Abstract
Little is known about the cell physiology of anammox bacteria growing at extremely low growth rates. Here, “Candidatus Brocadia sinica” and “Candidatus Scalindua sp.” were grown in continuous anaerobic membrane bioreactors (MBRs) with complete biomass retention to determine maintenance energy (i.e., power) requirements at near-zero growth rates. After prolonged retentostat cultivations, the specific growth rates (μ) of “Ca. B. sinica” and “Ca. Scalindua sp.” decreased to 0.000023 h−1 (doubling time of 1255 days) and 0.000157 h−1 (184 days), respectively. Under these near-zero growth conditions, substrate was continuously utilized to meet maintenance energy demands (me) of 6.7 ± 0.7 and 4.3 ± 0.7 kJ mole of biomass-C−1 h−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, which accorded with the theoretically predicted values of all anaerobic microorganisms (9.7 and 4.4 kJ mole of biomass-C−1 h−1at 37 °C and 28 °C, respectively). These me values correspond to 13.4 × 10−15 and 8.6 × 10−15 watts cell−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, which were five orders of magnitude higher than the basal power limit for natural settings (1.9 × 10−19 watts cells−1). Furthermore, the minimum substrate concentrations required for growth (Smin) were calculated to be 3.69 ± 0.21 and 0.09 ± 0.05 μM NO2− for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively. These results match the evidence that “Ca. Scalindua sp.” with lower maintenance power requirement and Smin are better adapted to energy-limited natural environments than “Ca. B. sinica”, suggesting the importance of these parameters on ecological niche differentiation in natural environments.
Similar content being viewed by others
Introduction
The anaerobic ammonium-oxidation (anammox) reaction has been utilized as an alternative energy-saving biological process for nitrogen removal from various wastewaters due to lower oxygen requirement, no external carbon source demand, and less sludge production. At present, more than 100 full-scale treatment plants have been in operation around the world [1, 2]. Anammox-based treatment processes are generally suitable for NH4+-rich wastewaters with low C/N ratio (<3.0) such as digester liquor and high-strength industrial wastewaters [3, 4]. Therefore, studies on microbial physiology and growth kinetics of anammox bacteria have been exclusively performed in batch and/or continuous cultures with ample substrates (NH4+ and NO2−) supply. Under these nutrient-rich conditions, the potential growth abilities of anammox bacteria like maximum specific growth rates (μmax) and maximum specific substrate utilization rates (qmax) have been widely reported [5, 6].
In contrast, little is known about cell physiology of anammox bacteria in oligotrophic natural environments, although anammox bacteria have been universally detected in natural freshwater [7, 8], marine [9,10,11,12], and terrestrial [13, 14] environments and known to be playing an important role in the global nitrogen cycle [15, 16]. In such natural habitats, the growth rates are expected to be extremely low due to severe substrate limitation. Studying cell physiology at such extremely low (near-zero) growth rates is important to understand their survival (maintenance of viability) strategies in natural environments. For successful survival, microorganisms must preferably possess high affinity to the limiting substrate (lower Km values), fast growth rates (higher μmax), and efficient substrate utilization rates. More importantly, maintenance energy (i.e., power) requirement and/or decay rate should be as low as possible [17, 18]. The maintenance energy is generally defined as the energy required for non-growth related processes to sustain the viability of cells (e.g., cell motility, osmoregulation, turnover of macromolecules such as enzymes and RNA, repair of proteins, molecular transport, and so on) other than new biomass synthesis [17, 19]. Therefore, it can be assumed that a relatively large fraction of energy is diverted to maintenance-related cellular processes at near-zero growth rates in energy-limited natural environments. Although a few studies have reported the decay rates of anammox activity (which is not endogenous decay) in batch starvation experiments [20,21,22,23], the maintenance power requirements of anammox bacteria have never been determined yet. The endogenous decay is defined as a reduction of active biomass directly coupled to a consumption of electron acceptor under starved conditions [23, 24], and thus is clearly different from the maintenance power requirement.
Most of the laboratory studies for the determination of maintenance power have been performed using chemostats. A retentostat with complete biomass retention was used to quantify the maintenance power requirements for autotrophic Nitrosomonas europaea and Nitrobacter winogradski [25]. Under the prolonged retentostat cultivation with a constant substrate supply, biomass accumulates to a certain degree, which gradually decreases the substrate availability per cell to a minimum power requirement only for maintenance [17, 26]. Under this condition, microorganisms can no longer grow but consume substrate to maintain themselves, implying the establishment of near-zero growth rates. Therefore, more environmentally relevant maintenance power requirements can be determined from the retentostat cultivation.
Anaerobic membrane bioreactors (MBRs) with the same reactor concept as the retentostat have been used to enrich slow-growing anammox bacteria and determine their physiology and kinetic parameters [27,28,29,30], because the MBR allows the cultivation of free-living planktonic cells at environmentally relevant low substrate concentrations [31,32,33]. However, anaerobic MBRs have never been used to measure the maintenance power requirements of anammox bacteria.
In the present study, “Ca. Brocadia sinica” (commonly found in the substrate-rich wastewater treatment processes) [29] and “Ca. Scalindua sp.” (commonly found in substrate-limiting oceanic environments) [34] were continuously cultivated in anaerobic MBRs with a constant substrate loading rate for long periods to determine the maintenance power requirements at near-zero growth rates. The results revealed that “Ca. B. sinica” exhibited 1.6 times higher maintenance power requirement than “Ca. Scalindua sp.”, which can explain their ecological niche differentiation.
Materials and methods
Anaerobic membrane bioreactor (MBR)
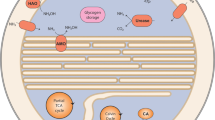
Two identical anaerobic membrane bioreactors (MBRs) were constructed and operated for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively as previously described [30, 33] (Fig. 1A). Briefly, a 2-L wide mouth reagent bottle (Sanyo, Japan) was used as the culture vessel (a working volume of 1.5 L). To completely retain biomass, a membrane module composed of 300 polyethylene hollow fiber membranes (pore size, 0.1 μm; tube diameter, 1 mm; length, 70 mm) was installed in the bottle. The membrane module was directly connected to a peristaltic pump (MP-1000, EYELA, Tokyo, Japan) which was actuated by a water level sensor (HL-S1A, ASONE, Japan) to maintain a constant water level in the MBR.
Biomass was completely retained in the reactor by polyethylene hollow fiber membranes (pore size = 0.1 μm) (A). A N2 purged gas bag was connected to the inorganic medium tank (10 L) to avoid O2 contamination. Confocal laser scanning microscope images of FISH analysis for “Ca. B. sinica” (B) and “Ca. S. japonica” (C), showing highly enriched and well-dispersed planktonic form of cultures. FISH analysis was performed with FITC-labeled EUB mix probe composed of equimolar EUB338, EUB338II, and EUB338III (green) for most members of Eubacteria and TRITC-labeled amx820 probe (red) for anammox bacteria. Anammox bacteria were shown in yellow. Scale bars represent 20 μm.
