Abstract
Predicting whether microbial invaders will colonize an environment is critical for managing natural and engineered ecosystems, and controlling infectious disease. Invaders often face competition by resident microbes. But how invasions play out in communities dominated by facilitative interactions is less clear. We previously showed that growth medium toxicity can promote facilitation between four bacterial species, as species that cannot grow alone rely on others to survive. Following the same logic, here we allowed other bacterial species to invade the four-species community and found that invaders could more easily colonize a toxic medium when the community was present. In a more benign environment instead, invasive species that could survive alone colonized more successfully when the residents were absent. Next, we asked whether early colonists could exclude future ones through a priority effect, by inoculating the invaders into the resident community only after its members had co-evolved for 44 weeks. Compared to the ancestral community, the co-evolved resident community was more competitive toward invaders and less affected by them. Our experiments show how communities may assemble by facilitating one another in harsh, sterile environments, but that arriving after community members have co-evolved can limit invasion success.
Similar content being viewed by others
Introduction
Successful colonization of invader microorganisms into sterile environments or existing microbial communities are common and can impact ecosystem diversity and function, potentially with significant consequences [1,2,3]. A better understanding of the factors driving microbial invasions may help to prevent the spread and establishment of invasive species, or to aid the intentional introduction of a new species for a desired purpose. For example, it might be desirable to prevent the invasion of a species that reduces the efficiency of a bioremediation system [4], or to promote the colonization of probiotic species in the intestinal microbiome of a patient [5, 6].
What determines the ability of an invasive species to colonize an existing ecosystem depends on the characteristics of both the invading species and the resident community [7, 8]. Many theoretical and empirical studies have established factors that influence invasion outcome, such as propagule pressure [9,10,11,12,13,14], resident community productivity [15], genotypic richness of invaders [13, 16] or the resident community [3, 13, 17,18,19,20], community niche coverage [3, 21, 22], and abiotic conditions (e.g., the presence of antibiotics [23]).
Invasion success may also depend on the sign and strength of interactions between resident community members, and between residents and invaders [24]. Previous studies tend to find that invaders compete with resident species [3, 13, 17, 20, 23, 25, 26], which is consistent with competition being prevalent in microbial communities [27,28,29]. However, in sterile environments, early colonizers can facilitate the arrival of other species [30,31,32,33,34,35]. This occurs when new communities assemble and groups of species follow one another in so-called “successions”, for example in the formation of dental plaque [36, 37] or marine particle communities [32, 33]. Facilitation likely occurs in newly assembling communities, as sterile environments are typically difficult to colonize, for example if they have an extreme pH, contain toxic compounds, or are lacking in easily accessible nutrients or water. Pioneer species may alter the environment in ways that facilitate invasion by new species that would otherwise not survive [24, 38,39,40,41]. This is in line with the Stress Gradient Hypothesis (SGH), which predicts that species are more likely to interact positively in stressful environments [42,43,44,45,46,47,48]. The link between the SGH and microbial invasion has, however, not yet been explored experimentally.
As more species colonize the environment and species diversity increases, previously available niches begin to fill up, such that competition is expected to increase and invasion success to drop. The negative relationship between invasion success and species richness and diversity have been well-established [3, 13, 17,18,19,20]. As time passes, resident species may co-evolve to reduce niche overlap and availability in a way that would prevent further invasion. The Community Monopolization Hypothesis predicts that early colonizers adapt to use available resources efficiently, yielding a competitive advantage against later-arriving species [31, 49,50,51,52,53], also known as a “priority effect” [31, 53].
Besides invasion success, it is important to consider whether invaders perturb the resident community, possibly changing community structure and function [24, 54, 55], even if the invader does not manage to establish [56]. One prediction is that co-evolved resident species would be less perturbed by species invasion, presumably due to increased niche coverage [49]. Experimentally disentangling the role of the different factors discussed above on invasion success and robustness against invasion can be challenging.
Here we aim to test the effect of the two less-well understood factors (the SGH and priority effects) on bacterial invasion success and resistance by studying invasion into a synthetic bacterial community whose composition is fixed at four species: Agrobacterium tumefaciens, Comamonas testosteroni, Microbacterium saperdae, and Ochrobactrum anthropi. These four species can grow and bioremediate metal working fluids (MWF) [47, 57], an industrial fluid used in metal manufacturing. MWFs contain mineral oils, emulsifiers, and biocides, some of which are toxic to bacteria. In previous work [47], we showed that when the four species were grown together in this toxic environment, they facilitated each other’s survival compared to when they were alone. Instead, when we added amino acids to make the environment more permissive, competition between species increased. This system allows us to study biological invasion while experimentally manipulating environmental conditions to control interactions between community members and holding all other factors constant. Another advantage of this system is that the four species can coexist over evolutionary time-scales, allowing us to explore the effect of community co-evolution on microbial invasion.
Using four invader species, Aeromonas caviae, Klebsiella pneumoniae, Providencia rettgeri, and Pseudomonas fulva that were isolated from waste MWF (chosen from a set of 20 based on our ability to distinguish them from the resident species), we first show that the resident community facilitates invasion of species that cannot grow alone, but inhibits those that can. Whether or not species could grow alone was modulated by changes in the growth medium. Second, after co-evolving the four resident species for 44 weeks, we found that invasions were still possible in MWF, but the growth of the invaders was inhibited relative to the ancestral community and the co-evolved resident species were less affected by invasions. Together, our results show that facilitative communities are easier to invade than competitive ones, but that a co-evolved community is more robust to invasion compared to an ancestral one.
