Abstract
As selection frequently favors noncooperating defectors in mixed populations with cooperators, mechanisms that promote cooperation stability clearly exist. One potential mechanism is bacterial cell-to-cell communication, quorum sensing (QS), which can allow cooperators to prevent invasion by defectors. However, the impact of QS on widespread maintenance of cooperation in well-mixed conditions has not been experimentally demonstrated over extended evolutionary timescales. Here, we use wild-type (WT) Vibrio campbellii that regulates cooperation with QS and an unconditional cooperating (UC) mutant to examine the evolutionary origins and dynamics of novel defectors during a long-term evolution experiment. We found that UC lineages were completely outcompeted by defectors, whereas functioning QS enabled the maintenance of cooperative variants in most WT populations. Sequencing evolved populations revealed multiple luxR mutations that swept the UC lineages. However, the evolution of mutant lineages with reduced levels of bioluminescence (dims) occurred in many WT lineages. These dim variants also decreased other cooperative phenotypes regulated by QS, including protease production, indicating they result from changes to QS regulation. This diminished investment phenotype optimizes a tradeoff between cooperative input and growth output and suggests that decreasing the cost of QS could be a favorable strategy for maintaining the cooperative behaviors it regulates.
Similar content being viewed by others
Introduction
Quorum sensing (QS) is a widely distributed form of cell–cell communication used among bacteria [1,2,3,4]. It relies upon the production of and response to small signaling molecules called autoinducers (AI) that allow cells to alter their patterns of gene expression in response to changes in population density [5,6,7]. In Vibrios, many cellular behaviors have been shown to be regulated by QS, including biofilm formation, secreted products such as surfactants, virulence factors, and extracellular enzymes, and bioluminescence [8,9,10,11,12,13,14,15,16]. Many products that QS regulates are metabolically costly and potentially beneficial even to population members that do not contribute to their production, thus qualifying as cooperative behaviors. Theory predicts that investment in signaling should be highest in mixtures of cooperators (producers) and defectors (nonproducers), where it would provide maximum information about levels of relatedness within a population [17], while other studies suggest that selection should favor decreased investment in QS [18]. Natural bacterial populations often contain mixtures of cooperators and defectors for public goods and QS [19, 20], raising questions over the nature and extent to which QS is under selection in natural environments. These observations suggest that selection in nature favors the evolution and persistence of defectors, but that they cannot completely sweep natural populations, leading to coexistence of different cooperative strategies.
We have previously shown that functional QS in mixed populations of Vibrios stabilizes cooperative behaviors in the presence of genetically engineered defectors [21], and QS can also promote the increase of cooperative behaviors when competing strains undergo dispersal events or population bottlenecks [22]. These investigations of cooperation and defection in V. campbellii (formerly Vibrio harveyi) utilized competitions of mixed populations including the engineered ΔluxR mutant lacking the gene for the QS master regulator that induces high-cell-density genes. Individual ΔluxR cells are defectors for positively QS-regulated cooperative behaviors. Deletion mutants of QS master regulators have also been shown to invade as defectors in experimental systems, including Pseudomonas aeruginosa and Vibrio cholerae [23,24,25,26,27]. Although such studies have been informative in discovering tradeoffs associated with QS maintenance, mutations in master regulator genes can also bear significant pleiotropic fitness costs as they often regulate large numbers of genes [3, 28,29,30]. For instance, the LuxR regulon in V. campbellii consists of around 10% of the genome [29, 31]. Because regulons this large are costly to express, they are controlled by an extensive regulatory network including multiple layers of regulators, including LuxO, AphA, and small RNA regulation impacting LuxR expression, suggesting multiple points where natural selection may act to tune this QS network. This architecture also means that the QS regulon includes genes relevant to both public and private traits, and this coregulation potentially provides a means to stabilize cooperative behaviors, although this has not been explicitly demonstrated in Vibrios [30]. In addition, although using seeded, engineered defectors allows tests of predicted competitive outcomes, it does not directly establish the ecological potential for QS defection or test if, in fact, it evolves and how. Nor does it inform the evolutionary origin and maintenance of the trait (in this case, QS regulation of a cooperative public good), but merely how it contributes to growth in a given environment.
Based on results from short-term selection experiments, we hypothesized that fitter defectors would evolve to potentially invade V. campbellii cooperator strains during extended growth in media containing casein as the sole carbon source, an environment where population yield is dependent on the extent of QS-regulated protease production [21, 22]. To examine this hypothesis, we initiated replicate populations of V. campbellii cells in well-mixed conditions passaged for 2000 generations from two cooperator genotypes, the wild-type (WT) strain with functional QS and the unconditional cooperator (UC) strain lacking the key QS signal response proteins LuxO and LuxU, such that it is genetically locked in the high-cell-density QS state leading to constitutive cooperation. We found that the UC strain was rapidly swept by de novo evolved defectors. However, the WT strain reduced defector invasion and maintained cooperation due to the evolution of “dim” mutants that decreased investment in cooperative traits by modulating the QS pathway. These results suggest that QS control of cooperation provides a molecular mechanism affording greater evolutionary potential for cooperators to compete with defectors by enabling bacteria to tune investment in cooperation.
Materials and methods
Evolution experiments
Six replicate populations of WT, ΔluxOU, and corresponding lacZ-tagged strains (arrayed in alternating fashion to monitor against population cross-contamination) of V. campbellii (previously V. harveyi [32]) were passaged in M9 salt medium (Difco) with 2% sodium chloride supplemented with casamino acids (Difco) or sodium caseinate (Sigma) (M9-casein and M9-CAA). This produced 48 populations (Table S1). Cultures (1 mL) were passaged in 96 deep-well plates, and diluted daily 1000-fold into identical media (~10 generations/day). Frozen stocks (20% glycerol) were made periodically and stored at −80 °C. Populations were sampled to determine growth, bioluminescence, and protease production.
