Abstract
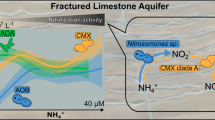
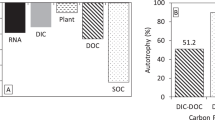
Biological nitrogen fixation (BNF), the conversion of N2 into bioavailable nitrogen (N), is the main process for replenishing N loss in the biosphere. However, BNF in groundwater systems remains poorly understood. In this study, we examined the activity, abundance, and community composition of diazotrophs in groundwater in the Hetao Plain of Inner Mongolia using 15N tracing methods, reverse transcription qPCR (RT-qPCR), and metagenomic/metatranscriptomic analyses. 15N2 tracing incubation of near in situ groundwater (9.5–585.4 nmol N L−1 h−1) and N2-fixer enrichment and isolates (13.2–1728.4 nmol N g−1 h−1, as directly verified by single-cell resonance Raman spectroscopy), suggested that BNF is a non-negligible source of N in groundwater in this region. The expression of nifH genes ranged from 3.4 × 103 to 1.2 × 106 copies L−1 and was tightly correlated with dissolved oxygen (DO), Fe(II), and NH4+. Diazotrophs in groundwater were chiefly aerobes or facultative anaerobes, dominated by Stutzerimonas, Pseudomonas, Paraburkholderia, Klebsiella, Rhodopseudomonas, Azoarcus, and additional uncultured populations. Active diazotrophs, which prefer reducing conditions, were more metabolically diverse and potentially associated with nitrification, sulfur/arsenic mobilization, Fe(II) transport, and CH4 oxidation. Our results highlight the importance of diazotrophs in subsurface geochemical cycles.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw data have been submitted to the NCBI database under BioProject numbers PRJNA937386, PRJNA996345, PRJNA996367, PRJNA882225, and PRJNA884812.
References
Mosley O, Gios E, Close M, Weaver L, Daughney C, Handley K. Nitrogen cycling and microbial cooperation in the terrestrial subsurface. ISME J. 2022;16:2561–73.
Dong X, Zhang C, Peng Y, Zhang H-X, Shi L-D, Wei G, et al. Phylogenetically and catabolically diverse diazotrophs reside in deep-sea cold seep sediments. Nat Commun. 2022;13:4885.
Gier J, Sommer S, Löscher C, Dale A, Schmitz R, Treude T. Nitrogen fixation in sediments along a depth transect through the Peruvian oxygen minimum zone. Biogeosciences. 2016;13:4065–80.
Tian L, Yan Z, Wang C, Xu S, Jiang H. Habitat heterogeneity induces regional differences in sediment nitrogen fixation in eutrophic freshwater lake. Sci Total Environ. 2021;772:145594.
Capone DG, Burns JA, Montoya JP, Subramaniam A, Mahaffey C, Gunderson T, et al. Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem Cy. 2005;19:GB2024.
Foster RA, Kuypers MMM, Vagner T, Paerl RW, Musat N, Zehr JP. Nitrogen fixation and transfer in open ocean diatom–cyanobacterial symbioses. ISME J. 2011;5:1484–93.
Zehr J, Shilova I, Farnelid H, Muñoz Marin M, Turk-Kubo K. Unusual marine unicellular symbiosis with the nitrogen-fixing cyanobacterium UCYN-A. Nat Microbiol. 2016;2:16214.
Steenhoudt O, Vanderleyden J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol Rev. 2000;24:487–506.
Zhang X, Ward BB, Sigman DM. Global nitrogen cycle: Critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chem Rev. 2020;120:5308–51.
Hou L, Wang R, Yin G, Liu M, Zheng Y. Nitrogen fixation in the intertidal sediments of the Yangtze Estuary: Occurrence and environmental implications. J Geophys Res-Biogeo. 2018;123:936–44.
Saiz E, Sgouridis F, Drijfhout FP, Ullah S. Biological nitrogen fixation in peatlands: Comparison between acetylene reduction assay and 15N2 assimilation methods. Soil Biol Biochem. 2019;131:157–65.
