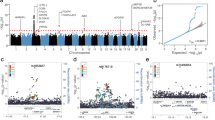
Abstract
We have identified an underrecognized severe adverse drug reaction (ADR) of trimethoprim-sulfamethoxazole (TMP-SMX) associated respiratory failure in previously healthy children and young adults. We investigated potential genetic risk factors associated with TMP-SMX induced respiratory failure in a cohort of seven patients. We explored whole genome sequence among seven patients representing nearly half of all reported cases worldwide and 63 unrelated control individuals in two stages: (1) human leukocyte antigen (HLA) locus variation as several other ADRs have been associated HLA genetic variants and (2) coding variation to catalog and explore potential rare variants contributing to this devastating reaction. All cases were either heterozygous (carriers) or homozygous for the common HLA-B*07:02–HLA-C*07:02 haplotype. Despite the small sample size, this observation is statistically significant both in conservative comparison to maximum reported population frequencies (binomial P = 0.00017 for HLA-B and P = 0.00028 for HLA-C) and to our control population assessed by same HLA genotyping approach (binomial P = 0.000001 for HLA-B and P = 0.000018 for HLA-C). No gene elsewhere in the genome harnessed shared rare case enriched coding variation. Our results suggests that HLA-B*07:02 and HLA-C*07:02 are necessary for a patient to develop respiratory failure due to TMP-SMX.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Frey N, Bircher A, Bodmer M, Jick SS, Meier CR, Spoendlin J. Antibiotic drug use and the risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: a population-based case-control study. J Invest Dermatol. 2018;138:1207–9.
Lin YF, Yang CH, Sindy H, Lin JY, Rosaline Hui CY, Tsai YC, et al. Severe cutaneous adverse reactions related to systemic antibiotics. Clin Infect Dis. 2014;58:1377–85.
Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–9.
Keisu M, Wiholm BE, Palmblad J. Trimethoprim-sulphamethoxazole-associated blood dyscrasias. Ten years’ experience of the Swedish spontaneous reporting system. J Intern Med. 1990;228:353–60.
Pirmohamed M, Friedmann PS, Molokhia M, Loke YK, Smith C, Phillips E, et al. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharm Ther. 2011;89:896–901.
Li YJ, Phillips E, Dellinger A, Nicoletti P, Schutte R, Li D, et al. HLA-B*14:01 and HLA-B*35:01 are associated with trimethoprim-sulfamethoxazole induced liver injury. Hepatology. 2021;73:268–81.
Wang CW, Tassaneeyakul W, Chen CB, Chen WT, Teng YC, Huang CY, et al. Whole genome sequencing identifies genetic variants associated with co-trimoxazole hypersensitivity in Asians. J Allergy Clin Immunol. 2021;147:1402–12.
Miller JO, Taylor J, Goldman JL. Severe acute respiratory failure in healthy adolescents exposed to trimethoprim-sulfamethoxazole. Pediatrics. 2019;143:e20183242.
Miller JO, Shih AR, Mino-Kenudson M, Taylor MS, Goldman JL. Trimethoprim-sulfamethoxazole-associated fulminant respiratory failure in children and young adults. Am J respiratory Crit care Med. 2021;203:918–21.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharm Ther. 1981;30:239–45.
Gonzalez-Galarza FF, McCabe A, Santos E, Jones J, Takeshita L, Ortega-Rivera ND, et al. Allele frequency net database (AFND) 2020 update: gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 2020;48:D783–8.
Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharm Ther. 2008;84:362–9.
Larjo A, Eveleigh R, Kilpelainen E, Kwan T, Pastinen T, Koskela S, et al. Accuracy of programs for the determination of human leukocyte antigen alleles from next-generation sequencing data. Front Immunol. 2017;8:1815.
Daly AK. Pharmacogenomics of adverse drug reactions. Genome Med. 2013;5:5.
Gonzalez-Galarza FF, McCabe A, Melo Dos Santos EJ, Jones AR, Middleton D. A snapshot of human leukocyte antigen (HLA) diversity using data from the Allele Frequency Net Database. Hum Immunol. 2020;82:496–504.
Alfirevic A, Gonzalez-Galarza F, Bell C, Martinsson K, Platt V, Bretland G, et al. In silico analysis of HLA associations with drug-induced liver injury: use of a HLA-genotyped DNA archive from healthy volunteers. Genome Med. 2012;4:51.
Abacavir sulfate drug insert. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020977s024,020978s028lbl.pdf.
Carbamazepine drug insert. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/016608s101,018281s048lbl.pdf.
Karnes JH, Miller MA, White KD, Konvinse KC, Pavlos RK, Redwood AJ, et al. Applications of Immunopharmacogenomics: predicting, preventing, and understanding immune-mediated adverse drug reactions. Annu Rev Pharm Toxicol. 2019;59:463–86.
Acknowledgements
We would like to thank the patients and families who contributed to this work.
Funding
The research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM129783 (JLG). Imputed HLA control data for Fig. 1 was provided under the scope of P50GM115305 (EJP).
Author information
Authors and Affiliations
Contributions
Participated in research design: JLG, JOM, NM, RE, AG, EJP, and TP. Conducted experiments: NM, RE, AG, EJP, and TP. Performed data analysis: JLG, NM, RE, AG, EJP, and TP. Wrote or contributed to the writing of the manuscript: JLG, JOM, NM, RE, AG, EJP, and TP.
Corresponding author
Ethics declarations
Competing interests
EJP reports grants from National Institutes of Health (P50GM115305, R01HG010863, R01AI152183, R21AI139021, U01AI154659) and from the National Health and Medical Research Council of Australia. She receives Royalties from Uptodate and consulting fees from Janssen, Vertex, Biocryst and Regeneron. She is co-director of IIID Pty Ltd that holds a patent for HLA-B*57:01 testing for abacavir hypersensitivity, and has a patent pending for Detection of Human Leukocyte Antigen-A*32:01 in connection with Diagnosing Drug Reaction with Eosinophilia and Systemic Symptoms without any financial remuneration and not directly related to the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goldman, J.L., Miller, J.O., Miller, N. et al. HLA-B*07:02 and HLA-C*07:02 are associated with trimethoprim-sulfamethoxazole respiratory failure. Pharmacogenomics J 22, 124–129 (2022). https://doi.org/10.1038/s41397-022-00266-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-022-00266-8
This article is cited by
-
HLA-B*07:02 and HLA-C*07:02 associated with cotrimoxazole-induced respiratory failure
Reactions Weekly (2022)