Artificial induction of QS
When used, HAI-1 (N-(3-hydroxybutyryl)-homoserine lactone, Sigma-Aldrich 74359) and AI-2 (3A-methyl-5,6-dihydro-furo[2,3-D][1,3,2]dioxaborole-2,2,6,6A-tetraol, B. Bassler) were added to 10 µM as previously described [33]. In competition experiments, populations were seeded with 1% ΔluxR as portion of the populations and diluted to 1/1000 of carrying capacity in M9-casein to initiate [21].
Growth productivity estimates
Growth was measured by sample dilution, plating on LB agar, and enumeration of resulting colonies or by measuring absorbances (600 nm) of population subsamples in a Beckman Coulter spectrophotometer (Model DU730) and in PerkinElmer EnVision or SpectraMax M5 plate readers.
Phenotype measurements
Protease production
Extracellular protease production of clones from populations at generations 870 was determined by FITC-caseinase assays (Sigma-Aldrich, PF0100) as previously described [21]. Concentrations were estimated by normalization to trypsin standard curves. Monocultures were passaged in LB (24 h), diluted 1000-fold into M9-casein media (24 h), and filtered culture supernatants were assayed. Data from evolved variants were fit with a Michaelis–Menten curve, using GraphPad Prism 8.4.1 software.
Bioluminescence measurements
Bioluminescence was measured in 100 μL culture (or a tenfold dilution series) in an EnVision Multilabel Plate Reader (PerkinElmer). Clonal bioluminescence was also qualitatively assessed by examining colonies on LB agar in an AlphaImager HP light box (ProteinSimple, chemoluminescence filter, no visible light applied). Colonies were phenotypically classified (bright, dim, defector) based upon visual examination.
Bioluminescence for lux curves were measured with the EnVision plate reader. Cultures were grown in LB, then diluted 1/1000 into LB+/− cell free 10% supernatants from a high-cell-density WT strain cultured filtered with a 0.22 μM filter in 96-well plates and luminescence was monitored during regrowth over the course of 8 h.
Defector determination
In Fig. 1, ΔluxOU and ΔluxR descendants were identified by screening for chloramphenicol resistance (LB-chloramphenicol, 10 μg/mL)). The ΔluxOU strain was distinguished from ΔluxR by colony PCR of selected clones.
The frequency of defectors or dims when cooperator QS was natively regulated (WT, circles), artificially induced by supplementation with exogenous autoinducers (WT + AI, triangles), and genetically induced (ΔluxOU, UC, squares). Two lines are displayed for each treatment group: open shapes represent the frequency of ΔluxR defectors, while solid shapes represent the frequency of evolved dim variants. Error bars represent 95% confidence intervals (N = 5 biological replicates).
Sequencing and bioinformatic analysis
Illumina NextEra barcoding kits were used to generate samples for population sequencing 12 WT and 6 UC progenitor populations taken at 80, 200, 870, and 2000 generations of evolution. Sequencing was completed on an Illumina NextSeq500 to minimum depth of 30× coverage. Reads were filtered for quality and analyzed with Trimmomatic, cutadapt, and seqtk (https://github.com/lh3/seqtk). Mutations were identified using breseq [v0.27.2] in polymorphism mode, with a mutation threshold of 5% or greater [34,35,36].
Results
Artificial induction of QS increases selection for reduced investment in cooperation
Previous studies with V. campbellii indicated that functional QS could significantly inhibit invasion by defector cells during competition, whereas an unconditional cooperator (UC, ΔluxOU) strain was rapidly invaded [21]. One primary explanation of this result is that premature production of protease by the UC strain at low densities enhanced defector invasion. By extension, we hypothesized that artificial premature induction of the high-density QS state would decrease the ability of WT to prevent defector invasion. The addition of exogenous AI signal molecules that activate the QS response would then be expected to elicit a constitutive QS phenotype resembling that of the UC mutant. To test this prediction, we used supplementation with 10 μM of HAI-1 and AI-2, the two predominant AIs produced in V. campbellii [33, 37]. Addition of AIs resulted in WT bioluminescence being virtually indistinguishable from UC at all densities during growth, demonstrating that QS was constitutively induced by their addition (Fig. S1C).
We conducted multiround competition experiments (7 passages/70 total generations), akin to previous studies [21], of WT with the nonluminescent ΔluxR defector (at 99 and 1% frequencies, respectively) with and without AI supplementation. Although this experiment was structured to test the interaction between the seeded strains, and particularly to see if the defector could invade from its rare starting frequency, its duration allows the potential for novel genotypic evolution to occur. With AI supplementation, ΔluxR defectors did increase in frequency, starting at 20 generations and reaching an average of ~60% of the population by 50 generations (Fig. 1). However, at 50 generations, we began to observe colonies that exhibited reduced but still detectable levels of bioluminescence (“dim” variants, blue solid line). Screening for chloramphenicol resistance allowed us to distinguish between ΔluxR (CmR) and WT (CmS), leading us to conclude that the dim variants had evolved from the WT parent. Ultimately, dims rose to majorities in the WT population, outcompeting the other strains present, including the ΔluxR defector. When competed against UC, ΔluxR rapidly increased in the frequency to 100% of the population, and no dim variants were observed (dashed red line). The invasion of dims in the WT/ΔluxR competition at generations 50–70 corresponded with reductions in population growth yield (Fig. S1A, blue line). However, this decrease in growth yield is not as extensive as the collapse that occurs when ΔluxR invades UC (Fig. S1A, red line). In the WT treatment with no AI added, ΔluxR defectors did ultimately begin to increase in frequency after 50 generations (Fig. 1), but this increase was rapidly followed by the evolution of WT dims (solid black line). At the end of this short-term evolution experiment, examination of numerous WT evolved isolates showed a range of bioluminescent phenotypes. We also observed reduced bioluminescence for the evolved bright clones compared to the parental WT strain, suggesting that even population members lacking a qualitatively distinct dim phenotype were under selection for decreased QS investment under the artificially high signal level condition (Fig. S1B). These experiments suggest that a primary strategy to combat defector invasion is modulation of a functional QS signaling network to decrease investment in cooperation without complete loss of function, thereby optimizing the apparent tradeoff between metabolic investment into cooperation and resulting growth output.