Tsoy OV, Ravcheev DA, Čuklina J, Gelfand MS. Nitrogen fixation and molecular oxygen: Comparative genomic reconstruction of transcription regulation in Alphaproteobacteria. Front Microbiol. 2016;7:1343.
Boyd E, Peters J. New insights into the evolutionary history of biological nitrogen fixation. Front Microbiol. 2013;4:201.
Newell S, Pritchard K, Foster S, Fulweiler W. Molecular evidence for sediment nitrogen fixation in a temperate New England estuary. PeerJ. 2016;4:e1615.
Reed S, Cleveland C, Townsend A. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu Rev Ecol Evol Syst. 2011;42:489–512.
Houlton BZ, Wang Y-P, Vitousek PM, Field CB. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature. 2008;454:327–30.
Smercina D, Evans S, Friesen M, Tiemann L. To fix or not to fix: Controls on free-living nitrogen-fixation in the rhizosphere. Appl Environ Microbiol. 2019;85:e02546–18.
Kapili B, Barnett S, Buckley D, Dekas A. Evidence for phylogenetically and catabolically diverse active diazotrophs in deep-sea sediment. ISME J. 2020;14:971–83.
Bertics V, Löscher C, Salonen I, Dale A, Gier J, Schmitz RA, et al. Occurrence of benthic microbial nitrogen fixation coupled to sulfate reduction in the seasonally hypoxic Eckernförde Bay, Baltic Sea. Biogeosciences. 2013;10:1243–58.
Gier J, Löscher C, Dale A, Sommer S, Lomnitz U, Treude T. Benthic dinitrogen fixation traversing the oxygen minimum zone off Mauritania (NW Africa). Front Mar Sci. 2017;4:390.
Fulweiler W, Brown S, Nixon S, Jenkins B. Evidence and a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Mar Ecol Prog Ser. 2013;482:57–68.
Jia R, Wang K, Li L, Qu Z, Shen W, Qu D. Abundance and community succession of nitrogen-fixing bacteria in ferrihydrite enriched cultures of paddy soils is closely related to Fe(III)-reduction. Sci Total Environ. 2020;720:137633.
Sun X, Kong T, Häggblom M, Kolton M, Li F, Dong Y, et al. Chemolithoautotropic diazotrophy dominates the nitrogen fixation process in mine tailings. Environ Sci Technol. 2020;54:6082–93.
Alley W, Healy R, LaBaugh J, Reilly T. Flow and storage in groundwater systems. Science. 2002;296:1985–90.
Griebler C, Lueders T. Microbial biodiversity in groundwater ecosystems. Freshw Biol. 2009;54:649–77.
Nai H, Xin J, Liu Y, Zheng X, Lin Z. Distribution and molecular chemodiversity of dissolved organic nitrogen in the vadose zone-groundwater system of a fluvial plain, northern China: Implications for understanding its loss pathway to groundwater. Sci Total Environ. 2020;723:137928.
Jiang Z, Shen X, Shi B, Cui M, Wang Y, Li P. Arsenic mobilization and transformation by ammonium-generating bacteria isolated from high arsenic groundwater in Hetao Plain, China. Int J Environ Res Public Health. 2022;19:9606.
Kumar S, Herrmann M, Blohm A, Hilke I, Frosch T, Trumbore S, et al. Thiosulfate- and hydrogen-driven autotrophic denitrification by a microbial consortium enriched from groundwater of an oligotrophic limestone aquifer. FEMS Microbiol Ecol. 2018; 94; https://doi.org/10.1093/femsec/fiy141.
Wang S, Radny D, Huang S, Zhuang L, Zhao S, Berg M, et al. Nitrogen loss by anaerobic ammonium oxidation in unconfined aquifer soils. Sci Rep. 2017;7:40173.
Han L-L, Wang H, Ge L, Xu M, Tang J-M, Li L, et al. Transition of source/sink processes and fate of ammonium in groundwater along with redox gradients. Water Res. 2023;231:119600.