MBR cultivation
Planktonic “Ca. B. sinica” and “Ca. Scalindua sp.” biomass (initial concentration; 0.02–0.04 g-protein L−1), which have been maintained in our laboratory [33,34,35], were inoculated into respective MBRs. The mineral medium was continuously fed into the MBRs at a hydraulic retention time (HRT) of 1 d. The mineral medium for “Ca. B. sinica” contained (mg L−1): (NH4)2SO4, (variable), NaNO2 (variable), FeSO4 ∙ 7H2O (9), EDTA ∙ 2Na (5.0), NaCl (1.0), KCl (1.4), CaCl2 ∙ 2H2O (1.4), MgSO4 ∙ 7H2O (1.0), NaHCO3 (84), KH2PO4 (54) and 0.5 mL L−1 trace elements solution II [36]. The inorganic nutrient medium for “Ca. Scalindua sp.” contained (mg L−1): (NH4)2SO4, (variable), NaNO2 (variable), CaCl2 ∙ 2H2O (114), MgSO4 ∙ 7H2O (99), KHCO3 (500), KH2PO4 (24.4), 0.5 mL L−1 trace element solution I & II [36] and 25 g L−1 of SEALIFE (Marine Tech, Tokyo, Japan) as an artificial sea salt [37].
To promote the biomass growth, the concentrations of NH4+ and NO2− were gradually increased from 1.86 mM to 18.6 mM, which corresponds to nitrogen loading rates (NLRs) of 0.18–1.78 mmol (NH4+ + NO2−) L−1 h−1. In order to attain the near-zero growth rate, the NLR was fixed at the point when enough active biomass was obtained, which was around 20 days after the beginning of operation. This time is defined as the start of retentostat cultivation. Retentostat cultivation experiment was performed in duplicate to confirm the reproducibility for each anammox bacterium. Since equimolar amounts of NH4+ and NO2− were supplied, NO2− was a limiting substrate in all experiments. The MBR culture was continuously stirred with a magnetic stirrer to maintain homogeneous planktonic biomass. The culture was continuously purged with a mixed Ar and CO2 (95:5) gas to prevent oxygen contamination. The MBRs of “Ca. B. sinica” and “Ca. Scalindua sp.” were operated at their optimal temperature (37 °C and 28 °C, respectively). Sludge retention time (SRT) was not controlled. pH was also not controlled, but it was relatively stable throughout the cultivation ranging 7.6–7.8 for “Ca. B. sinica” culture and 7.8–8.0 for “Ca. Scalindua sp.” culture, respectively.
Chemical analyses
Influent and effluent (permeate) samples were collected from MBRs to determine NH4+, NO2− and NO3− concentrations. NH4+ and NO2− concentrations were determined by the colorimetric method with the indophenol and hypochlorous acid [38] and the N-(1-Naphthyl)ethylene diamine [39], respectively, after the samples were filtered through 0.2 μm pore membranes (Advantec, Tokyo, Japan). NO3- concentration was analyzed using ion-exchange chromatography (IC-2010, TOSOH, Tokyo, Japan) with an TSKgel IC-Anion HS column (TOSOH) [40].
For measurement of biomass concentration (Cx,total), MBR culture (2 mL) was withdrawn and centrifuged at 12,000 rpm for 5 min. Harvested cell pellet was mixed with 1% (w/v) SDS solution, and total biomass protein was extracted at 99 °C for 15 min. Extracted protein was collected as a supernatant after centrifugation at 16,400 rpm for 15 min and stored at −80 °C until measurement. Protein concentration was measured by the DC protein assay kit (Bio-Rad, USA) using the bovine serum albumin (BSA) as the protein standard.
Cell viability of anammox bacteria
The viability of anammox bacteria in MBR cultures was determined based on the live/dead staining using the LIVE/DEAD™ BacLight™ Bacterial Viability Kit assay (L-7012, Invitrogen™, USA). MBR cell cultures (2 mL) were collected, washed twice with the N2 purged mineral medium without ammonium and nitrite, and mixed with 3 μL of stain solution containing equal volumes of SYTO® 9 dye (green) and propidium iodide (PI, red) solution. The stained cell samples were analyzed by an epi-fluorescence microscope (Axiovert 200, Carl Zeiss Microscopy) equipped with the Filter Set 09 (Excitation BP 450-490, Beam Splitter FT510, Emission LP515, Carl Zeiss Microscopy). The numbers of green and red stained cells were manually counted for more than ten samples, and the average ratios (viability) were calculated. The percentage of the anammox bacteria in the MBR culture (total bacteria) was determined based on fluorescence in situ hybridization (FISH) analysis [41]. FISH analysis was performed with FITC-labeled EUB mix probe composed of equimolar EUB338, EUB338II, and EUB338III (green) for most members of Eubacteria and TRITC-labeled AMX820 probe (red) for anammox bacteria as previously described [42]. The hybridized cells were observed with a confocal laser scanning microscope (TCS SP8, Leica, Japan) equipped with Ar ion and He-Ne laser. The both probes-hybridized anammox bacterial cells (yellow) and other bacterial cells (green) were directly counted at least 500 cells in randomly chosen 10 microscopic fields of slides prepared for each sample, and the average percentages of anammox bacteria were calculated. The measurements of 16S rRNA gene copy numbers with quantitative PCR confirmed that “Ca. B. sinica” and “Ca. Scalindua sp.” accounted for more than 99% of the anammox population in respective MBR cultures [35]. Therefore, the measured biomass concentration (Cx,total, measured as protein concentration; g-protein L−1) was converted to the concentrations of viable anammox bacteria (CAMX,viable) by multiplying biomass concentration (Cx,total), the viability (%) determined from the LIVE/DEAD assay, and the percentage of anammox bacteria from the FISH analysis:
Hereafter, for convenience of explanation, biomass concentration (Cx) refers to the concentrations of viable anammox bacteria (CAMX,viable).
Maintenance coefficient (m s)
The substrate is practically utilized for both growth and maintenance process. Microbial maintenance has been described in different ways with different assumptions, which was critically reviewed by van Bodegom [17]. When the substrate utilization rate for maintenance process is assumed to be constant and independent of the growth rate, it is denoted as maintenance coefficient (ms) and can be described as follows [43, 44]:
where qs is the specific substrate consumption rate (mmol NH4+ g-protein−1 h−1), μ is the specific growth rate (h−1), ms is maintenance coefficient (mmol NH4+ g-protein−1 h−1) and Ymax is the maximum biomass growth yield (g-protein (mol NH4+)−1). According to Eq. (2), ms and Ymax can be determined by plotting the values of qs against μ, whose relation is linear [44]. Thus, the y-axis intercept determines ms, and the reciprocal of the slope of the regression line represents Ymax. If μ were zero, then qs becomes equal to ms. Thus, ms can be also directly determined from the MBR retentostat cultivation experiments in which the zero growth rate is achieved. The following relationship between Ymax and ms can be obtained from Eq. (2).