Long-term experimental evolution in strains with different levels of QS function results in different population dynamics
The short-term evolution experiment demonstrated that the WT strain could evolve to produce fitter variants that could withstand defector invasion, even under artificially induced conditions. We hypothesized that the evolution of such variants could play a critical role in the maintenance of cooperation within experimental populations in conditions where cheating is possible. To better understand how the evolution of decreased investment in cooperation can occur, we initiated a longer-term evolution experiment in 12 replicate populations of each of two cooperator strains: WT with a functional QS system and the UC that constitutively activates QS. Importantly, no defectors were added at the start of the experiment and must evolve naturally from the parental cooperator strains. In addition, this experiment was performed in two different environments: M9-casein in which QS-regulated protease production is required for high growth yields and M9 + casamino acids (M9-CAA) which contains hydrolyzed casein and does not require protease production (and by extension, QS) to reach high growth yields. These populations were passaged for approximately ten generations daily for a duration of 2000 generations in total.
We expected selection for defectors to be stronger in M9-casein media than in M9-CAA because of the higher potential gains in growth conveyed by protease production in this environment [21]. We used bioluminescence of individual isolates to indicate QS-mediated cooperation. Defectors were defined as cells not producing any visible bioluminescence, and thus QS-regulated cooperative behaviors, while cooperators were defined as cells maintaining some level of bioluminescence, even if reduced from that of the parental strain. As predicted, defectors evolved rapidly and fixed in all UC M9-casein populations (Fig. 2; cooperators are black, defectors are gray), with two interesting exceptions (discussed in the next section). Figure S2A shows images of plated isolated colonies displaying their bioluminescence signal across all 12 UC M9-casein populations at different generations of the experiments, demonstrating rapid loss of bioluminescence (defector colonies shown in blue).
Each panel represents a replicate among the 12 WT and 12 UC lineages. Cumulative frequencies of cooperators (black, gauged by visible bioluminescence, including both bright and dim clones) and defectors (gray, completely nonbioluminescent) are tracked over the course of a 2000 generation evolution experiment.
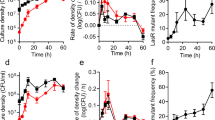
Shortly after defectors appeared in the UC lineages, defectors also became detectable in WT M9-casein populations (Fig. 2). However, the rates at which defectors increased in the WT populations varied greatly and they generally did not sweep the WT populations, with 11/12 populations maintaining cooperators for the entire experiment. Defectors reached an average of 46% across the treatment, although their frequencies varied widely among experimental populations (range ≤ 0.01–100%; Figs. 2 and S3A). Despite the maintenance of cooperation in WT populations, the average population-level intensity and frequency of bioluminescent genotypes decreased over time due to the tension provided by selection for defection (Figs. S2B and 3). In populations evolved from WT where defectors were frequent, cooperators and defectors coexisted, which has been observed in other experimental studies [38,39,40,41,42]. Conversely, in the asocial M9-CAA control condition, cooperator strains were consistently selected against because QS-regulated public goods are not essential in this growth condition and thus are a costly behavior that does not enhance fitness (Fig. S3C–E). In addition, the more rapid invasion of defectors into UC populations in M9-casein than in M9-CAA was consistent with our prediction of stronger selection for defectors in that environment. For the remainder of this study, we will focus specifically on the populations evolved in M9-casein.
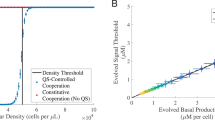
WT populations are represented by circles while UC populations are triangles. Linear regression analysis showed a strong and continuous correlation between these properties for WT populations (R2 = 0.82, p < 0.001). The numbers on the graph correspond to the population numbers of the two outlying UC lineages.
The rise of defectors in populations from both genotypic backgrounds resulted in lower daily population densities once defectors became detectable (Fig. S3B). For UC populations, this was because defectors swept those populations and defection corresponds to decreased casein breakdown and thereby limits nutrient availability. However, drops were also observed in WT populations where defectors predominantly did not sweep. This suggests that cooperators contributed less to public goods in WT populations, either due to the decrease in cooperator frequency or because there was active selection to lessen investment in public goods. The latter is supported by the evolution of dim variants and their increase to sizable portions of their populations.