Materials and methods
Study system
The four bacterial species used to assemble the resident community were isolated from MWF [57] and are referred to as: Agrobacterium tumefaciens str. MWF001, Comamonas testosteroni str. MWF001, Microbacterium saperdae str. MWF001, and Ochrobactrum anthropi str. MWF001 (as in ref. [47]). The additional four bacterial species, used to invade the resident community, were kindly donated by Peter Küenzi from Blaser Swisslube AG, Hasle-Rüegsau and we identified them using MALDI-TOF MS performed at Mabritec AG, Switzerland as: Aeromonas caviae, Klebsiella pneumoniae, Providencia rettgeri, and Pseudomonas fulva. We name these four strains str. Blaser001. As mentioned in the main text, these species were chosen from a set of 20 isolates, based on our ability to design selective plates on which the invader but not the resident species would grow (see Table 1). The choice of invader species might favor species that differ metabolically from the residents, which could potentially increase invasion ability, but we expect this effect to be small, given how different the selective media were from MWF. The MWF (Castrol Hysol XF, acquired in 2016) was prepared at a concentration of 0.5% (v/v), diluted in water with the addition of selected salts and metal traces to support bacterial growth. We also used MWF medium supplemented with 1% casamino acids (Difco, UK) (MWF + AA). These media were prepared as in ref. [47].
Experimental setup
To assemble the resident community, a single isolated colony of each species was selected and inoculated in 10 ml of Tryptic Soy Broth (TSB) in Erlenmeyer flasks (50 ml), then incubated overnight at 28 °C (200 rpm). To achieve exponentially growing bacteria, with a final concentration of ∼106–107 CFU/ml, each bacterial species was inoculated at an OD600 of 0.05 measured by spectrophotometry (Ultrospec 10, Amersham Biosciences), in 20 ml of TSB in Erlenmeyer flasks (100 ml) and cultivated at 28 °C, shaken at 200 rpm. After 3 h, 200 μl of each of the four resident species were combined and centrifuged (5 min, 10,000 rcf). The bacterial pellet was resuspended in 30 ml of MWF or MWF + AA into borosilicate glass tubes (16 × 125 mm, 30 ml).
Transfers
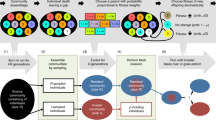
All communities (the four-species or the three-species resident communities) were incubated at 28 °C and shaken at 200 rpm for seven days in either MWF or MWF + AA medium. Every week, 300 μl (1%) of the week-old culture was transferred into fresh medium and the growth cycle repeated. Each week, we also harvested 1 ml of each culture, spun it down at 10,000 rcf for 5 min, resuspended it in glycerol 25% (diluted in PBS) and stocked it at −80 °C for future analyses. This was repeated for 44 transfers (weeks) to co-evolve the resident communities or for four transfers in the invasion assays. The evolutionary experiment was conducted in five replicate culture tubes for each condition (three- or four-species community), of which we show only one here (Fig. S1). After the 44 weeks, we isolated one colony of each species, which we refer to as A. tumefaciens str. MWF431, C. testosteroni str. MWF431, M. saperdae str. MWF431, and O. anthropi str. MWF431 for the four-species co-evolved community; and A. tumefaciens str. MWF351, C. testosteroni str. MWF351, M. saperdae str. MWF351, and O. anthropi str. MWF351 for the three-species co-evolved community. This design choice simplified our experiments, but means that we cannot test how intraspecies diversity affects invasion success.
Invasion assays
Invasion was performed after 2 days of the first transfer of the resident community. One single colony of each invader species was selected and inoculated in 10 ml of TSB in Erlenmeyer flasks (50 ml) and incubated overnight at 28 °C, shaken at 200 rpm. To achieve exponentially growing bacteria, with a final concentration of ∼106–107 CFU/ml, each invader strain was inoculated at an OD600 of 0.05 measured by spectrophotometry (Ultrospec 10, Amersham Biosciences), in 20 ml of TSB in Erlenmeyer flasks (100 ml) and cultivated at 28 °C (200 rpm). After 3 h, 200 μl of the invader species were centrifuged (5 min, 10,000 rcf). The bacterial pellet was resuspended in the same medium of the resident community. In total, 200 μl of this suspension were then added to the culture tubes, with or without the resident communities. For the experiments where propagule pressure was changed (Figs. S2 and S3), we took either 2 ml or 200 μl for propagule size 107 or 106, respectively, centrifuged, and resuspended them. For propagule size 105 or 104, we instead aliquoted 20 μl of cell suspension into 180 μl of PBS and diluted it once more in PBS for 104, before centrifuging and re-suspending in the growth medium of the resident community.