Xiong Y, Du Y, Deng Y, Ma T, Wang Y. Feammox in alluvial-lacustrine aquifer system: Nitrogen/iron isotopic and biogeochemical evidences. Water Res. 2022;222:118867.
Li P, Jiang Z, Wang Y, Deng Y, Van Nostrand J, Yuan T, et al. Analysis of the functional gene structure and metabolic potential of microbial community in high arsenic groundwater. Water Res. 2017;123:268–76.
Xin J, Liu Y, Chen F, Duan Y, Wei G, Zheng X, et al. The missing nitrogen pieces: A critical review on the distribution, transformation, and budget of nitrogen in the vadose zone-groundwater system. Water Res. 2019;165:114977.
Zhu G, Wang S, Li Y, Zhuang L, Zhao S, Wang C, et al. Microbial pathways for nitrogen loss in an upland soil. Environ Microbiol. 2018;20:1723–38.
Zhou W, Xia L, Yan X. Vertical distribution of denitrification end-products in paddy soils. Sci Total Environ. 2017;576:462–71.
Mosley OE, Gios E, Weaver L, Close M, Daughney C, van der Raaij R, et al. Metabolic diversity and aero-tolerance in anammox bacteria from geochemically distinct aquifers. mSystems. 2022;7:e01255–21.
Jiang Z, Li P, Wang Y, Liu H, Wei D, Yuan C, et al. Arsenic mobilization in a high arsenic groundwater revealed by metagenomic and Geochip analyses. Sci Rep. 2019;9:12972.
Dong Y, Sanford RA, Connor L, Chee-Sanford J, Wimmer BT, Iranmanesh A, et al. Differential structure and functional gene response to geochemistry associated with the suspended and attached shallow aquifer microbiomes from the Illinois Basin, IL. Water Res. 2021;202:117431.
Méheust R, Castelle C, Carnevali P, Farag I, He C, Chen L, et al. Groundwater Elusimicrobia are metabolically diverse compared to gut microbiome Elusimicrobia and some have a novel nitrogenase paralog. ISME J. 2020;14:2907–22.
Bae H-S, Rash B, Rainey F, Nobre M, Tiago I, da Costa M, et al. Description of Azospira restricta sp. nov., a nitrogen-fixing bacterium isolated from groundwater. Int J Syst Evol Microbiol. 2007;57:1521–26.
Pedron R, Luchi E, Albiac MA, Di Cagno R, Catorci D, Esposito A, et al. Mesorhizobium comanense sp. nov., isolated from groundwater. Int J Syst Evol Microbiol. 2021; 71; https://doi.org/10.1099/ijsem.0.005131.
Xiu W, Wu M, Nixon SL, Lloyd JR, Bassil NM, Gai R, et al. Genome-resolved metagenomic analysis of groundwater: Insights into arsenic mobilization in biogeochemical interaction networks. Environ Sci Technol. 2022;56:10105–19.
Chen M, Lu Y, Jiao N, Tian J, Kao S-J, Zhang Y. Biogeographic drivers of diazotrophs in the western Pacific Ocean. Limnol Oceanogr. 2019;64:1403–21.
Wang Y, Zhang G, Wang H, Cheng Y, Liu H, Jiang Z, et al. Effects of different dissolved organic matter on microbial communities and arsenic mobilization in aquifers. J Hazard Mater. 2021;411:125146.
Poly F, Monrozier LJ, Bally R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol. 2001;152:95–103.
Uritskiy GV, DiRuggiero J, Taylor J. MetaWRAP—a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome. 2018;6:158.
Jiang Z, Zhong S, Shen X, Cui M, Wang Y, Li J. Microbially mediated arsenic mobilization in the clay layer and underlying aquifer in the Hetao Basin, Inner Mongolia, China. Sci Total Environ. 2022;836:155597.
Wang H, Li P, Liu X, Zhang J, Stein LY, Gu J-D. An overlooked influence of reactive oxygen species on ammonia-oxidizing microbial communities in redox-fluctuating aquifers. Water Res. 2023;233:119734.