Alternative strategists have strong effects on population-level phenotypes
Because the evolved populations were started from clonal WT or UC cooperators, they initially had similarly high levels of bioluminescence (~2.6 × 109 RLU/mL) and reached high densities on M9-casein (~7.2 × 108 CFU/mL, Fig. 3). Beyond cooperation alone, this also impacts resulting population biology, as population size influences mutation availability and the strength of selection. By ~30 generations and then over the course of the experiment, populations diverged in both properties. At 2000 generations, ten UC populations were phenotypically indistinguishable in both growth (lower yield) and bioluminescence (undetectable) from ΔluxR (Fig. 3, solid black triangles). By contrast, the other two UC populations (UCcas16, UCcas19) experienced comparable early drops in population density, but rebounded and ultimately experienced large increases in yields, with ending population yields among the highest of all the experimental populations (Fig. S3B). UCcas16 and UCcas19 also had extremely low levels of bioluminescence, in some cases only detectable in a sensitive plate reader (Fig. 3). The WT populations display a diverse range of population-level bioluminescence readings at 2000 generations (Fig. 3, solid black circles). Linear regression analysis showed a strong positive relationship between population bioluminescence and maximum density for WT populations (R2 = 0.82, Fig. 3). These results reflect the strong dependence of population-level outcomes, including overall growth upon the types and relative representation of QS phenotype(s) found within the experimental populations; the trend is particularly apparent and continuous among the WT populations.
Evolution of luxR drives evolutionary dynamics in UC but not WT lineages
We sequenced population samples from temporal transects across the duration of our evolution experiment at 0, 80, 200, 870, and 2000 generations. This included all 12 WT populations, which displayed a wide range of defector frequencies and population-level phenotypes, and six UC lineages, which appeared to have been swept by defectors. We found strikingly different patterns of defector evolution between the two genotypic treatment groups. Specifically, all UC populations were rapidly displaced by luxR mutant genotypes while no luxR mutant alleles exceeded 40% frequency in WT populations (Figs. 4 and S4, Table S2). In fact, only half of the WT populations evolved luxR mutations at any generation analyzed, and only rose to intermediate frequencies (5–40%) in those populations. In total, 21 distinct mutations were identified at the luxR locus, and many appeared to be deleterious to its function (i.e., premature stop codon, frameshift, deletion). Of these mutations, 14 were observed in both the UC and WT lineages (Table S2). In addition, in many cases multiple mutant luxR alleles occurred simultaneously within populations (Fig. S4). The luxR mutations that occurred early in the experiment in UC populations were ultimately replaced by different variants through clonal interference [43,44,45,46,47]. For example, in four UC populations, mutations that fell within the luxR gene coding sequence (CDS) were outcompeted by mutations that likely imparted regulatory changes. These putative luxR regulatory mutants that had mutations upstream (UCcas14, UCcas16) or downstream (UCcas19, UCcas22) of the CDS outcompeted alternative mutations that fell within the CDS. In the WT populations where defectors fixed (WTcas09) or nearly fixed (WTcas07), no luxR mutations were present at detectable levels.
In contrast to the luxR mutations selected in the UC background, 10/12 WT populations exhibited mutations in luxO, which encodes a QS regulatory component that ultimately represses luxR translation at low cell density (Table S3). For the most part (all but WTcas09), the frequencies of these mutants fluctuated over time and did not reach majorities in their populations. This finding suggests that a primary target for the evolved dim phenotype is mutated luxO. These results demonstrate that although mutation of luxR is an effective defector strategy in competition against UC mutants, this strategy cannot sweep WT lineages. Instead, the presence of a functional QS pathway selects for effects like diminished QS activity (rather than complete loss) of QS by frequently targeting alternative components such as luxO.
Additional regulatory targets occurred among both WT and UC populations (Table S3). These included a GntR family transcriptional regulator (VIBHAR_RS03920), the two-component sensor histidine kinase BarA (VIBHAR_RS16525), the two-component system sensor VIBHAR_RS23260, the Rsd/AlgQ family anti-sigma factor VIBHAR_RS01060, and an alternative TetR/AcrR family transcriptional regulator VIBHAR_RS19645. The direct impact of all these mutations on QS signaling remains to be determined.
Evolved dims and defectors possess multiple phenotypic differences from ancestral cooperator strains
As noted, the rise of nonluminescent defectors in evolved populations led to large drops in population densities (Fig. S3B, D). Our shorter-term selection experiments showed that evolved dims and nonluminescent defectors can contribute to lower population growth yields (Figs. 1 and S1). Because bioluminescence and protease production are induced by QS at high cell density in the ancestor, we wondered whether these traits remained correlated in evolved clones. To test this idea, evolved clones from the different bioluminescence phenotypic classes from all populations (at generation 870) were measured for bioluminescence and were consistent with their visual classification (Fig. S5A). Protease production was measured when cultures reached their carrying capacity in M9-casein. Evolved bright isolates maintained high levels of protease production while nonluminescent defector clones tested produced low levels of protease analogous to the ΔluxR defector (Fig. 5A), confirming that these clones were in fact general QS-defectors. Alternatively, evolved dim variants exhibited intermediate levels of protease production, similar to their bioluminescent phenotype. This result suggests that the causative mutations altered multiple traits simultaneously rather than separate mutations impacting each process individually. We also examined bioluminescence over time in evolved isolates and saw that dims responded to both density and supplemented AIs (like WT), though to a reduced extent (Fig. S5B).
Evolved clones that were visually classified as bright, dim, and dark were assessed for (A) extracellular protease levels by measuring the release of FITC-conjugated to casein molecules and measuring fluorescence. B Cell growth (measured by absorbance at 600 nm) was plotted as a function of protease production and fitted with a Michaelis–Menten curve. The 95% confidence interval of values for the parent WT and UC strains is shown by the shaded box in (B), while the luxR mutant is indicated by the triangles. The evolved clones consisted of multiple genotypes that arose in different populations grouped as brights, dims, or defectors as indicated.