Quantifying bacterial abundance
The abundance of each resident or invader species was quantified before the inoculation in the MWF or MWF + AA (before combining resident species) and before each transfer using serial dilution and selective plating (Table 1). We also quantified population sizes of the resident species at the time of invasion (Fig. S4C) and the same approach was used to quantify growth curves shown in Figs. S5–S8. To define invasion outcomes we used an invasion threshold representing the dynamics of an invader species with a growth rate of 0 (its abundance changes only due to dilution, i.e., 100-fold decrease every transfer from the initial population size). By subtracting this threshold value from the abundance of the invader species at transfer four, the invasion is defined as successful if >0 (the growth rate is positive) or failed if ≤0 (the growth rate is 0 or negative). We used a Kruskal–Wallis test to assess whether effects were significant. Raw CFU/ml data and the results of all statistical tests are listed in Dataset 1.
Quantifying growth rates
We quantified bacterial growth rates in Fig. S5 in two ways: first, we took the difference in CFU/ml between all the measurements on consecutive days that we had and took the maximum value (panels B and E). We also computed the fold change between all consecutive CFU/mL measurements and divided that by the number of days between measurements (panels C and F).
Quantifying pairwise interactions
Pairwise interactions between species (Figs. S6G, S7G, and S8F) were quantified as in ref. [47]. Briefly, arrow thickness indicates the interaction strength measured as the ten-fold change in area under the growth curve (AUC, plotted in Figs. S6F, S7F, and S8E), with the color showing the sign of fold-change and the p values resulting from a Kruskal–Wallis test comparing each species alone and with a given partner species. We use the AUC to represent species growth, as we have found it to adequately summarize species’ effects on one another, combining growth rate, yield, and lag phase length [47].
Results
The resident community facilitates the invasion of species that cannot grow alone
We first ask whether each of the four invader species (A. caviae, K. pneumoniae, P. rettgeri, and P. fulva), could colonize MWF and to what extent the resident community promotes or inhibits invasion. The resident community was cultured in MWF for 1 week, with 1% of the population transferred into fresh media once a week for a total of 4 weeks (see Methods). Each invader species was inoculated individually into three replicate microcosms of the resident community 48 h after the first transfer, presumably during the community’s exponential growth phase (Fig. S9A). As a control treatment, we inoculated each of the invader species into sterile MWF and performed transfers in parallel (Fig. S9B). We quantified the abundance of all species at inoculation and before each transfer (see Methods; invaders in Fig. 1A and residents in Fig. S10A, left).
A Red lines: abundances of the four invader species in MWF at different transfers, quantified at first inoculation and before each transfer. The four invader species were grown alone or inoculated into the growing resident community after it had been transferred once and until transfer four (one transfer every 7 days). At each transfer, the culture is diluted 100-fold. The experiments were done in parallel except for the invader alone, which was done separately. Black lines: theoretical expectation of what would happen if the invader did not grow at all and was just diluted, which we call the “invasion threshold”, whether invading alone (dotted) and into the community (solid). We count an invasion as successful if the abundance of the invader at the end of the transfers is higher than the invasion threshold. B Blue lines: abundances of the four invader species in MWF + AA at different transfers when alone (dashed) and into the community (solid). Black lines: the theoretical invasion threshold simulating a non-growing invader. There is only one invasion threshold as experiments were all conducted in parallel and had the same starting population sizes (compared to A). C To exclude the effect of the choice of dilution rate on invasion success, we calculate the “invasion magnitude”: the abundance of each invader at transfer four minus its corresponding invasion threshold. If this number is higher or lower than zero, then the invasion is successful (+) or failed (−), respectively. From left to right: A. caviae (Ac), K. pneumoniae (Kp), P. rettgeri (Pr), P. fulva (Pf). Statistical significance is marked above the data points (p values: *<0.05, NS not significant). For abundance of resident species see Fig. S2.
P. fulva was the only one of the four invader species that could colonize the MWF when alone (Fig. 1A (red dotted line), C). Instead, when the community was present, the number of successful invasions increased: A. caviae, K. pneumoniae, and P. fulva colonized the MWF containing the resident community, while P. rettgeri still did not (Fig. 1A (red solid line), C). These results are in line with our previous findings [47] that species that cannot grow alone in MWF are likely to be facilitated by other species, explaining why in some cases invasion is only successful when the community is present.
Residents inhibit invaders that can grow alone in a more permissive medium
MWFs are designed to prevent bacterial contamination and include biocides [58], which make them quite toxic. This explains why only one of the invader species was able to grow alone in the MWF medium. To explore invasion in a less harsh environment, we enriched the medium by adding 1% casamino acids (MWF + AA). Casamino acids are a nutrient source for three out of the four resident community members and, according to previous work [47, 59], we expect more negative interactions in a more permissive medium. We found that K. pneumoniae, P. fulva, and P. rettgeri could colonize MWF + AA alone, while A. caviae still suffered from the environmental toxicity (invaders in Fig. 1B, C and residents in Fig. S10A, right). The three species that were able to colonize alone were still able to invade the community, but significantly less well compared to when the community was absent (Kruskal–Wallis, all p values < 0.05, Fig. 1C). Consistent with previous work [47], our results suggest that in this more permissive environment, the community competes with the invaders.
A resident community co-evolved in MWF is more competitive toward invaders
The capacity to colonize a resident community might depend on community history: resident species that have adapted to one another in a given environment may be more likely to exclude future colonists through a priority effect [49,50,51]. To test this hypothesis, we extend the pre-invasion phase to 44 weeks, allowing the four resident species to adapt to MWF and to each other (see Methods). Next, we mixed one co-evolved isolate of each species and call this the “co-evolved community” (Fig. S9C).
