migraine. A double-blind, placebo-controlled study. JAMA. 1983;250:2500–2.
Yu W, Horowitz SH. Treatment of sporadic hemiplegic migraine with calcium-channel blocker verapamil. Neurology. 2003;60:120–1.
Petersen AS, Barloese MCJ, Snoer A, Soerensen AMS, Jensen RH. Verapamil and cluster headache: still a mystery. a narrative review of efficacy, mechanisms and perspectives. Headache. 2019;59:1198–211.
Gabai IJ, Spierings EL. Prophylactic treatment of cluster headache with verapamil. Headache. 1989;29:167–8.
Jónsdóttir M, Meyer JS, Rogers RL. Efficacy, side effects and tolerance compared during headache treatment with three different calcium blockers. Headache. 1987;27:364–9.
Fotuhi M, Glaun B, Quan SY, Sofare T. Vestibular migraine: a critical review of treatment trials. J Neurol. 2009;256:711–6.
Pomes LM, Guglielmetti M, Bertamino E, Simmaco M, Borro M, Martelletti P. Optimising migraine treatment: from drug-drug interactions to personalized medicine. J Headache Pain. 2019;20:56.
Cutrer FM, Moyer AM, Atkinson EJ, Wang L, Tian S, Wu Y, et al. Genetic variants related to successful migraine prophylaxis with verapamil. Mol Genet Genomic Med. 2021;9:e1680.
Busse D, Cosme J, Beaune P, Kroemer HK, Eichelbaum M. Cytochromes of the P450 2C subfamily are the major enzymes involved in the O-demethylation of verapamil in humans. Naunyn Schmiedebergs Arch Pharm. 1995;353:116–21.
Jin Y, Wang YH, Miao J, Li L, Kovacs RJ, Marunde R, et al. Cytochrome P450 3A5 genotype is associated with verapamil response in healthy subjects. Clin Pharm Ther. 2007;82:579–85.
Kroemer HK, Gautier JC, Beaune P, Henderson C, Wolf CR, Eichelbaum M. Identification of P450 enzymes involved in metabolism of verapamil in humans. Naunyn Schmiedebergs Arch Pharm. 1993;348:332–7.
Tracy TS, Korzekwa KR, Gonzalez FJ, Wainer IW. Cytochrome P450 isoforms involved in metabolism of the enantiomers of verapamil and norverapamil. Br J Clin Pharm. 1999;47:545–52.
Zhao LM, He XJ, Qiu F, Sun YX, Li-Ling J. Influence of ABCB1 gene polymorphisms on the pharmacokinetics of verapamil among healthy Chinese Han ethnic subjects. Br J Clin Pharm. 2009;68:395–401.
Borlak J, Walles M, Levsen K, Thum T. Verapamil: metabolism in cultures of primary human coronary arterial endothelial cells. Drug Metab Dispos. 2003;31:888–91.
Zhou Y, Ingelman-Sundberg M, Lauschke VM. Worldwide distribution of Cytochrome P450 Alleles: A meta-analysis of population-scale sequencing projects. Clin Pharm Ther. 2017;102:688–700.
Amitriptyline [package insert]. Prinston, NJ: Sandoz Inc.; 2014.
PAMELOR® (nortriptyline) [package insert]. Hazelwood, MO: Mallinckrodt Pharmaceuticals; 2019.
EFFEXOR XR® (venlafaxine) [package insert]. Philadelphia, PA: Pfizer; 2021.
Hicks JK, Sangkuhl K, Swen JJ, Ellingrod VL, Müller DJ, Shimoda K, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharm Ther. 2017;102:37–44.
Jiang F, Kim HD, Na HS, Lee SY, Seo DW, Choi JY, et al. The influences of CYP2D6 genotypes and drug interactions on the pharmacokinetics of venlafaxine: exploring predictive biomarkers for treatment outcomes. Psychopharmacology. 2015;232:1899–909.
The Royal Dutch Pharmacists Association-Pharmacogenetics Working Group (DPWG). Dutch guidelines August 2019 update.
CALAN® SR (verapamil) [package insert]. New York, NY: Pfizer; 2019.
Fuhr U, Müller-Peltzer H, Kern R, Lopez-Rojas P, Jünemann M, Harder S, et al. Effects of grapefruit juice and smoking on verapamil concentrations in steady state. Eur J Clin Pharm. 2002;58:45–53.
Benowitz NL, Peng M, Jacob P 3rd. Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin Pharm Ther. 2003;74:468–74.
Bauer M, Karch R, Zeitlinger M, Philippe C, Römermann K, Stanek J, et al. Approaching complete inhibition of P-glycoprotein at the human blood-brain barrier: an (R)-[11C]verapamil PET study. J Cereb Blood Flow Metab. 2015;35:743–6.
Eriksen MK, Thomsen LL, Olesen J. Sensitivity and specificity of the new international diagnostic criteria for migraine with aura. J Neurol Neurosurg Psychiatry. 2005;76:212–7.
Kelman L. The aura: a tertiary care study of 952 migraine patients. Cephalalgia. 2004;24:728–34.
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
Y-CC was responsible for designing the review protocol, screening the patients to identify the final cohort, conducting the chart review, extracting the data, and writing the manuscript. HW and JNM performed the data analysis. CER, AJS and FMC reviewed and provided feedback on the manuscript. CCC was responsible for designing the review protocol, assisting the chart review, and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Robertson has served on advisory boards for Impel, Linpharma, Satsuma, Biohaven, Eli Lilly, and Lundbeck. She has received research funding from Teva, Pfizer, and Lundbeck. She receives compensation as an author and associate editor of UpToDate. Dr. Starling has received consulting fees from Allergan, Amgen, Axsome Therapeutics, Eli Lilly & Company, Everyday Health, Impel, Lundbeck, Med-IQ, Medscape, Neurolief, Novartis, Satsuma, Teva, and Theranica. Dr. Chiang serves on the advisory board for Satsuma and eNeura. Other authors declare no competing financial interests. This paper did not receive any funding.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, YC., Wang, H., Mandrekar, J.N. et al. Pharmacogenomic study—A pilot study of the effect of pharmacogenomic phenotypes on the adequate dosing of verapamil for migraine prevention. Pharmacogenomics J 24, 11 (2024). https://doi.org/10.1038/s41397-024-00331-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-024-00331-4