To further understand how varying levels of protease activity impacted growth, we characterized growth phenotypes of these clones in M9-casein when grown in monocultures and compared it to their protease production phenotypes (Fig. 5B). We also measured several replicates of the control genotypes (WT, ΔluxOU, ΔluxR) for comparison. Among all strains, we found that there was a saturating relationship between the two traits, where clones that produced more protease also grew better in M9-casein media (Michaelis–Menten curve fit, R2 = 0.93). With a Km of 30.85 nM, approximately a third of the amount of protease produced by the founding cooperator strains (mean = 106.87–110.70 nM) corresponded to half of the maximum growth yield observed at 24 h. Beyond this amount, and especially beyond ~50 nM protease (resulting in >80–90% growth of the founding cooperator strains), led to diminishing additional benefits in growth yield. As expected, the ΔluxR strain (Fig. 5B, triangles) displayed relatively low levels of both quantities and the ancestral cooperator strains both possessed high levels of protease production and growth in M9-casein media (dashed box). Evolved defector clones had lower levels of growth and protease production, largely resembling the defector control (squares). Bright variants evolved from the WT parent demonstrated a range of protease production and cell growth that generally resembled the parental cooperators (open circles), while dim variants evolved from WT all produced less protease and growth than the parental cooperators, although most of these variants were increased relative to the nonluminescent defectors (gray circles). Together, these results suggest that evolved WT variants, and especially dims, are selected to optimize the tradeoff between cooperation and growth in the experimental environment.
Discussion
Cooperation suffers from an inherent tradeoff between the cost incurred by individuals against the population-level benefit conferred from performing a particular behavior. As a result, optimizing costs versus benefits provides a challenge to maintaining cooperative behaviors through natural selection. Thus, cooperation is a theoretical challenge for evolutionary biology to explain [48,49,50,51,52,53,54,55]. This study investigated the impacts of QS upon the stability of cooperative behaviors that it regulates over longer evolutionary timescales.
This study also posed the following questions: if defectors were given the opportunity to evolve de novo, would they? If so, would the evolutionary paths and mutational targets be comparable between the WT and UC cooperating lineages? To examine the evolution cooperators and defectors, and the resulting impacts on phenotypes, dynamics, and selected genotypes, we incorporated both short-term examination of interactions between cooperators and engineered defectors and longer-term evolution experiments. Nearly midway through the evolution experiment (generation 870), a comparison of protease production versus growth for evolved clones revealed a nonlinear saturating curve, reminiscent of Monod curves used to describe microbial growth (Fig. 5b, [56]). The coordinates to which many clones evolved is where increases in growth as a function of protease production begin to plateau. This portion of the curve resembles a Pareto front [57,58,59], suggesting that the evolved WT sublineages with decreased investment in protease production were selected by reducing QS activity. Further, correlated decreases in bioluminescence (both maxima and response over time) and protease activity suggest an overall decrease in QS, rather than a decoupling of light production from LuxR regulation. We interpret the dim phenotype as an optimization to the selection regime, where populations that best balance pressures to maximize growth rates and minimize exploitation (namely, maximizing inclusive fitness, [7]), perform well over time. Because QS is reduced but not eliminated, dims likely benefit from reducing cost of QS investment while also avoiding pleiotropic costs experienced by luxR knockout genotypes.
We predicted that defectors would be less likely to evolve and rise to detectable frequencies in WT populations due to our previous competition results, as well as other reported mechanisms of cheater control such as policing and metabolic constraint [15, 60,61,62,63,64]. We found that this prediction was partially correct in the conditions tested. Defectors did evolve and increase in the presence of WT, but in significantly reduced proportions compared to the UC ancestor (Fig. 2). All UC populations were rapidly invaded and ultimately swept by evolved variants with dramatically reduced QS activity (Fig. 3), and in nearly all cases were indistinguishable in phenotype from the engineered ΔluxR defector strain used as a control. This provides support that QS can act as a form of cheater restraint.
We sought to determine the genetic nature of the evolved dims and defectors by sequencing 18 experimental populations. Our shorter-term competition results indicated that UC cannot prevent cheating by ΔluxR defectors in the M9-casein environment [21]. It also predicts that the evolved dim variants that invaded WT populations were fitter against the WT ancestor than the ΔluxR strain (Fig. 1). Sequencing supported these conclusions as the UC populations were indeed universally swept by luxR mutations. Though the exact nature of luxR mutations varied between lineages, several were clearly loss-of-function mutations, suggesting decreased cooperation was beneficial (Fig. S4, Table S2). Based on measured frequencies, in WT populations that were swept/nearly swept by defectors, luxR mutant alleles could not have accounted for the defector phenotypes present. Because dims outcompeted the ΔluxR defectors in the shorter-term evolution experiment (Fig. 1), their intermediate QS phenotypes have a role in preserving cooperation in populations where they evolve by outcompeting luxR null variants. This is likely due to their ability to minimize QS cost and exploitation by defectors without suffering the pleiotropic costs that defectors tend to experience. Among the higher frequency luxR alleles, many of them were putative regulatory mutations that mapped to the gene’s promoter region (or even as synonymous changes to the luxR start codon), rather than changes to the gene CDS [31]. Alternatively, the frequent mutations to luxO in WT populations demonstrates that impacting LuxO activity is a beneficial strategy to modulate QS pathways and investment into cooperation without the complete loss of cooperation caused by luxR null mutations (Fig. S4, Table S3). Interestingly, mutations altering the LuxO homolog in Vibrio cholerae that produce cells with lower QS activity, analogous the dims we describe here, spontaneously arose in the WT C6706 strain and were mistakenly disseminated to different laboratories, illustrating that LuxO is a frequent target of selection to modulate QS in Vibrios [65, 66].