We now ask to what extent the invader species can colonize the co-evolved resident community compared to the ancestral one. We found that while P. rettgeri could colonize neither, A. caviae, K. pneumoniae, and P. fulva colonized both the ancestral and co- evolved communities (invaders in Fig. 2A, B and residents in Fig. S10B). However, all three invader species had a smaller invasion magnitude in the co-evolved compared to the ancestral community (Kurskal–Wallis, A. caviae p value < 0.0005, K. pneumoniae p value < 0.0005, P. fulva p value < 0.05, Fig. 2B). The invasion outcome for A. caviae was initially inconclusive, where in one out of two biological replicates the invader went extinct when inoculated into the ancestral community (Fig. S2A, B). We tested whether this was due to variability in propagule pressure [13], but found no evidence for this, as different invasion population sizes of A. caviae all converged to a similar population size at transfer four (invaders in Fig. S3 and residents in Fig. S11). We therefore concluded that the death of A. caviae in one biological replicate might have been due to a technical error (Fig. S2).
A, C Red/gray lines: invader species were grown alone (red dashed), or inoculated into the ancestral (red solid) or the co-evolved community (gray solid) in MWF. Cultures were diluted 100-fold in fresh MWF every 7 days for a total of four transfers. The experiments were all conducted in parallel. Black lines: the theoretical invasion threshold simulating a non-growing invader. B, D Invasion magnitude (abundance at transfer four minus the invasion threshold) alone “A”, into the ancestral community “C”, or into the evolved community “E”. Positive or negative invasion magnitudes indicate successful (+) or failed (−) invasions, respectively. From left to right: A. caviae (Ac), K. pneumoniae (Kp), P. rettgeri (Pr), P. fulva (Pf), O. anthropi (Oa). Statistical significances are marked above the data points (p values: *<0.05, NS not significant, N/A not applicable). See Fig. 1 caption for more details. In A and B, the resident community consists of a co-culture of four ancestral or evolved clonal populations, while in C and D, the community contained only three clonal populations (four residents, without O. anthropi), either ancestral or evolved. For abundance of the four-species or three-species resident communities see Figs. S2B or S6, respectively.
We next wondered whether the pattern observed for A. caviae, K. pneumoniae, and P. fulva was specific to these invader species colonizing our community of four resident species, or whether there was anything particular about the four resident species. One way to explore this is to exclude one species from the co-evolutionary process and allow it to invade at a later stage. We did this by co-evolving three of the resident species, A. tumefaciens, C. testosteroni, and M. saperdae together in MWF for 44 weeks, excluding O. anthropi. Next, we combined single isolates of the three co-evolved species and invaded the wild-type O. anthropi into this co-evolved three-species community (Fig. S9D). As before, O. anthropi could not colonize the MWF when alone (as in ref. [47], Fig. 2C), but invaded successfully when inoculated into the ancestral or the co-evolved community of three. Consistent with the previous invasion assays (Fig. 2A, B) and our hypothesis that a co-evolved community is more difficult to invade, O. anthropi grew significantly worse when it was inoculated into the co-evolved three-species community compared to the corresponding ancestral one (invader O. anthropi in Fig. 2C, D and residents in Fig. S12).
In summary, while invasions into a community co-evolved in MWF are still possible, co-evolved community members inhibit invading species more than their ancestors.
Co-evolved communities are less affected by invasion compared to their ancestors
So far, we have focused on the effect of the resident community on the invading species. Next, we consider how robust the resident community is to these invasion events.
In all our treatments, the resident species were maintained over the four transfers (Figs. S10 and S12). At transfer four (representing cumulative effects), the abundance of two of the ancestral residents, A. tumefaciens and O. anthropi, was significantly lower when invaded by P. fulva (t-test, both p value < 0.005, Figs. 3A and S13A). Otherwise, we detected no significant changes in their final abundance following invasion by other species. This lack of perturbation was also observed for C. testosteroni. M. saperdae instead had a greater final population size in the presence of most invaders (t-test, A. caviae, P. rettgeri, and P. fulva, all p values < 0.005, Fig. 3A and Fig. S13A). This is not unexpected, as we know that M. saperdae strongly depends on other species to grow in MWF [47].
Each panel represents the total population size (CFU/ml) at transfer four of a resident member in the ancestral community (A), the co-evolved community (B), the ancestral three-species community (C) and the co-evolved three-species community (D). The full datasets are in Figs. S2 and S6. All experiments were performed in MWF. The bacterial abundance of community members without any invader species is represented by colored dots and once invaded by black dots (invader species indicated in brackets). From left to right: A. tumefaciens (At), C. testosteroni (Ct), M. saperdae (Ms), O. anthropi (Oa), A. caviae (Ac), K. pneumoniae (Kp), P. rettgeri (Pr), P. fulva (Pf). We compared the data points of each species when invaded to the corresponding data points when co-cultured with other community members but without invasion. Statistical significance is marked above the data points (p values: *<0.05, **<0.01, NS not significant).