Y is the apparent (observed) growth yield (g-protein (mol NH4+)−1) with consideration of substrate consumption for both growth and maintenance. This relationship indicates that Y becomes equal to Ymax when μ is zero.
Specific maintenance rate (a)
When it is assumed that microbial maintenance directly influences the growth rate, Eq. (S1) can be modified as follows:
where Cx is the biomass concentration (g-protein L−1), Cs is the residual ammonium or nitrite concentration in MBR (mM), a is the specific maintenance rate (h−1), which was originally termed as “endogenous metabolism” [45]. Thus, this is analogous to the decay rate (expressed as a negative growth rate) and can be linked to ms as follows:[17]
The a actually includes relative decay (death) rate when it is experimentally determined from biomass and substrate mass balances [17].
Minimum substrate concentration (S min)
Furthermore, a minimum substrate concentration reflecting maintenance metabolism (Smin), at which the net bacterial growth is zero, can be proposed as follows: [17]
According to Eq. (6). and (7), the values of a and Smin can be calculated by substituting the values of ms and Ymax, which were determined in the MBR retentostat cultivation experiments in this study. Ks is a half-saturation constant of limiting substrate (μM).
Estimation of specific growth rate in MBR cultivation
For steady state conditions (i.e., dCs/dt = 0), the biomass accumulation during retentostat cultivation can be simulated using the following van Verseveld equation (46), which is derived from Eq. (S1–S3 in the Supplemental Information) and Eq. (2).
It should be noted that Cx (g-protein L−1) refers to the concentration of viable anammox bacteria (CAMX,viable) as mentioned above (Eq. (1)). In order to determine the specific growth rate (μ) using Eq. (S1), Cx was fitted with the van Verseveld equation to determine the derivative (dCx/dt) of Eq. (S1) [46]. Actually, the fitting was done with Cx = A · e B· t + C, which is the same shape as Eq (8), using the R software (R Foundation for Statistical Computing, Vienna) and by minimizing the sum of squares of errors by varying A, B, and C [19].
Results and discussion
MBR (retentostat) cultivation
Free-living planktonic cells of enriched “Ca. B. sinica” and “Ca. Scalindua sp.” were continuously cultivated in the MBRs (retentostats) with complete biomass retention to quantify the maintenance coefficients (ms) (Fig. 1A–C). Two independent MBR retentostat cultivation experiments were performed for 64 and 168 days (34 and 144 days in the retentostat mode with a constant nitrogen (NH4+ + NO2−) loading rate (NLR)) for “Ca. B. sinica” and 83 and 120 days (62 and 100 days) for “Ca. Scalindua sp.”, respectively to confirm the reproducibility. In the second experiments, the wider ranges of specific growth rates were examined by extending the length of retentostat cultivations. Thus, the results of the second runs were shown in the main text, whereas ones of the first runs can be seen in the Supplemental Information (Fig. S1A, B).
Nitrogen loading rate (NLR) was gradually increased by increasing the influent NH4+ and NO2− concentrations to promote the growth of “Ca. B. sinica” and “Ca. Scalindua sp.” in MRBs (Fig. 2A, B). After about 20 days, the NLRs were fixed at ca. 1.2–1.6 mmol (NH4+ + NO2-) L−1 h−1, and then the MBRs were further operated for 144 days for “Ca. B. sinica” and 100 days for “Ca. Scalindua sp.”, respectively. Nitrogen removal ratios of both MBRs were 87.6 ± 1.8% for “Ca. B. sinica” and 86.7 ± 1.8% for “Ca. Scalindua sp.”, respectively. The permeate NO2- concentrations were always close to zero μM, except for abnormally high values caused by oxygen inhibition due to the shortage of Ar/CO2 gas supply.
Performance of”Ca. B. sinica” MBR (A) and “Ca. Scalindua sp.” MBR (B) during 168 days and 120 days continuous operation, respectively. Orange lines, blue areas, red dots, and black dots represent the nitrogen (NH4+ and NO2−) loading rate (NLR), nitrogen (NH4+ and NO2−) removal rate (NRR), NH4+ concentration and NO2− concentration in the MBRs, respectively. The unexpected oxygen contamination resulted in sudden increases in nitrite concentration. Data of the second experiment (Run-2) were shown.
In the “Ca. B. sinica” MBR cultures, the stoichiometric ratio of consumed nitrite and ammonium (ΔNO2−/ΔNH4+) was 1.10 ± 0.07 for the first run and 1.20 ± 0.08 for the second run, respectively (Fig. S2A). In addition, the ratio of produced nitrate and consumed ammonium (ΔNO3−/ΔNH4+) was 0.16 ± 0.02 for the first run and 0.14 ± 0.02 for the second run, respectively. In the “Ca. Scalindua sp.” MBR cultures, the ΔNO2−/ΔNH4+ ratio was 1.13 ± 0.03 for the first run and 1.19 ± 0.08 for the second run, respectively (Fig. S2B). Furthermore, the ΔNO3−/ΔNH4+ ratio was 0.12 ± 0.02 for the first run and 0.11 ± 0.03 for the second run, respectively. These values are very close to the proposed stoichiometric ratios of anammox process (1.15 and 0.16, respectively) [47], which indicates that nitrogen conversions observed in both MBRs were highly attributed to anammox bacteria.
Biomass accumulation in MBRs
“Ca. B. sinica” biomass progressively accumulated in the MBR and gradually levelled off after NLR was fixed at ca. 1.2–1.6 mmol (NH4+ + NO2−) L−1 h−1 (Fig. 3A), whereas “Ca. Scalindua sp.” biomass steadily increased even after 100 days (Fig. 3B). It is expected that the complete biomass retention in MBR resulted in accumulation of non-viable cells upon prolonged retentostat cultivation, which do not consume substrate either for growth and maintenance. Therefore, the viability of cultured biomass was determined with the LIVE/DEAD™ BacLight™ Bacterial Viability Kit. The results revealed that the viabilities of both species were stable and high throughout the retentostat cultivations. The average viabilities of “Ca. B. sinica” cultures were 90.3 ± 5.7% for the first run and 88.6 ± 5.7% for the second run, respectively. Similarly, the average viabilities of “Ca. Scalindua sp.” cultures were 84.0 ± 7.2% for the first run and 83.3 ± 7.9% for the second run, respectively. The slightly lower viability of “Ca. Scalindua sp.” cultures might indicate that non-viable biomass accumulated to some extent. This partly explains the continuous increase of “Ca. Scalindua sp.” biomass even after 100 days of cultivation (Fig. 3B). Furthermore, FISH analyses revealed that “Ca. B. sinica” made up 87.5 ± 4.0% and 86.8 ± 6.8% of total biomass in the first and second run, respectively. Similarly, “Ca. Scalindua sp.” made up 85.0 ± 3.8% and 87.1 ± 1.9% of total biomass in the first and second run, respectively. Then viable biomass concentrations of respective anammox bacteria were calculated using Eq. (1). Hereafter, the biomass denotes the viable biomass concentrations of respective anammox bacteria.