In the long-term evolution experiment, dim variants were especially enriched for in the WT-casein treatment. Overall, 8 of the 12 WT lineages (Table S1) possessed notable population-level dimming or harbored significant dim subpopulations by generation 2000. Notably, WT populations that were swept/nearly swept by defectors by generation 2000 (WTcas07, WTcas09) did not evolve dims. Dim variants were substantially rarer among UC lineages: only two lineages (UCcas16 and UCcas19) displayed any measurable bioluminescence and were so dim that it was difficult to visually detect (Fig. 3). Like true defectors, they exhibited characteristic decreases in population productivity and exhibited no measurable protease activity. However, these populations later regained growth levels and ultimately became among the top performing of all the experimental populations (Fig. S2B, top two red lines). Thus, it seems likely that these extreme dim variants acquired beneficial mutations and became proficient for growth in M9-casein (Figs. S2B and 3). One possibility that we are currently exploring is that protease produced by members of those lineages was a privatized form that remained associated with the producing cell.
The genetic mutations underlying the dim phenotype varied across populations and many regulatory gene targets exist among potential source mutations. In addition, the observed phenotypic range dims exhibited was quite wide (Fig. 5), suggesting their phenotypes can be conferred by a potentially broad set of mutations and/or gene targets. Evolved mutations included several regulatory genes, suggesting that there are multiple regulatory routes that can impact the degree to which resulting genotypes activate QS (Table S3). Although some of the regulatory targets have not been previously linked to QS in V. campbellii, BarA orthologs have previously described connections to QS signaling in V. cholerae, where it has been shown to regulate expression of the QS master regulator HapR, analogous to LuxR in V. campbellii [61, 67]. In addition, the GntR homolog in Serratia has an observed connection between QS and gluconate metabolism [68]. LuxO can interact with alternative sigma factors (σ54 and σS) in V. campbellii and other Vibrios [69], suggesting further connections between QS, starvation, and carbon metabolism [70]. Overall, the luxR locus does not appear to be the most selectively favored mutational target in WT populations. However, other regulatory genes such those listed above and in Table S3 appear to be common targets. We predict that these targets are more advantageous because they may elicit subtle effects on luxR expression, resulting in a dim phenotype and maintaining cooperation and robust growth.
We observed the evolution of dim variants in M9-casein, where the cost and benefit of cooperative investment is high because this environment requires cooperative protease production for maximum utilization [21]. These evolved dims exhibit reduced cooperation and could potentially outcompete and exploit more extensively cooperating genotypes. However, QS and the regulation of cooperation are maintained in dims, so cooperation in dim-containing populations was preserved. As dims frequently evolved in this study, reduced cooperation appears to be under selection in these experimental conditions. Genotypes with functioning QS were more capable of evolving in response to selective pressure in a robust and repeatable manner. Broadly, the optimization of cooperation seen in dims acts as a mechanism to maintain cooperation even when selection favors defection: the results of this study were observed in well-mixed populations where factors that can favor cooperation, including spatial structure, biofilm formation, and migration did not occur [22, 71, 72]. This speaks to the robustness of V. campbellii’s QS regulatory network, even over long temporal scales in conditions that provide strong selection against cooperation, providing justification for why QS-mediated cooperation is so frequently maintained among microbes. Moreover, we predict that QS promotion of cooperative traits to be even more robust in natural environments where features including spatial structure and migration are commonplace.
Finally, the results of our study provide perspective on the selective forces affecting natural populations where differing degrees of QS are often observed. Particularly, diminished investment in QS has been observed in situ: in Pseudomonas aeruginosa infections of cystic fibrosis patient lungs, and in diminished infectivity and bioluminescence of natural Vibrio isolates [19, 20, 73,74,75,76,77,78,79]. Importantly, we show that functioning QS systems provide robustness against the complete collapse of regulated cooperative behaviors and offer an explanation for why mixes of cooperators and defectors, as well as intermediate phenotypes like the dims we describe, are observed in nature.
Change history
06 April 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41396-021-00972-4
References
Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–75.
Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–46.
Schuster M, Greenberg EP. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int J Med Microbiol. 2006;296:73–81.
Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222.
Redfield RJ. Is quorum sensing a side effect of diffusion sensing?. Trends Microbiol. 2002;10:365–70.
Hense BA, Kuttler C, Müller J, Rothballer M, Hartmann A, Kreft J. Does efficiency sensing unify diffusion and quorum sensing?. Nat Rev Microbiol. 2007;5:230–39.
West SA, Winzer K, Gardner A, Diggle SP. Quorum sensing and the confusion about diffusion. Trends Microbiol. 2012;20:586–94.
Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002. https://doi.org/10.1016/s0092-8674(02)00829-2.
Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 2002;99:3129–34.
Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol. 2003;50:101–4.
Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell. 2003;5:647–56.
Henke JM, Bassler BL. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J Bacteriol. 2004a;186:3794–805.
Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J Bacteriol. 2004b;186:6902–14.
Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33.
Xavier JB, Kim W, Foster KR. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol Microbiol. 2011. https://doi.org/10.1111/j.1365-2958.2010.07436.x.
Srivastava D, Waters CM. A tangled web: regulatory connections between quorum sensing and cyclic di-GMP. J Bacteriol. 2012;194:4485–93.
Brown SP, Johnstone RA. Cooperation in the dark: signalling and collective action in quorum-sensing bacteria. Proc R Soc Lond. 2001. https://doi.org/10.1098/rspb.2001.1609.
Popat R, Pollitt EJG, Harrison F, Naghra H, Hong K, Chan K, et al. Conflict of interest and signal interference lead to the breakdown of honest signaling. Evolution. 2015;69:2371–83.