Once the community had co-evolved, the abundances of A. tumefaciens and M. saperdae were no longer significantly affected by the invasion of P. fulva (Fig. 3B). The abundance of O. anthropi was still lower following invasion by P. fulva, but significantly less compared to the ancestor (ancestral vs. co-evolved, t-test, p value = 0.0167, Fig. 3A, B, last column). In addition, M. saperdae was no longer significantly positively affected by any of the invaders. This may be because the co-evolved M. saperdae grows significantly better within the resident community (Fig. 3A, B). The co-evolved three-species community behaved similarly: while the abundance of ancestral A. tumefaciens was significantly lower following the invasion of O. anthropi (t-test, p value < 0.05), its co-evolved counterpart was not (Fig. 3C, D). Altogether, co-evolved resident communities were more robust to invasion compared to ancestral ones.
Discussion
Studies on microbial invasion often focus on how resident community composition and species richness affect invasion outcomes. Here, we chose instead to work with a resident community whose composition was fixed at the same four species and ask how their environment—specifically environmental harshness—and their common evolutionary history affect invasion success and resistance.
By increasing the permissiveness of a harsh medium (MWF) through the addition of amino acids (MWF + AA), the number of invader species able to grow alone increased from a single one to three out of four species. In almost all cases where invaders died alone, the resident community facilitated their survival and growth. Unfortunately, we do not yet know why more nutrients or the presence of the resident community increased species survival. The resident species might be producing additional resources or removing toxic compounds [60]. Regardless of the mechanism, though, if invaders could survive alone in the more permissive environment, they experienced a net negative effect if the community was present.
The observation that species that couldn’t survive alone benefitted from those that could is consistent with our previous research [47] and more generally with the SGH, which can now be linked to invasion ecology due to our ability to modulate environmental harshness and inter-species interactions: in a harsh environment colonized by few species, invasion success may be high, as niches are still available and invaders can rely on the presence of the residents to survive. This is expected if early-arriving species improve the environment, facilitating the growth of others that are less well adapted to it [38,39,40,41]. Although MWF is particularly toxic, we expect many sterile environments to be “harsh” for many species, as every environment poses challenges to species that are not adapted to it. Nevertheless, facilitation may not always dominate, as it is possible that first colonizers alter the environment in a way that inhibits future invaders [31], or that late colonizers out-compete earlier ones and replace them [61], but this is not what we observe here.
Our new intuition might then help to explain why the assembly of many natural microbial communities is often highly predictable [32, 33, 36, 37, 62]. Assuming that only few species can act as pioneers and improve the environment in similar ways, the following colonizers may come from a predictable set of species. For example, microbial colonization of the healthy mammalian gut displays specific patterns of species arrival [62, 63]. But is a sterile gut a harsh environment? Microorganisms colonizing a newborn gut must survive the acidic conditions of the stomach, the host’s immune system and bile acids, and cholesterol produced by the host that are toxic for most microbial species [64]. A few specialized Lactobacillus and Bifidobacterium species produce bile resistance proteins [65], which allow them to colonize the gut and may facilitate the arrival of other species [66]. Similar dynamics may occur in other systems where strong ecosystem perturbations clear the ground for new communities to assemble, such as following antibiotic treatments, or the heavy pollution of soils. However, it remains to be seen whether first colonizers facilitate future arrivals as would be predicted by the SGH [42, 47, 67], or whether it is more of a race to fill available niches.
Once a community has assembled despite the challenging environment, we next asked whether the timing of invasion matters. In our experiments, early invaders fared better than those colonizing a community whose species had co-evolved, and co-evolved species were less perturbed by (more robust against) the invaders. Our findings corroborate several theoretical studies on the Community Monopolization Hypothesis [49, 50, 52] and provide rare experimental support to it. To our knowledge, this hypothesis has so far only been experimentally tested with single microbial species invading an ancestral or evolved second species [53, 68].
But what makes the co-evolved MWF community more resistant and robust to invasion? Possible explanations are summarized in Fig. 4. First, more productive communities (greater population size) are expected to be harder to invade (Fig. 4A) [15]. Although we observed no significant differences in productivity between ancestral and co-evolved communities at the time of transfer (Fig. S4A, B), co-evolved A. tumefaciens and M. saperdae (but not C. testosteroni and O. anthropi) grew faster during the first days (Fig. S5) and population sizes at the time of invasion were significantly greater in the co-evolved communities (Fig. S4C).
A Because the community might have evolved greater productivity (population size); B because within-species diversity may be higher with additional strains occupying available niches; C because some evolved strains may have evolved antagonistic phenotypes to compete with other community members; or D because evolved species better cover available niches. More than one of these mechanisms could hold simultaneously. Created with BioRender.com.
While productivity is a plausible explanation for reduced invasion success in co-evolved communities, it need not be the only one [15]. Another explanation could be that communities with higher species- and strain-level diversity tend to be more robust against invasions (Fig. 4B) [3, 13, 69]. However, diversity cannot explain invasion outcomes in our system: by using single isolates from the co-evolved communities, our experiments had the same species- and strain-level diversity in all treatments (Fig. S9A, C).
It could also be that co-evolved communities are more resistant to invasion because co-evolved residents actively inhibit other species through interference competition (Fig. 4C) [70]. This is difficult to test here, as spent media experiments are challenging with MWF. But if this were true and species had evolved to produce broad spectrum inhibitory molecules, we might expect the resident species to interact negatively with one another. On measuring pairwise interactions between ancestral (Fig. S6G) and co-evolved species (Figs. S7G and S8F, see Methods), however, we only observed that positive interactions weakened between A. tumefaciens and C. testosteroni and increased in the other two species (Figs. S7G and S8G), making this scenario less plausible.