Biomass accumulations of “Ca. B. sinica” (A) and “Ca. Scalindua sp.” (B) in MBRs after the NLR was fixed at ca. 1.2–1.6 mmol (NH4+ + NO2−) L−1 h−1 (operation day 24 and 20 for “Ca. B. sinica” and “Ca. Scalindua sp.” in Fig. 2 were defined as time zero in this Figure). Black dots indicate the measured biomass concentration (g-protein L−1). The broken lines are biomass concentrations fitted with the van Verseveld equation (Eq. (8)). Specific growth rate, μ (h−1), was calculated from the fitted biomass concentration (green line). At the end of retentostat cultivation, μ of”Ca. B. sinica” and “Ca. Scalindua sp.” approached near-zero.
The specific growth rates (μ) were estimated based on the fitted biomass accumulation curves (see “Materials and methods”) (Fig. 3A, B). They asymptotically decreased and approached near-zero. At the end of the retentostat cultivation, μ was estimated to be 0.000023 h−1 at 144 day for “Ca. B. sinica” and 0.000157 h−1 at 100 day for “Ca. Scalindua sp.”, corresponding to a doubling time of 1255 days and 184 days, respectively. Apparently, this implies that the near-zero growing cultures (defined as μ is less than 0.00019 h−1, corresponding the doubling time of more than 150 days in this study) were obtained by decreasing the substrate availability per amount of biomass. However, it should be noted that these bacterial cultures were not starved, because the substrates were continuously supplied and consumed. The similar results as those shown in Fig. 3 were observed in the first MBR runs (Fig. S3A, B).
Maintenance coefficient (m s)
The specific NH4+ consumption rates (qs) of “Ca. B. sinica” and “Ca. Scalindua sp.” are plotted against the cultivation time (t) (Fig. 4A, Fig. S4A, B). Both qs values decreased and approached asymptotes at near-zero growth rates, which are specific substrate (NH4+) utilization rates for only maintenance-related processes and known as the maintenance coefficient (ms: mmol-NH4+ g-protein−1 h−1) [17, 19, 43]. The average asymptotic qs value, that is, ms was directly determined at the near-zero growth rates (the doubling time was > 150 days) to be 1.26 ± 0.06 mmol-NH4+ g-protein−1 h−1 for “Ca. B. sinica” and 0.93 ± 0.11 mmol-NH4+ g-protein−1 h−1 for “Ca. Scalindua sp.”, respectively (Fig. 4A). According to Eq. (2)., the maintenance coefficient (ms) can be also extrapolated by plotting the qs against the estimated specific growth rate (μ) (Fig. 4B). Linear regression lines with an R2 of >0.99 and >0.99 were obtained for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively. The Y-intercept gives ms (qs = ms at μ = 0), which was found to be 1.27 and 0.83 mmol-NH4+ g-protein−1 h−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively (Table 1). These values correspond well to those directly determined from the qs – t relationship (Fig. 4A). To the best of our knowledge, this is the first experimental data on the maintenance coefficients for anammox bacteria.
Specific NH4+ consumption rates (qs) in MBR culture of “Ca. B. sinica” (filled circle) and “Ca. Scalindua sp.” (open circle), which asymptotically approached the maintenance coefficient (ms) during prolonged retentostat cultivation (A). Relationship between specific NH4+ consumption rate (qs) and specific growth rate (μ) of”Ca. B. sinica” (filled circle) and “Ca. Scalindua sp.” (open circle), respectively (B). Y-axis intersect of the linear regression line determines the maintenance rate (ms). A reciprocal of the slope of the regression line is the maximum biomass yield (Ymax).
Maximum biomass growth yield (Y max)
The Ymax represented by 1/slope of the regression lines (Eq. 2) were found to be 1.01 and 1.02 g-protein (mol-NH4+)−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively (Fig. 4B) (Table 1). According to Eq. (3), Ymax becomes equal to the apparent (observed) growth yield (Y) with consideration of substrate consumption for both growth and maintenance when specific growth rate (μ) is zero. Assuming the protein content of biomass is 0.60 g-protein (g-biomass dry weight)−1 [29] and 1 mole of biomass-C equals to 24.6 g dry biomass [48], the extrapolated Ymax can be converted to 0.068 mol-biomass-C (mol of NH4+)−1 for “Ca. B. sinica” and 0.069 mol-biomass-C (mol of NH4+)−1 for “Ca. Scalindua sp.”, respectively (Table 1). The independent duplicate MBR runs showed a reproducible result for “Ca. B. sinica” but slightly higher Ymax for “Ca. Scalindua sp.” probably due to the accumulation of non-viable biomass to some extent (Fig. S5A, B). These Ymax values were very close to the reported Y values for “Ca. B. sinica” (0.063 mol-biomass-C (mol of NH4+)−1) [29], one for “Ca. Jettenia caeni” (0.056 mol-biomass-C (mol of NH4+)−1) [27], and one for “Ca. Kuenenia stuttgartiensis” (0.066 mol-biomass-C (mol of NH4+)−1) [49], but higher than one for “Ca. Scalindua sp.” (0.030 mol-biomass-C (mol of NH4+)−1 at 28 °C and 2.8% salinity) [34, 50]. The Y values of “Ca. Scalindua sp.” were highly dependent on salinity and in the range 0.010–0.019 mol-biomass-C (mol of NH4+)−1 under low salinity conditions (≤1%) [50]. The maximum biomass yields Ymax of anammox bacteria obtained in this study are also similar to the observed Y values for chemolithotrophic growth of aerobic ammonium-oxidizing bacteria (AOB) (0.034–0.082 mol-biomass-C (mol of NH4+)−1) and nitrite-oxidizing bacteria (NOB) (0.020 mol-biomass-C (mol of NO2−)−1) [25, 51, 52].
Specific maintenance (decay) rate (a)
Based on Eq. (6). with the obtained ms and Ymax, the specific maintenance rates (a) were calculated to be 0.0014 ± 0.0001 h−1 and 0.0012 ± 0.0005 h−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively (Table 1). These values were one order of magnitude higher than the death rate (0.00046 ± 0.00004 h−1, defined as a decrease rate of amount of viable bacteria under anaerobic starvation conditions) reported by Wang et al. [23].