Montoya JP, Voss M, Kahler P, Capone DG. A simple, high-precision, high-sensitivity tracer assay for N(inf2) fixation. Appl Environ Microbiol. 1996;62:986–93.
Wu S, Moge D, Wan X, Selden C, Benavides M, Bonnet S, et al. Insights into nitrogen fixation below the euphotic zone: Trials in an oligotrophic marginal sea and global compilation. Biogeosciences Discuss. 2021;2021:1–31.
Berthelot H, Benavides M, Moisander P, Grosso O, Bonnet S. High Nitrogen fixation rates in the particulate and dissolved pools in the Western Tropical Pacific (Solomon and Bismarck Seas). Geophys Res Lett. 2017;44:8414–23.
Knapp AN, Sigman DM, Lipschultz F. N isotopic composition of dissolved organic nitrogen and nitrate at the Bermuda Atlantic Time-series Study site. Global Biogeochem Cy. 2005;19:1–15.
Xu MN, Wu Y, Zheng LW, Zheng Z, Zhao H, Laws EA, et al. Quantification of multiple simultaneously occurring nitrogen flows in the euphotic ocean. Biogeosciences. 2017;14:1021–38.
Lu Y, Wen Z, Shi D, Lin W, Bonnet S, Dai M, et al. Biogeography of N2 fixation influenced by the western boundary current intrusion in the South China Sea. J Geophys Res: Oceans. 2019;124:6983–96.
Lu Y, Wen Z, Shi D, Chen M, Zhang Y, Bonnet S, et al. Effect of light on N2 fixation and net nitrogen release of Trichodesmium in a field study. Biogeosciences. 2018;15:1–12.
Dabundo R, Lehmann MF, Treibergs L, Tobias CR, Altabet MA, Moisander PH, et al. The contamination of commercial 15N2 gas stocks with 15N–labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS ONE. 2014;9:e110335.
Fulweiler RWW, Heiss EM, Rogener MK, Newell SE, Wilhelm SW. Examining the impact of acetylene on N-fixation and the active sediment microbial community. Front Microbiol. 2015;6:418.
Cui L, Yang K, Li H-Z, Zhang H, Su J-Q, Paraskevaidi M, et al. Functional single-cell approach to probing nitrogen-fixing bacteria in soil communities by resonance raman spectroscopy with 15N2 labeling. Anal Chem. 2018;90:5082–89.
Cui L, Yang K, Zhou G, Huang W. Surface-enhanced raman spectroscopy combined with stable isotope probing to monitor nitrogen assimilation at both bulk and single-cell level. Anal Chem. 2017;89:5793–800.
Csardi G, Nepusz T. The Igraph software package for complex network research. Interjournal Complex Systems. 2006;1695:1–9.
Newman MEJ. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103:8577–82.
Gao Z, Weng H, Guo H. Unraveling influences of nitrogen cycling on arsenic enrichment in groundwater from the Hetao Basin using geochemical and multi-isotopic approaches. J Hydrol. 2021;595:125981.
Aharoni I, Dahan O, Siebner H. Continuous monitoring of dissolved inorganic nitrogen (DIN) transformations along the waste-vadose zone - groundwater path of an uncontrolled landfill, using multiple N-species isotopic analysis. Water Res. 2022;219:118508.
Carrey R, Ballesté E, Blanch AR, Lucena F, Pons P, López JM, et al. Combining multi-isotopic and molecular source tracking methods to identify nitrate pollution sources in surface and groundwater. Water Res. 2021;188:116537.
Larmola T, Leppänen SM, Tuittila E-S, Aarva M, Merilä P, Fritze H, et al. Methanotrophy induces nitrogen fixation during peatland development. Proc Natl Acad Sci USA. 2014;111:734–39.
Oremland Ronald S, Taylor, Barrie F. Inhibition of methanogenesis in marine sediments by acetylene and ethylene: Validity of the acetylene reduction assay for anaerobic microcosms. Appl Microbiol. 1975;30:707–09.
Ho A, Bodelier PLE. Diazotrophic methanotrophs in peatlands: the missing link? Plant Soil. 2015;389:419–23.