A final reason for reduced invasion success in co-evolved communities is that the co-evolving residents may have partitioned the available niches among themselves, leaving little “space” for new arrivals (Fig. 4D) [31]. Further investigation, possibly using metabolomics analyses, would be needed to clarify whether this is the case and to more mechanistically understand resistance against invasion in our system. Taken together, although our experimental data cannot conclusively test all the different explanations (Fig. 4), we find support for the role of community productivity [15], but cannot exclude increased interference competitive or niche coverage as additional factors reducing invasion success.
Our fixed-species experimental design revealed some interesting patterns of invasion success, but also has its limitations. One confounding factor is that adding amino acids to the growth medium allowed more species to grow alone, but also provided new or larger niches for invader species to occupy. This is reflected in the higher overall invasion magnitude of species in MWF + AA compared to MWF (Fig. 1C). But despite these additional niches, invasion magnitude was still lower when the community was present compared to its absence (Fig. 1C). This made it difficult to interpret how invaders affected the resident community grown in MWF + AA: the effects varied depending on the invader species and the resident species with no clear pattern (Fig. S13). Further exploring the mechanisms behind the interactions in our system and developing a theoretical basis for what to expect may help to understand these effects.
One could also question whether a small synthetic community is representative of natural communities and their diversity. A mathematical model in our previous study indicated that competition would increase with a higher number of species in MWF [47] and perhaps we would expect invasions to be less successful in this context. This would also align with experiments involving larger communities that presumably occupy more niches and leave fewer resources for the invader [13, 17,18,19,20, 25]. Nevertheless, our community could help to understand the first phases of community assembly, when only few species have colonized.
Another weakness of our study is the arbitrary choice to perform four transfers at a 1% dilution rate. To compensate, we were careful to define our measures independently of these choices, such that we could compare between treatments rather than considering absolute measures of invasion. We quantified “invasion success”, representing absolute population increase or decrease and “invasion magnitude”, which compares population sizes between treatments at the end of the experiment. Another possibility would have been to extend the length of the experiment to observe whether invaders eventually went extinct or established themselves. However, as we were interested in the ecological dynamics of invasion separately from the evolutionary dynamics of the resident community, we decided to keep the invasion time-scale short and assume that species’ genetic adaptation to the environment and each other was negligible. In reality, of course, invaders might acquire mutations that increase invasion success.
In conclusion, we used a model system to disentangle interactions between species and measure their effect on microbial invasion. This revealed that a small, facilitative resident community can improve the environment for species that would otherwise be unable to colonize. However, a community whose residents have adapted to the environment and each other is more difficult to invade. Our work provides new experimental support for the Community Monopolization Hypothesis [42, 49, 71] and by linking invasion ecology with the SGH, provides a fresh perspective on community assembly as a sequence of invasion events into a harsh environment, where facilitation may be dominant at first as species complement each other, but decreases as niches are occupied through co-evolution or through invasion.
Data availability
All data used in this manuscript are provided in Dataset 1.
References
Thakur MP, van der Putten WH, Cobben MMP, van Kleunen M, Geisen S. Microbial invasions in terrestrial ecosystems. Nat Rev Microbiol. 2019;17:621–31.
Mooney HA, Cleland EE. The evolutionary impact of invasive species. Proc Natl Acad Sci USA. 2001;98:5446–51.
Mallon CA, Elsas JDV, Salles JF. Microbial invasions: the process, patterns, and mechanisms. Trends Microbiol. 2015;23:719–29.
O’Brien S, Hodgson DJ, Buckling A. Social evolution of toxic metal bioremediation in Pseudomonas aeruginosa. Proc R Soc B Biol Sci. 2014;281:20140858.
Walter J, Maldonado-Gómez MX, Martínez I. To engraft or not to engraft: an ecological framework for gut microbiome modulation with live microbes. Curr Opin Biotechnol. 2018;49:129–39.
van der Goot E, van Spronsen FJ, Falcão Salles J, van der Zee EA. A microbial community ecology perspective on the gut-microbiome-brain axis. Front Endocrinol. 2020;11:611.
Williamson M, Fitter A. The varying success of invaders. Ecology. 1996;77:1661–6.
Catford JA, Jansson R, Nilsson C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib. 2009;15:22–40.
Simberloff D. The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst. 2009;40:81–102.
Acosta F, Zamor RM, Najar FZ, Roe BA, Hambright KD. Dynamics of an experimental microbial invasion. Proc Natl Acad Sci USA. 2015;112:11594–9.
Barney JN, Ho MW, Atwater DZ. Propagule pressure cannot always overcome biotic resistance: the role of density-dependent establishment in four invasive species. Weed Res. 2016;56:208–18.
Ketola T, Saarinen K, Lindström L. Propagule pressure increase and phylogenetic diversity decrease community’s susceptibility to invasion. BMC Ecol. 2017;17:1–7.
Vila JCC, Jones ML, Patel M, Bell T, Rosindell J. Uncovering the rules of microbial community invasions. Nat Ecol Evol. 2019;3:1162–71.