Retentostat vs. chemostat cultures
In general, the maintenance coefficients (ms) determined in chemostat cultures were higher than those obtained from the retentostat cultures [25, 53, 54]. This is because ms is conventionally determined by mathematically extrapolating the Y-intercept of the qs – μ linear relationships like Fig. 4B. In chemostat studies, minimum growth is required to compensate the continuous overflow of biomass; thus a qs value cannot be reliably deduced at near-zero growth rate [55]. In addition, chemostat operation at dilution rates of < 0.05 h−1 causes heterogeneous distribution of nutrient and biomass culture in space and time such as biomass flocculation and internal wall growth [53, 56]. Thus, the maintenance coefficients derived from chemostat cultures generally reflect the maintenance energy requirement at relatively high metabolic (growth) rates, which differ widely from physiological state in energy-limited natural ecosystems [53, 55]. Many cellular functions are dependent on metabolic (growth) rates [30, 57]. In general, the maintenance energy requirements for growing bacteria were higher than those for non-growing bacteria [17].
In retentostat cultures, near-zero growth can be achieved (Fig. 3) and thus only the most essential functions are maintained such as the repair of proteins and RNA components [30, 57]. Thus, the retentostat cultivation enables more accurate assessment of the growth rate independent maintenance energy requirement. In the present retentostat study, the ms values deduced from the qs – μ linear relationships (Fig. 4B) were almost identical with those directly determined from the qs – t relationship (Fig. 4A). This is because only qs values at μ < 0.01 h−1 for “Ca. B. sinica” and μ < 0.005 h−1 for “Ca. Scalindua sp.” were used to extrapolate the qs values at μ = 0 (Fig. 3A, B), which are low enough to assume to be “zero growth”. Thus, the retentostat study provides insights into more realistic cell physiology at near-zero growth rates (i.e., natural environments).
Gibbs energy-based maintenance energy requirements (m e)
The maintenance coefficients (ms) can be converted to Gibbs energy equivalent as follows [48]:
me is the biomass specific Gibbs free energy consumption for maintenance (kJ mole of biomass-C−1 h−1) and ΔGr is the available Gibbs free energy for anammox reaction (NH4+ + NO2− → N2 + H2O) in a MBR system. In this study, the values of ΔGr in the MBR system was calculated from the following Eqs. (10) and (11)
where ΔGr0 is the standard Gibbs free energy of the anammox reaction at respective cultivation temperatures (i.e., 37 °C for “Ca. B. sinica” or 28 °C for “Ca. Scalindua sp.”, respectively), which are calculated from the standard Gibbs energies of every reactants and products [58,59,60]. R is the gas constant, T is temperature (K), and a is activity [59]. The activity of species i (ai) can be related to the measured concentration of i (Ci) as expressed by Eq. (11), where Ci0 represents the standard state concentration (1 M), and γi is the activity coefficient (unitless), which is a function of ionic strength and temperature [59]. The ionic strength of culture medium was ~0.1 M for “Ca. B. sinica” and 0.7 M for “Ca. Scalindua sp.”, respectively. The actually measured concentrations of NH4+, NO2− and N2 during near-zero growth (the doubling time was >150 days) were used to determine the respective activities. Since the MBR culture was continuously purged with a mixed Ar and CO2 (95:5%) gas to prevent oxygen contamination, PN2 was expected to be negligibly low (assuming <10−4 Pa). To determine me, the obtained ms, in mmole of NH4+ per g-protein per hour, was first converted to mD in mole of NH4+ per mole of biomass-C per hour (Table 2). Assuming the protein content of biomass is 0.6 g-protein (g-dry biomass)−1 [29] and 1 mole of biomass-C = 24.6 g dry biomass [48], mD was calculated to be 0.020 and 0.013 mole of NH4+ mole of biomass-C−1 h−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively. Thus, the me can be calculated to be 6.7 and 4.3 kJ mole of biomass-C−1 h−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively (Table 2). “Ca. B. sinica” was cultured at 37 °C and ≈ 0% salinity, whereas “Ca. Scalindua sp.” was cultured at 28 °C and 2.5% salinity. The higher me value for “Ca. B. sinica” could be attributed to the higher culture temperature even though the higher salinity medium was used for “Ca. Scalindua sp.”, which increases the maintenance energy requirement due to osmoregulation and repair of proteins [17].
The estimated me values were in good agreement with the ones theoretically predicted for all anaerobic microorganisms grown at 28 °C and 37 °C, i.e., 4.4 and 9.7 kJ mole of biomass-C−1 h−1, respectively [48]. The me values obtained for anammox bacteria were also in the same range as the ones for aerobic nitrifying bacteria, Nitrosomonas europaea and Nitrobacter winogradskyi, which were determined using retentostats at 20–25 °C. (Table 2). However, the maintenance energy requirements for anammox bacteria were higher than that ones for Syntrophobacter fumaroxidans syntrophically grown with Methanospirillum hungatei in a chemostat fed with propionic acid at 37 °C (0.14–0.20 kJ mole of biomass-C−1 h−1) and Pelobacter acetylenicus syntrophically grown with different H2 oxidizers in chemostats at 28 °C (1.27–2.12 kJ mol biomass-C−1 h−1) [61]. This suggests that syntrophic bacteria generally require much less energy to survive in methanogenic environments. It should be noted that maintenance energy requirements depend on the actual growth rates [17, 62], chemostat-based estimates of maintenance energy tend to exhibit higher values due to higher growth rates as mentioned above.
For better comparison with the maintenance energy requirements of other microorganisms, these me values were further converted into the unit of cell-specific maintenance power requirement (Pcs, J s−1 or watts (W) per cell) with assuming 86 fg-C per cell [63], resulting in 13.4 × 10−15 and 8.6 × 10−15 W cell−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively (Table 2). The cell-specific maintenance power requirements (Pcs) for a variety of microbial metabolisms determined in the laboratories were summarized by LaRowe and Amend (2015), in which Pcs range over more than 5 orders of magnitude (10−12–10−17 W cell−1). The obtained maintenance power requirements of anammox bacteria were similar to ones of ammonium-oxidizing bacteria (28 × 10−15 W cell−1) and ones of anaerobes (0.36–8.6 × 10−15 W cell−1) but 1–2 orders of magnitude lower than ones of aerobic heterotrophs (49–4700 × 10−15 W cell−1) [63]. The lowest Pcs value determined in the laboratory measurement was 1.9 × 10−17 W cell−1 for a green sulfur bacterium (an anoxygenic phototroph) [63, 64].