Farías L, Faúndez J, Fernández C, Cornejo M, Sanhueza S, Carrasco C. Biological N2O fixation in the Eastern South Pacific Ocean and marine cyanobacterial cultures. PLoS ONE. 2013;8:e63956.
Desloover J, Roobroeck D, Heylen K, Puig S, Boeckx P, Verstraete W, et al. Pathway of nitrous oxide consumption in isolated Pseudomonas stutzeri strains under anoxic and oxic conditions. Environ Microbiol. 2014;16:3143–52.
Hynes RK, Knowles R. Effect of acetylene on autotrophic and heterotrophic nitrification. Can J Microbiol. 1982;28:334–40.
Shiozaki T, Ijichi M, Kodama T, Takeda S, Furuya K. Heterotrophic bacteria as major nitrogen fixers in the euphotic zone of the Indian Ocean. Global Biogeochem Cy. 2014;28:1096–110.
Sipler R, Gong D, Baer S, Sanderson M, Roberts Q, Mulholland M, et al. Preliminary estimates of the contribution of Arctic nitrogen fixation to the global nitrogen budget. Limnol Oceanogr Lett. 2017;2:159–66.
Shiozaki T, Bombar D, Riemann L, Hashihama F, Takeda S, Yamaguchi T, et al. Basin scale variability of active diazotrophs and nitrogen fixation in the North Pacific, from the tropics to the subarctic Bering Sea. Global Biogeochem Cy. 2017;31:996–1009.
Moore M, Mills M, Achterberg E, Geider R, Laroche J, Lucas M, et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat Geosci. 2009;2:867–71.
Wen Z, Lin W, Shen R, Hong H, Kao S-J, Shi D. Nitrogen fixation in two coastal upwelling regions of the Taiwan Strait. Sci Rep. 2017;7:17601.
Chen Y-L, Chen H-Y, Tuo S-H, Ohki K. Seasonal dynamics of new production from Trichodesmium N2 fixation and nitrate uptake in the upstream Kuroshio and South China Sea basin. Limnol Oceanogr. 2008;53:1705–21.
Kumar P, Singh A, Ramesh R, Nallathambi T. N2 fixation in the eastern Arabian sea: Probable role of heterotrophic diazotrophs. Front Mar Sci. 2017;4:80.
Geisler E, Rahav E, Bar Zeev E. Contribution of heterotrophic diazotrophs to N2 fixation in a eutrophic river: free-living vs. aggregate-associated. Front Microbiol. 2022;13:779820.
Jabir T, Vipindas PV, Jesmi Y, Valliyodan S, Parambath PM, Singh A, et al. Nutrient stoichiometry (N:P) controls nitrogen fixation and distribution of diazotrophs in a tropical eutrophic estuary. Mar Pollut Bull. 2020;151:110799.
Wang R, Li X, Hou L, Liu M, Zheng Y, Yin G, et al. Nitrogen fixation in surface sediments of the East China Sea: Occurrence and environmental implications. Mar Pollut Bull. 2018;137:542–48.
Liu C, li N, Shao X, Gao D, Xia J, Cui Q, et al. Effects of coastal marsh conversion to shrimp aquaculture ponds on sediment nitrogen fixation. Front Mar Sci. 2022;9:1034145.
Glodowska M, Stopelli E, Straub D, Vu Thi D, Trang PTK, Viet PH, et al. Arsenic behavior in groundwater in Hanoi (Vietnam) influenced by a complex biogeochemical network of iron, methane, and sulfur cycling. J Hazard Mater. 2021;407:124398.
Karl DM, Church MJ. Ecosystem structure and dynamics in the north Pacific Subtropical Gyre: New views of an old ocean. Ecosystems. 2017;20:433–57.
Karl D, Letelier R, Tupas L, Dore J, Christian J, Hebel D. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature. 1997;388:533–38.
Zhu C, Friman V-P, Li L, Xu Q, Guo J, Guo S, et al. Meta-analysis of diazotrophic signatures across terrestrial ecosystems at the continental scale. Environ Microbiol. 2022;24:2013–28.