Dressler MD, Conde J, Eldakar OT, Smith RP. Timing between successive introduction events determines establishment success in bacteria with an Allee effect. Proc R Soc B Biol Sci. 2019;286:20190598.
Jones ML, Rivett DW, Pascual-Garria A, Bell T. Relationships between community composition, productivity and invasion resistance in semi-natural bacterial microcosms. eLife. 2021;10:e71811.
Rivett DW, Jones ML, Ramoneda J, Mombrikotb SB, Ransome E, Bell T. Elevated success of multispecies bacterial invasions impacts community composition during ecological succession. Ecol Lett. 2018;21:516–24.
Case TJ. Invasion resistance arises in strongly interacting species-rich model competition communities. Proc Natl Acad Sci USA. 1990;87:9610–4.
Jousset A, Schulz W, Scheu S, Eisenhauer N. Intraspecific genotypic richness and relatedness predict the invasibility of microbial communities. ISME J. 2011;5:1108–14.
Eisenhauer N, Scheu S, Jousset A. Bacterial diversity stabilizes community productivity. PLoS ONE. 2012;7:e34517.
Wei Z, Yang T, Friman VP, Xu Y, Shen Q, Jousset A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat Comm. 2015;6:8413.
Amalfitano S, Coci M, Corno G, Luna GM. A microbial perspective on biological invasions in aquatic ecosystems. Hydrobiologia. 2015;746:13–22.
Li SP, Tan J, Yang X, Ma C, Jiang L. Niche and fitness differences determine invasion success and impact in laboratory bacterial communities. ISME J. 2019;13:402–12.
Baumgartner M, Pfrunder-Cardozo KR, Hall AR. Microbial community composition interacts with local abiotic conditions to drive colonization resistance in human gut microbiome samples. Proc R Soc B Biol Sci. 2021;288:20203106.
Kurkjian HM, Akbari MJ, Momeni B. The impact of interactions on invasion and colonization resistance in microbial communities. PLoS Comp Biol. 2021;17:e1008643.
Tilman D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA. 2004;101:10854–61.
van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci USA. 2012;109:1159–64.
Foster KR, Bell T. Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol. 2012;22:1845–50.
Mitri S, Foster KR. The genotypic view of social interactions in microbial communities. Annu Rev Microbiol. 2013;43:247–73.
Kehe J, Ortiz A, Kulesa A, Gore J, Blainey PC, Friedman J. Positive interactions are common among culturable bacteria. Sci Adv. 2021;7:7159.
Connell JH, Slatyer RO. Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat. 1977;111:1119–44.
Debray R, Socolar Y, Kaulbach G, Guzman A, Hernandez CA, Curley R, et al. Priority effects in microbiome assembly. Nat Rev Microbiol. 2022;20:109–21.
Datta MS, Sliwerska E, Gore J, Polz MF, Cordero OX. Microbial interactions lead to rapid micro-scale successions on model marine particles. Nat Commun. 2016;7:11965.
Enke TN, Datta MS, Schwartzman J, Cermak N, Schmitz D, Barrere J, et al. Modular assembly of polysaccharide-degrading marine microbial communities. Curr Biol. 2019;29:1528–35.e6.
Mazumdar V, Amar S, Segrè D. Metabolic proximity in the order of colonization of a microbial community. PLoS ONE. 2013;8:e77617.
Gralka M, Szabo R, Stocker R, Cordero OX. Trophic interactions and the drivers of microbial community assembly. Curr Biol. 2020;30:R1176–88.
Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11:94–100.
Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell–cell distance. Nat Rev Microbiol. 2010;8:471–80.
Monier JM, Lindow SE. Aggregates of resident bacteria facilitate survival of immigrant bacteria on leaf surfaces. Micro Ecol. 2005;49:343–52.
Poza-Carrion C, Suslow T, Lindow S. Resident bacteria on leaves enhance survival of immigrant cells of Salmonella enterica. Phytopathology. 2013;103:341–51.
Li M, Wei Z, Wang J, Jousset A, Friman V, Xu Y, et al. Facilitation promotes invasions in plant-associated microbial communities. Ecol Lett. 2019;22:149–58.
Estrela S, Vila JCC, Lu N, Bajić D, Rebolleda-Gómez M, Chang CY, et al. Functional attractors in microbial community assembly. Cell Syst. 2022;13:29–42.e7.
Bertness MD, Callaway R. Positive interactions in communities. Trends Ecol Evol. 1994;9:191–3.
Zaneveld JR, McMinds R, Thurber RV. Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nat Microbiol. 2017;2:1–8.
de Vries FT, Griffiths RI, Bailey M, Craig H, Girlanda M, Gweon HS, et al. Soil bacterial networks are less stable under drought than fungal networks. Nat Commun. 2018;9:3033.
Goldford JE, Lu N, Bajić D, Estrela S, Tikhonov M, Sanchez-Gorostiaga A, et al. Emergent simplicity in microbial community assembly. Science. 2018;361:469–74.
Nguyen LTT, Broughton K, Osanai Y, Anderson IC, Bange MP, Tissue DT, et al. Effects of elevated temperature and elevated CO2 on soil nitrification and ammonia-oxidizing microbial communities in field-grown crop. Sci Total Environ. 2019;675:81–9.