It has been suggested that the maintenance power requirements in natural environments can be 3–6 order of magnitude lower than those obtained from laboratory settings like chemostat and even retentostat culture studies [63, 65,66,67, 68]. Accordingly, the maintenance energy requirement of anammox bacteria in natural environments could be much lower than the ones obtained in the present laboratory study. For example, the cell-specific metabolic rates of anammox bacteria (dominated by “Ca Scalindua sediminis”) in ∼80,000-y-old subsurface sediment were reported to be 10−16–10−18 mol NH4 cell−1 d−1 [68], which are at least 2–4 orders of magnitude lower than those measured in the present study (2.7–4.1 × 10−14 mol of NH4 cell−1 d−1). Furthermore, LaRowe and Amend reported the much lower cell-specific maintenance power requirement of 1.9 × 10−19 W cell−1 in the marine sediments in the South Pacific Gyre [67], which was 2 orders of magnitude lower than the lowest value determined in the laboratory [63, 64]. Therefore, further efforts such as transcriptomics and proteomics should be made to bridge the current gap of cell physiology at extremely slow growth rates and maintenance power requirements between the laboratory settings and the natural settings.
Minimum substrate concentration required for growth (S min)
Smin becomes important at near-zero growth rate. Since NO2− is usually less abundant than NH4+ in natural environments [6, 69], Smin for nitrite, above which net biomass growth occurs, were calculated for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively, based on Eq. (7). (Table 3). For this calculation, the specific maintenance rate (a) was derived in advance from Eq. (6). using the above-mentioned values of maintenance coefficient (ms) and maximum biomass yield (Ymax). Smin can be introduced in the Monod equation as follows to assess its impact on the specific growth rate (μ) as a function of nitrite concentration [17, 70] (Fig. 5):
The specific growth rate (μ) and limiting substrate (nitrite) concentration relationship of “Ca. B. sinica” (A) and “Ca. Scalindua sp.” (B), respectively. The μ were simulated using the original Monod model (red line) and Monod model extended with Smin (blue line), respectively. Inserted figures are magnified views of lower nitrite concentration ranges.
The values of half-saturation constant (Ks) for nitrite and maximum specific growth rate (μmax) for “Ca. B. sinica” and “Ca. Scalindua sp.” used for simulation were taken from the literature [6] and listed in Table 3. Average residual NO2− concentrations in MBRs at near-zero growth rates (reflecting “actually measured Smin”) were 1.93 ± 2.07 μM NO2− for “Ca. B. sinica” and 0.50 ± 0.86 μM NO2− for “Ca. Scalindua sp.”, respectively. These measured values were comparable to those calculated Smin from Eq. (7). (Table 3). The calculated Smin for “Ca. Scalindua sp.” was significantly lower (Smin = 0.09 ± 0.05 μM NO2−) than one for “Ca. B. sinica” (Smin = 3.69 ± 0.21 μM NO2−) due to lower Ks values. Thus, “Ca. Scalindua sp.” with lower Smin seems to be able to uptake nitrite more efficiently to satisfy its maintenance power requirement and thus to survive better in the natural environments such as energy-limited oceanic oxygen minimum zones (OMZs) where typical NO2− concentrations were < 3.0 μM [71]. In contrast, “Ca. B. sinica” with higher Smin had difficulty to cope with low NO2− concentrations, and thus has been commonly found in the substrate-rich wastewater treatment processes [16, 35, 40].
Minimum substrate input rate to sustain biomass
Substrate is consumed for both biomass growth and maintenance process as described as Eq. (2). (qs = μ/Ymax + ms). According to this linear relationship, the minimum substrate consumption rate or “input rate” required to sustain the biomass can be defined as SCRmin = qs x = ms x, assuming zero growth rate (μ = 0) (blue lines in Fig. 6), below which the biomass decreases due to biomass decay. For example, to maintain the biomass of 20 mg-protein L−1, SCRmin were 28.6 and 18.6 μmol NH4+ L−1 h−1 for “Ca. B. sinica” and “Ca. Scalindua sp.”, respectively, showing “Ca. B. sinica” requires 1.5 times more substrate input to sustain the same amount of biomass due to higher ms. In contrast, the maximum substrate consumption rate (SCRmax) can be calculated from Eq. (2). (SCRmax = qs x = (μmax/Ymax + ms) x), assuming the maximum specific growth rate (red lines in Fig. 6), above which substrate remains in the culture medium (i.e., MBR effluent). Thus, wastewater treatment plants should be operated below the SCRmax to achieve superior effluent water quality. The SCRmax of “Ca. B. sinica” was 2.4 times higher than that of “Ca. Scalindua sp.” due to higher maximum specific growth rate (μmax) and maintenance coefficient (ms), implying that “Ca. B. sinica” could be a better anammox species for wastewater treatment.
Relationships between substrate consumption rate (SCR) and biomass concentration (x) of “Ca. B. sinica” (A) and “Ca. Scalindua sp” (B). Red lines represent the maximum substrate consumption rates (SCRmax) as a function of biomass concentration, above which the substrate remains in the culture media (i.e., MBR effluent). SCRmax were determined based on ms, Ymax, and μmax. Blue lines represent the minimum substrate consumption rates (SCRmin) or “input rates” to sustain the biomass at zero growth rates (μ = 0), below which the biomass concentration decreases due to biomass decay.
In conclusion, anammox bacteria in natural environments must have physiological mechanisms that allow them to survive and maintain their activity and biomass under extremely low substrate concentrations (energy fluxes). Minimizing the maintenance power requirement could be one of the survival strategies. “Ca. Scalindua sp.”, which has been commonly found in energy-limiting oceanic environments, exhibited less maintenance power requirement than “Ca. B. sinica”, which has been commonly found in substrate-rich wastewater treatment processes. This indicates the importance of maintenance power requirement on ecological niche differentiation in natural environments.
References
Lackner S, Gilbert EM, Vlaeminck SE, Joss A, Horn H, van Loosdrecht MCM. Full-scale partial nitritation/anammox experience - an application survey. Water Res. 2014;55:292–303.
Ali M, Okabe S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere. 2015;141:144–53.
Ni S, Sung S, Yue Q, Gao B. Substrate removal evaluation of granular anammox process in a pilot-scale upflow anaerobic sludge blanket reactor. Ecol Eng 2012;38:30–36.
Wang B, Peng Y, Guo Y, Yuan Y, Zhao M, Wang S. Impact of partial nitritation degree and C/N ratio on simultaneous sludge fermentation, denitrification and anammox process. Bioresour Technol. 2016;219:411–9.
Zhang L, Narita Y, Gao L, Ali M, Oshiki M, Okabe S. Maximum specific growth rate of anammox bacteria revisited. Water Res. 2017;116:296–303.
Zhang L, Okabe S. Ecological niche differentiation among anammox bacteria. Water Res. 2020;171:115468.
Sun W, Xu MY, Wu WM, Guo J, Xia CY, Sun GP, et al. Molecular diversity and distribution of anammox community in sediments of the Dongjiang River, a drinking water source of Hong Kong. J Appl Microbiol. 2014;116:464–76.