Cordero OX, Ventouras L, DeLong EF, Polz MF. Public good dynamics drive evolution of iron acquisition strategies in natural bacterioplankton populations. Proc Natl Acad Sci USA. 2012;109:20059–64.
Wang Y, Wang H, Cui Z, Chen H, Zhong Z, Kan B, et al. The prevalence of functional quorum-sensing systems in recently emerged Vibrio cholerae toxigenic strains. Environ Microbiol Rep. 2011;3:218–22.
Bruger EL, Waters CM. Bacterial quorum sensing stabilizes cooperation by optimizing growth strategies. Appl Environ Microbiol. 2016;82:6498–506.
Bruger EL, Waters CM. Maximizing growth yield and dispersal via quorum sensing promotes cooperation in Vibrio bacteria. Appl Environ Microbiol. 2018;84. https://doi.org/10.1128/AEM.00402-18.
Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–14.
Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natil Acad Sci USA. 2007. https://doi.org/10.1073/pnas.0705653104.
Katzianer DS, Wang H, Carey RM, Zhu J. ‘Quorum non-sensing’: social cheating and deception in Vibrio cholerae. Appl Environ Microbiol. 2015;81:3856–62.
Pollak S, Omer-Bendori S, Even-Tov E, Lipsman V, Bareia T, Ben-Zion I, et al. Facultative cheating supports the coexistence of diverse quorum-sensing alleles. Proc Natl Acad Sci USA. 2016;113:2152–57.
Duan F, March JC. Engineered bacterial communication prevents Vibrio cholerae virulence in an infant mouse model. Proc Natl Acad Sci USA. 2010;107:11260–4.
Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol. 2003;185:2080–95.
van Kessel JC, Rutherford ST, Shao Y, Utria AF, Bassler BL. Individual and combined roles of the master regulators AphA and LuxR in control of the Vibrio harveyi quorum-sensing regulon. J Bacteriol. 2013;195:436–43.
dos Santos M, Ghoul M, West SA. Pleiotropy, cooperation, and the social evolution of genetic architecture. PLoS Biol. 2018;16:e2006671.
Rutherford ST, van Kessel JC, Shao Y, Bassler BL. AphA and LuxR/HapR reciprocally control quorum sensing in Vibrios. Genes Dev. 2011;25:397–408.
Lin B, Wang Z, Malanoski AP, O’Grady EA, Wimpee CF, Vuddhakul V, et al. Comparative genomic analyses identify the Vibrio harveyi genome sequenced strains BAA-1116 and HY01 as Vibrio campbellii. Environ Microbiol Rep. 2009. https://doi.org/10.1111/j.1758-2229.2009.00100.x.
Waters CM, Bassler BL. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev. 2006;20:2754–67.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12.
Deatherage DE, Barrick JE. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. In: Engineering and analyzing multicellular systems. New York, NY: Humana Press; 2014. p. 165–88.
Mok KC, Wingreen NS, Bassler BL. Vibrio harveyi quorum sensing: a coincidence detector for two autoinducers controls gene expression. EMBO J. 2003;22:870–81.
Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–7.
Turner PE, Chao L. Escape from prisoner’s dilemma in RNA Phage phi6. Am Nat. 2003;161:497–505.
MacLean RC, Gudelj I. Resource competition and social conflict in experimental populations of yeast. Nature. 2006;441:498–501.
Czárán T, Hoekstra RF. Microbial communication, cooperation and cheating: quorum sensing drives the evolution of cooperation in bacteria. PloS ONE. 2009;4:e6655.
Gore J, Youk H, van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459:253–56.
de Visser JAGM, Rozen DE. Clonal interference and the periodic selection of new beneficial mutations in Escherichia coli. Genetics. 2006;172:2093–100.
Park S, Krug J. Clonal interference in large populations. Proc Natl Acad Sci USA. 2007;104:18135–40.
Fogle CA, Nagle JL, Desai MM. Clonal interference, multiple mutations and adaptation in large asexual populations. Genetics. 2008;180:2163–73.
Kao KC, Sherlock G. Molecular characterization of clonal interference during adaptive evolution in asexual populations of Saccharomyces cerevisiae. Nat Genet. 2008;40:1499–504.
Lang GI, Rice DP, Hickman MJ, Sodergren E, Weinstock GM, Botstein D, et al. Pervasive genetic hitchhiking and clonal interference in forty evolving yeast populations. Nature. 2013;500:571–74.
Hamilton WD. The evolution of altruistic behavior. Am Nat. 1963;97:354–56.
Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964a;7:1–16.
Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964b;7:17–52.
Hardin G. The tragedy of the commons. Science. 1968;162:1243–48.
West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4:597–607.
West SA, Griffin AS, Gardner A. Evolutionary explanations for cooperation. Curr Biol. 2007;17:R661–72.
Rankin DJ, Bargum K, Kokko H. The tragedy of the commons in evolutionary biology. Trends Ecol Evol. 2007;22:643–51.
MacLean RC. The tragedy of the commons in microbial populations: insights from theoretical, comparative and experimental studies. Heredity. 2008;100:471–7.
Tilman D. Tests of resource competition theory using four species of Lake Michigan algae. Ecology. 1981;62:802–15.
Shoval O, Sheftel H, Shinar G, Hart Y, Ramote O, Mayo A, et al. Evolutionary trade-offs, Pareto optimality, and the geometry of phenotype space. Science. 2012;336:1157–60.
Satterwhite RS, Cooper TF. Constraints on adaptation of Escherichia coli to mixed-resource environments increase over time. Evolution. 2015;69:2067–78.