Piccardi P, Vessman B, Mitri S. Toxicity drives facilitation between 4 bacterial species. Proc Natl Acad Sci USA. 2019;116:15979–84.
Hernandez DJ, David AS, Menges ES, Searcy CA, Afkhami ME. Environmental stress destabilizes microbial networks. ISME J. 2021;15:1722–34.
Urban MC, De Meester L. Community monopolization: Local adaptation enhances priority effects in an evolving metacommunity. Proc R Soc B Biol Sci. 2009;276:4129–38.
Vanoverbeke J, Urban MC, De Meester L. Community assembly is a race between immigration and adaptation: eco-evolutionary interactions across spatial scales. Ecography. 2016;39:858–70.
De Meester L, Vanoverbeke J, Kilsdonk LJ, Urban MC. Evolving perspectives on monopolization and priority effects. Trends Ecol Evol. 2016;31:136–46.
Loeuille N, Leibold MA. Evolution in metacommunities: on the relative importance of species sorting and monopolization in structuring communities. Am Nat. 2008;171:788–99.
Nadeau CP, Farkas TE, Makkay AM, Papke RT, Urban MC. Adaptation reduces competitive dominance and alters community assembly. Proc R Soc B Biol Sci. 2021;288:20203133.
Faillace CA, Morin PJ. Evolution alters the consequences of invasions in experimental communities. Nat Ecol Evol. 2017;1:0013.
Castledine M, Sierocinski P, Padfield D, Buckling A. Community coalescence: an eco-evolutionary perspective. Philos Trans R Soc B Biol Sci. 2020;375:20190252.
Amor DR, Ratzke C, Gore J. Transient invaders can induce shifts between alternative stable states of microbial communities. Sci Adv. 2020;6:eaay8676.
van der Gast CJ, Thompson IP. Effects of pH amendment on metal working fluid wastewater biological treatment using a defined bacterial consortium. Biotechnol Bioeng. 2005;89:357–66.
Saha R, Donofrio RS. The microbiology of metalworking fluids. Appl Microbiol Biotechnol. 2012;94:1119–30.
Ratzke C, Barrere J, Gore J. Strength of species interactions determines biodiversity and stability in microbial communities. Nat Ecol Evol. 2020;4:376–83.
Estrela S, Libby E, Van Cleve J, Débarre F, Deforet M, Harcombe WR, et al. Environmentally mediated social eilemmas. Trends Ecol Evol. 2019;34:6–18.
Enke TN, Leventhal GE, Metzger M, Saavedra JT, Cordero OX. Microscale ecology regulates particulate organic matter turnover in model marine microbial communities. Nat Commun. 2018;9:2743.
Furman O, Shenhav L, Sasson G, Kokou F, Honig H, Jacoby S, et al. Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat Commun. 2020;11:1–13.
Coyte KZ, Rao C, Rakoff-Nahoum S, Foster KR. Ecological rules for the assembly of microbiome communities. PLoS Biol. 2021;19:e3001116.
Molinero N, Ruiz L, Sánchez B, Margolles A, Delgado S. Intestinal bacteria interplay with bile and cholesterol metabolism: implications on host physiology. Front Physiol. 2019;10:185.
Ruiz L, Margolles A, Sánchez B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol. 2013;4:396.
Gérard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens. 2013;3:14–24.
Amarnath K, Narla AV, Pontrelli S, Dong J, Caglar T, Taylor BR, et al. Stress-induced cross-feeding of internal metabolites provides a dynamic mechanism of microbial cooperation. bioRxiv. 2021. https://doi.org/10.1101/2021.06.24.449802.
Fukami T, Beaumont HJ, Zhang XX, Rainey PB. Immigration history controls diversification in experimental adaptive radiation. Nature. 2007;446:436–9.
Bell T, Newman JA, Silverman BW, Turner SL, Lilley AK. The contribution of species richness and composition to bacterial services. Nature. 2005;436:1157–60.
Ghoul M, Mitri S. The ecology and evolution of microbial competition. Trends Microbiol. 2016;24:833–45.
Fukami T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Syst. 2015;46:1–23.
Acknowledgements
We thank Oliver Meacock, Shota Shibasaki, and three anonymous reviewers for useful and constructive feedback on the manuscript. We thank Christopher van der Gast and Ian Thompson for providing the four species used to assemble the resident community and Peter Küenzi for providing the four additional species used as invaders. We thank Flor-Inés Arias-Sánchez for designing the selective media. PP and GA were funded by the University of Lausanne, SM by European Research Council Starting Grant 715097. JMA received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 678841.
Funding
Open access funding provided by University of Lausanne.
Author information
Authors and Affiliations
Contributions
PP, GA, JMA and SM designed research. GA and PP performed the experiments. PP, GA, JMA and SM analyzed the data. PP and SM wrote the manuscript with input from GA and JMA.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piccardi, P., Alberti, G., Alexander, J.M. et al. Microbial invasion of a toxic medium is facilitated by a resident community but inhibited as the community co-evolves. ISME J 16, 2644–2652 (2022). https://doi.org/10.1038/s41396-022-01314-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-022-01314-8
This article is cited by
-
Transitivity and intransitivity in soil bacterial networks
The ISME Journal (2023)
-
Within-host adaptation alters priority effects within the tomato phyllosphere microbiome
Nature Ecology & Evolution (2023)