Messer L, Brown M, Furnas M, Carney R, McKinnon A, Seymour J. Diversity and activity of diazotrophs in Great Barrier reef surface waters. Front Microbiol. 2017;8:967.
Tan E, Hsu T-C, Huang X, Lin H-J, Kao S-J. Nitrogen transformations and removal efficiency enhancement of a constructed wetland in subtropical Taiwan. Sci Total Environ. 2017;601-602:1378–88.
Farnelid H, Turk-Kubo K, Ploug H, Ossolinski J, Collins J, Van Mooy B, et al. Diverse diazotrophs are present on sinking particles in the North Pacific subtropical gyre. ISME J. 2018;13:170–82.
Bonnet S, Dekaezemacker J, Turk-Kubo K, Moutin T, Hamersley M, Grosso O, et al. Aphotic N2 fixation in the eastern tropical South Pacific Ocean. PLoS ONE. 2013;8:e81265.
Huang F, Lin X, Hu W, Zeng F, He L, Yin KD. Nitrogen cycling processes in sediments of the Pearl River Estuary: Spatial variations, controlling factors, and environmental implications. CATENA. 2021;206:105545.
Moynihan MA, Goodkin NF, Morgan KM, Kho PYY, Lopes dos Santos A, Lauro FM, et al. Coral-associated nitrogen fixation rates and diazotrophic diversity on a nutrient-replete equatorial reef. ISME J. 2022;16:233–46.
Zehr J, Capone D. Changing perspectives in marine nitrogen fixation. Science. 2020;368:729.
Delmont TO, Pierella Karlusich JJ, Veseli I, Fuessel J, Eren AM, Foster RA, et al. Heterotrophic bacterial diazotrophs are more abundant than their cyanobacterial counterparts in metagenomes covering most of the sunlit ocean. ISME J. 2022;16:927–36.
Messer LF, Mahaffey C, M Robinson C, Jeffries TC, Baker KG, Bibiloni Isaksson J, et al. High levels of heterogeneity in diazotroph diversity and activity within a putative hotspot for marine nitrogen fixation. ISME J. 2016;10:1499–513.
Bentzon-Tilia M, Severin I, Hansen L, Riemann L. Genomics and ecophysiology of heterotrophic nitrogen-fixing bacteria isolated from estuarine surface water. mBio. 2015;6:e00929–15.
Yousuf J, Thajudeen J, Aneesa PA, Joseph A, Divya PS, Varghese A, et al. Diversity and activity of culturable nitrogen fixing heterotrophic bacteria from estuarine and coastal environments of Southeastern Arabian Sea (SEAS). Reg Stud Mar Sci. 2019; 33:100973.
Handley K, Verberkmoes N, Steefel C, Williams K, Sharon I, Miller C, et al. Biostimulation induces syntrophic interactions that impact C, S and N cycling in a sediment microbial community. ISME J. 2013;7:800–16.
Bombar D, Paerl R, Riemann L. Marine non-cyanobacterial diazotrophs: moving beyond molecular detection. Trends Microbiol. 2016;24:916–27.
Knapp AN. The sensitivity of marine N2 fixation to dissolved inorganic nitrogen. Front Microbiol. 2012;3:374.
McKinlay J, Harwood C. Inaugural Article: carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc Natl Acad Sci USA. 2010;107:11669–75.
Li Y, Guo L, Häggblom MM, Yang R, Li M, Sun X, et al. Serratia spp. are responsible for nitrogen fixation fueled by As(III) oxidation, a novel biogeochemical process identified in mine tailings. Environ Sci Technol. 2022;56:2033–43.
Bertics V, Sohm J, Treude T, Chow C-E, Fuhrman J, Ziebis W. Burrowing deeper into benthic nitrogen cycling: The impact of Bioturbation on nitrogen fixation coupled to sulfate reduction. Mar Ecol Prog Ser. 2010;409:1–15.
Farhan Ul Haque M, Hernández M, Crombie AT, Murrell JC. Identification of active gaseous-alkane degraders at natural gas seeps. ISME J. 2022;16:1705–16.