Zhu GB, Wang SY, Wang WD, Wang Y, Zhou LL, Jiang B, et al. Hotspots of anaerobic ammonium oxidation at land-freshwater interfaces. Nat Geosci. 2013;6:103–7.
Kuypers MMM, Lavik G, Woebken D, Schmid M, Fuchs BM, Amann R, et al. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc Natl Acad Sci USA. 2005;102:6478–83.
Schmid M, Risgaard-Petersen N, van de Vossenberg J, Kuypers MMM, Lavik G, Petersen J, et al. Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environ Microbiol. 2007;9:1476–84.
Dalsgaard T, Canfield DE, Petersen J, Thamdrup B, Acuña-González J. N2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica. Nature. 2003;422:606–8.
Kuypers MMM, Olav Sliekers A, Lavik G, Schmid M, Jørgensen BB, Gijs Kuenen J, et al. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature. 2003;422:608–11.
Humbert S, Tarnawski S, Fromin N, Mallet MP, Aragno M, Zopfi J. Molecular detection of anammox bacteria in terrestrial ecosystems: distribution and diversity. ISME J. 2010;4:450–4.
Zhu GB, Wang SY, Wang Y, Wang CX, Risgaard-Petersen N, Jetten MSM, et al. Anaerobic ammonia oxidation in a fertilized paddy soil. ISME J. 2011;5:1905–12.
Oshiki M, Satoh H, Okabe S. Ecology and physiology of anaerobic ammonium oxidizing bacteria. Environ Microbiol. 2016;18:2784–96.
Sonthiphand P, Hall MW, Neufeld JD. Biogeography of anaerobic ammonia-oxidizing (anammox) bacteria. Front Microbiol. 2014;5:1–14.
van Bodegom P. Microbial maintenance: A critical review on its quantification. Microb Ecol. 2007;53:513–23.
Wang G, Post WM. A theoretical reassessment of microbial maintenance and implications for microbial ecology modeling. FEMS Microbiol Ecol. 2012;81:610–7.
Overkamp W, Ercan O, Herber M, van Maris AJA, Kleerebezem M, Kuipers OP. Physiological and cell morphology adaptation of Bacillus subtilis at near-zero specific growth rates: a transcriptome analysis. Environ Microbiol. 2015;17:346–63.
Ma X, Wang Y, Zhou S, Yan Y, Lin X, Wu M. Endogenous metabolism of anaerobic ammonium oxidizing bacteria in response to short-term anaerobic and anoxic starvation stress. Chem Eng J. 2017;313:1233–41.
Ma X, Wang Y. Anammox bacteria exhibit capacity to withstand long-term starvation stress: a proteomic-based investigation of survival mechanisms. Chemosphere. 2018;211:952–61.
Xing B-S, Guo Q, Jiang X-Y, Chen Q-Q, He M-M, Wu L-M, et al. Long-term starvation and subsequent reactivation of anaerobic ammonium oxidation (anammox) granules. Chem Eng J. 2016;287:575–84.
Wang Q, Song K, Hao X, Wei J, Pijuan M, van Loosdrecht MCM, et al. Evaluating death and activity decay of Anammox bacteria during anaerobic and aerobic starvation. Chemosphere. 2018;201:25–31.
Lopez C, Pons MN, Morgenroth E. Endogenous processes during long-term starvation in activated sludge performing enhanced biological phosphorous removal. Water Res. 2006;40:1519–30.
Tappe W, Laverman A, Bohland M, Braster M, Rittershaus S, Groeneweg J, et al. Maintenance energy demand and starvation recovery dynamics of Nitrosomonas europaea and Nitrobacter winogradskyi cultivated in a retentostat with complete biomass retention. Appl Environ Microbiol. 1999;65:2471–7.
Vos T, Hakkaart XDV, de Hulster EAF, van Maris AJA, Pronk JT, Daran-Lapujade P. Maintenance-energy requirements and robustness of Saccharomyces cerevisiae at aerobic near-zero specific growth rates. Micro Cell Fact. 2016;15:111.
Ali M, Oshiki M, Awata T, Isobe K, Kimura Z, Yoshiaki H, et al. Physiological characterization of anaerobic ammonium oxidizing bacterium “Candidatus Jettenia caeni”. Environ Microbiol. 2015;17:2172–89.
Narita Y, Zhang L, Kimura, Ali M, Fujii T, Okabe S. Enrichment and physiological characterization of an anaerobic ammonium-oxidizing bacterium “Candidatus Brocadia sapporoensis”. Syst Appl Microbiol. 2017;40:448–57.
Oshiki M, Shimokawa M, Fujii N, Satoh H, Okabe S. Physiological characteristics of the anaerobic ammonium-oxidizing bacterium “Candidatus Brocadia sinica”. Microbiol. 2011;157:1706–13.
Okabe, S, Shafdar, AA, Kobayashi, K, Zhang, L, and Oshiki, M. Glycogen metabolism of the anammox bacterium “Candidatus Brocadia sinica” ISME J. 2020; https://doi.org/10.1038/s41396-020-00850-5.
van der Star WRL, Miclea AI, van Dongen UGJM, Muyzer G, Picioreanu C, van Loosdrecht MCM. The membrane bioreactor: a novel tool to grow anammox bacteria as free cells. Biotechnol Bioeng. 2008;101:286–94.
Zhang L, Okabe S. Rapid cultivation of free-living planktonic anammox cells. Water Res. 2017;127:204–10.
Oshiki M, Awata T, Kindaichi T, Satoh H, Okabe S. Cultivation of planktonic anaerobic ammonium oxidation (Anammox) bacteria using membrane bioreactor. Microbes Environ. 2013;28:436–43.
Awata T, Oshiki M, Kindaichi T, Ozaki N, Ohashi A, Okabe S. Physiological characterization of an anaerobic ammonium-oxidizing bacterium belonging to the “Candidatus Scalindua” group. Appl Environ Microbiol. 2013;79:4145–8.
Zhang L, Narita Y, Gao L, Ali M, Oshiki M, Ishii S, et al. Microbial competition among anammox baxteria in nitrite-limited bioreactors. Water Res. 2017;125:249–58.
Graaf AA, Van DE, Bruijn PDE, Robertson LA, Jetten MSM, Kuenen JG. Autotrophic growth of anaerobic in a fluidized bed reactor. Microbiol. 1996;142:2187–96.
Kindaichi T, Awata T, Suzuki Y, Tanabe K, Hatamoto M, Ozaki N, et al. Enrichment using an up-flow column reactor and community structure of marine anammox bacteria from coastal sediment. Microbes Environ. 2011;26:67–73.