Li Y, Petrov DA, Sherlock G. Single nucleotide mapping of trait space reveals Pareto fronts that constrain adaptation. Nat Ecol Evol. 2019;3:1–13.
Dandekar AA, Chugani S, Greenberg EP. Bacterial quorum sensing and metabolic incentives to cooperate. Science. 2012;338:264–6.
Wang M, Schaefer AL, Dandekar AA, Greenberg EP. Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. Proc Natl Acad Sci USA. 2015;112:2187–91.
Schluter J, Schoech AP, Foster KR, Mitri S. The evolution of quorum sensing as a mechanism to infer kinship. PLoS Comput Biol. 2016;12:e1004848.
Yan H, Asfahl KL, Li N, Sun F, Xiao J, Shen D, et al. Conditional quorum-sensing induction of a cyanide-insensitive terminal oxidase stabilizes cooperating populations of Pseudomonas aeruginosa. Nat Commun. 2019;10:4999.
Zhao K, Liu L, Chen X, Huang T, Du L, Lin J, et al. Behavioral heterogeneity in quorum sensing can stabilize social cooperation in microbial populations. BMC Biol. 2019;17:20.
Jung SA, Chapman CA, Ng WL. Quadruple quorum-sensing inputs control Vibrio cholerae virulence and maintain system robustness. PLoS Pathog. 2015;11:e1004837.
Stutzmann S, Blokesch M. Circulation of a quorum-sensing-impaired variant of Vibrio cholerae strain C6706 masks important phenotypes. mSphere. 2016;1:1–10.
Tsou AM, Liu Z, Cai T, Zhu J. The VarS/VarA two-component system modulates the activity of the Vibrio cholerae quorum-sensing transcriptional regulator HapR. Microbiology. 2011;157:1620.
Fineran PC, Everson L, Slater H, Salmond GP. A GntR family transcriptional regulator (PigT) controls gluconate-mediated repression and defines a new, independent pathway for regulation of the tripyrrole antibiotic, prodigiosin, in Serratia. Microbiology. 2005;151:3833–45.
Nguyen AN, Disconzi E, Charrière GM, Destoumieux-Garzón D, Bouloc P, Le Roux F, et al. csrB gene duplication drives the evolution of redundant regulatory pathways controlling expression of the major toxic secreted metalloproteases in Vibrio tasmaniensis lgp32. mSphere. 2018;3:e00582–18.
Cuthbertson L, Nodwell JR. The TetR family of regulators. Microbiol Mol Biol Rev. 2013;77:440–75.
Datta MS, Korolev KS, Cvijovic I, Dudley C, Gore J. Range expansion promotes cooperation in an experimental microbial metapopulation. Proc Natl Acad Sci USA. 2013;110:7354–59.
van Dyken JD, Müller MJI, Mack KML, Desai MM. Spatial population expansion promotes the evolution of cooperation in an experimental prisoner’s dilemma. Curr Biol. 2013;23:919–23.
Joelsson A, Liu Z, Zhu J. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect Immun. 2006;74:1141–7.
Feltner JB, Wolter DJ, Pope CE, Groleau MC, Smalley NE, Greenberg EP, et al. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. MBio. 2016;7:e01513–16.
Kostylev M, Kim DY, Smalley NE, Salukhe I, Greenberg EP, Dandekar AA. Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc Natl Acad Sci USA. 2019;116:7027–32.
Chen R, Déziel E, Groleau MC, Schaefer AL, Greenberg EP. Social cheating in a Pseudomonas aeruginosa quorum-sensing variant. Proc Natl Acad Sci USA. 2019;116:7021–6.
Talyzina NM, Ingvarsson PK, Zhu J, Wai SN, Andersson A. Molecular diversification in the quorum-sensing system of Vibrio cholerae: role of natural selection in the emergence of pandemic strains. Appl Environ Microbiol 2009;75:3808–12.
Keynan A, Hastings JW. The isolation and characterization of dark mutants of luminescent bacteria. Biol Bull. 1961;121:375.
Fidopiastis PM, Miyamoto CM, Jobling MG, Meighen EA, Ruby EG. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol Microbiol. 2002;45:131–43.
Acknowledgements
This study was supported by grants R01GM109259 to CMW, R01GM110444 to CMW and VSC, Frank Peabody and Dr. Marvin Hensley fellowships to ELB, and funding from the BEACON Center for the Study of Evolution in Action (NSF Cooperative Agreement DBI-0939454) to ELB and CMW. Sequencing was conducted at the Microbial Genome Sequencing center (MiGS) at the University of Pittsburgh. We thank B. Bassler for the generous gift of AI-2 reagent. We also thank J. Bazurto (https://orcid.org/0000-0001-9012-2260) and B. Koestler (https://orcid.org/0000-0001-7213-0953) for helpful suggestions and careful review of the manuscript.
Author information
Authors and Affiliations
Contributions
ELB, VSC, and CMW designed the experiments. ELB and DJS conducted the experiments. ELB analyzed the data. ELB, VSC, and CMW wrote/edited the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of the article was revised. The spelling of the Daniel J. Snyder was incorrect.
Supplementary information
Rights and permissions
About this article
Cite this article
Bruger, E.L., Snyder, D.J., Cooper, V.S. et al. Quorum sensing provides a molecular mechanism for evolution to tune and maintain investment in cooperation. ISME J 15, 1236–1247 (2021). https://doi.org/10.1038/s41396-020-00847-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-020-00847-0
This article is cited by
-
Bottom-up synthetic ecology study of microbial consortia to enhance lignocellulose bioconversion
Biotechnology for Biofuels and Bioproducts (2022)
-
Bacterial communication
Biology & Philosophy (2021)