Farhan Ul Haque M, Crombie AT, Ensminger SA, Baciu C, Murrell JC. Facultative methanotrophs are abundant at terrestrial natural gas seeps. Microbiome. 2018;6:118.
Bay SK, Dong X, Bradley JA, Leung PM, Grinter R, Jirapanjawat T, et al. Trace gas oxidizers are widespread and active members of soil microbial communities. Nat Microbiol. 2021;6:246–56.
Cui J, Zhang M, Chen L, Zhang S, Luo Y, Cao W, et al. Methanotrophs contribute to nitrogen fixation in emergent macrophytes. Front Microbiol. 2022;13:851424.
Fulweiler RW, Nixon SW, Buckley BA, Granger SL. Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature. 2007;448:180–82.
Yu X, Zhou J, Song W, Xu M, He Q, Peng Y, et al. SCycDB: A curated functional gene database for metagenomic profiling of sulfur cycling pathways. Mol Ecol Res. 2020;21:924–40.
Welsh D, Wellsbury P, Bourguès S, De Wit R, Herbert R. Relationship between porewater organic carbon content, sulphate reduction and nitrogen fixation (acetylene reduction) in the rhizosphere of Zostera noltii. Hydrobiologia. 1996;329:175–83.
Wang Y, Wei D, Li P, Jiang Z, Liu H, Qing C, et al. Diversity and arsenic-metabolizing gene clusters of indigenous arsenate-reducing bacteria in high arsenic groundwater of the Hetao Plain, Inner Mongolia. Ecotoxicology. 2021;30:1680–88.
Hoven R, Santini J. Arsenite oxidation by the heterotroph Hydrogenophaga sp. str. NT-14: the arsenite oxidase and its physiological electron acceptor. BBA Bioenergetics. 2004;1656:148–55.
Li L, Jia R, Qu Z, Li T, Shen W, Qu D. Coupling between nitrogen-fixing and iron(III)-reducing bacteria as revealed by the metabolically active bacterial community in flooded paddy soils amended with glucose. Sci Total Environ. 2020;716:137056.
Zhang H, Liu F, Zheng S, Chen L, Zhang X, Gong J. The differentiation of iron-reducing bacterial community and iron-reduction activity between riverine and marine sediments in the Yellow River estuary. Mar Life Sci Tech. 2019;2:87–96.
Liu H, Li P, Wang H, Qing C, Tan T, Shi B, et al. Arsenic mobilization affected by extracellular polymeric substances (EPS) of the dissimilatory iron reducing bacteria isolated from high arsenic groundwater. Sci Total Environ. 2020;735:139501.
Garber AI, Nealson KH, Okamoto A, McAllister SM, Chan CS, Barco RA, et al. FeGenie: A comprehensive tool for the identification of iron genes and iron gene neighborhoods in genome and metagenome assemblies. Front Microbiol. 2020;11:37.
Yan L, Herrmann M, Kampe B, Lehmann R, Totsche KU, Küsel K. Environmental selection shapes the formation of near-surface groundwater microbiomes. Water Res. 2020;170:115341.
Lovley DR. Organic matter mineralization with the reduction of ferric iron: A review. Geomicrobiol J. 1987;5:375–99.
Jones JG, Gardener S, Simon BM. Bacterial reduction of ferric iron in a stratified eutrophic lake. Microbiology. 1983;129:131–39.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant No 42177068, 91851115, 91851209, and 91851101).
Author information
Authors and Affiliations
Contributions
Conceptualization: PL, XL, and HW. Sample collection and methodology: HW, XL, L-LH, PL, YW, and ZJ. Analysis and writing-original draft preparation: XL and HW. Writing-review and editing and funding acquisition: PL, LC, and S-JK. 15N analysis: L-LH and S-JK. Single-cell resonance Raman-15N2 SIP analysis: KY and LC.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Li, P., Wang, H. et al. Nitrogen fixation and diazotroph diversity in groundwater systems. ISME J 17, 2023–2034 (2023). https://doi.org/10.1038/s41396-023-01513-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-023-01513-x