APHA. Standard Methods for the Examination of Water and Sewage, Washington DC,1998,
Nagaraja P, Shivaswamy M, Kumar H. Highly sensitive N-(1-Naphthyl)ethylene diamine method for the spectrophotometric determination of trace amounts of nitrite in various water samples. Intern J Environ Anal Chem. 2001;80:39–48.
Tsushima I, Ogasawara Y, Kindaichi T, Satoh H, Okabe S. Development of high-rate anaerobic ammonium-oxidizing (anammox) biofilm reactors. Water Res. 2007;41:1623–34.
Kindaichi T, Tsushima I, Ogasawara Y, Shimokawa M, Ozaki N, Satoh H, et al. In situ activity and spatial organization of anaerobic ammonium-oxidizing (anammox) bacteria in biofilms. Appl Environ Microbiol. 2007;73:4931–9.
Okabe S, Satoh H, Watanabe Y. In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol. 1999;65:3182–91.
Pirt SJ. Maintenance energy of bacteria in growing cultures. Proc R soc Lond B Biol Sci. 1965;163:224–31.
Pirt SJ. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol. 1982;133:300–2.
Herbert D, Elsworth R, Telling RC. The continuous culture of bacteria: a theoretical and experimental study. J Gen Microbiol. 1956;14:601–22.
van Verseveld HW, De Hollander JA, Frankena J, Braster M, Leeuwerik FJ, Stouthamer AH. Modeling of microbial substrate conversion, growth and product formation in a recycling fermentor. Antonie Van Leeuwenhoek. 1986;52:325–42.
Lotti T, Kleerebezem R, Lubello C, van Loosdrecht MCM. Physiological and kinetic characterization of a suspended cell anammox culture. Water Res. 2014;60:1–14.
Tijhuis L, Van Loosdrecht MCM, Heijnen JJ. A thermodynmically based correlation for maintenance Gibbs energy requirements in aerobic and anaerobic chemotrophic growth. Biotechnol Bioeng. 1993;42:509–19.
Strous M, Heijnen JJ, Kuenen JG, Jetten MSM. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl Microbiol Biotechnol. 1998;50:589–96.
Awata T, Kindaichi T, Ozaki N, Ohashi A. Biomass yield efficiency of the marine anammox bacterium, “Candidatus Scalindua sp.,” is affected by salinity. Microbes Environ. 2015;30:86–91.
Henze, M. Wastewater Treatment: Biological and chemical processes. New York, NY: Springer, 1997.
Vandekerckhove, TGL, Bodé, S, De Mulder, C, Vlaeminck, SE, Boon, N. 13C Incorporation as a tool to estimate biomass yields in thermophilic and mesophilic nitrifying communities. Front Microbiol. 2019;10:192.
Tappe W, Tomaschewski C, Rittershaus S, Groeneweg J. Cultivation of nitrifying bacteria in the retentostat, a simple fermentor with internal biomass retention. FEMS Microbiol Ecol. 1996;19:47–52.
Rebnegger C, Vos T, Graf AB, Valli M, Pronk JT, Daran-Lapujade P, et al. Picha pastoris exhibits high viability and a low maintenance energy requirement at near-zero specific growth rates. Appl Environ Microbiol. 2016;82:4570–83.
Lever MA, Rogers KL, Lloyd KG, Overmann J, Schink B, Thauer RK, et al. Life under extreme energy limitation: a synthesis of laboratory- and field-based investigations. FEMS Microbiol Rev. 2015;39:688–728.
Bulthuis BA, Frankena J, Koningstein GM, van Verseveld HW, Stouthamer AH. Instability of protease production in a rel1/rel2 pair of Bacillus licheniformis and associated morphological and physiological characteristics. Antonie Leeuwenhoek. 1988;54:95–111.
Kempes, CP, van Bodegom PM, Wolpert, D, Libby, E, Amend, J, Hoehler, T. Drivers of bacterial maintenance and minimal energy requirements. Front Microbiol. 2017;8:31.
Amend JP, Shock EL. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol Rev. 2001;25:175–243.
Amend JP, LaRowe DE. Minireview: demystifying microbial reaction energetics. Environ Microbiol. 2019;21:3539–47.
Kartal B, Keltjens JT. Anammox biochemistry: a tale of heme c proteins. Trends Biochem Sci. 2016;41:998–1011.
Scholten JCM, Conrad R. Energetics of syntrophic propionate oxidation in defined batch and chemostat coculture. Appl Environ Microbiol. 2000;66:2934–42.
LaRowe DE, Amend JP. The energetics of anabolism in natural settings. ISME J. 2016;10:1285–95.
LaRowe DE, Amend JP. Catabolic rates, population sizes and doubling/replacement times of microorganisms in natural settings. Am J Sci. 2015;315:167–203.
Marschall E, Jogler M, Henssge U, Overmann J. Large-scale distribution and activity patterns of an extremely low-light-adapted population of green sulfur bacteria in the Black Sea. Environ Microbiol. 2010;12:1348–62.
Bradley, JA, Arndt, S, Amend, JP, Burwicz, E, Dale, AW, Egger, M et al. Widespread energy limitation to life in global subseafloor sediments. Sci Adv. 2020;6:eaba0697.
Hoehler TM, Jorgensen BB. Microbial life under extreme energy limitation. Nat Rev Microbiol. 2013;11:83–94.
LaRowe, DE, Amend, JP. Power limits for microbial life. Front Microbiol 2015;6:718.
Zhao R, Mogollon JM, Abby SS, Schleper C, Biddle JF, Roerdink DL. et al. Geochemical transition zone powering microbial growth in subsurface sediments. Proc Natl Acad Sci USA. 2020;117:32617–26.
Pitcher A, Villanueva L, Hopmans EC, Schouten S, Reichart G-J, Sinninghe Damste JS. Niche segregation of ammonia-oxidizing archaea and anammox bacteria in the Arabian Sea oxygen minimum zone. ISME J. 2011;5:1896–904.
Füssel J, Lam P, Lavik G, Jensen MM, Holtappels M, Günter M, et al. Nitrite oxidation in the Namibian oxygen minimum zone. ISME J. 2012;6:1200–9.
Füchslin HP, Schneider C, Egli T. In glucose-limited continuous culture the minimum substrate concentration for growth, Smin, is crucial in the competition between the enterobacterium Escherichia coli and Chelatobacter heintzii, an environmentally abundant bacterium. ISME J. 2012;6:777–89.
Acknowledgements
This research was financially supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (A) Grant Number 19H0077609, which was granted to Satoshi Okabe.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Okabe, S., Kamigaito, A. & Kobayashi, K. Maintenance power requirements of anammox bacteria “Candidatus Brocadia sinica” and “Candidatus Scalindua sp.”. ISME J 15, 3566–3575 (2021). https://doi.org/10.1038/s41396-021-01031-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-021-01031-8