Abstract
Dichloromethane (DCM) is an anthropogenic pollutant with ozone destruction potential that is also formed naturally. Under anoxic conditions, fermentation of DCM to acetate and formate has been reported in axenic culture Dehalobacterium formicoaceticum, and to acetate, H2 and CO2 in mixed culture RM, which harbors the DCM degrader ‘Candidatus Dichloromethanomonas elyunquensis’. RM cultures produced 28.1 ± 2.3 μmol of acetate from 155.6 ± 9.3 μmol DCM, far less than the one third (i.e., about 51.9 µmol) predicted based on the assumed fermentation model, and observed in cultures of Dehalobacterium formicoaceticum. Temporal metabolite analyses using gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) spectroscopy revealed that no 13C-labeled acetate was formed in 13C-DCM-grown RM cultures, indicating acetate was not a direct product of DCM metabolism. The data were reconciled with DCM mineralization and H2 consumption via CO2 reduction to acetate and methane by homoacetogenic and methanogenic partner populations, respectively. In contrast, Dehalobacterium formicoaceticum produced 13C-labeled acetate and formate from 13C-DCM, consistent with a fermentation pathway. Free energy change calculations predicted that organisms with the mineralization pathway are the dominant DCM consumers in environments with H2 <100 ppmv. These findings have implications for carbon and electron flow in environments where DCM is introduced through natural production processes or anthropogenic activities.
Similar content being viewed by others
Introduction
Substantial amounts of dichloromethane (DCM) exist in the environment due to anthropogenic activities and natural formation [1,2,3,4]. The total amount of DCM produced by various sources (e.g., industry, biomass combustion, biotic formation in oceans and terrestrial ecosystems, abiotic formation) exceeds 900 gigagrams per year (Gg y–1) [3]. DCM emissions from global coastal oceans and tropical terrestrial mangrove forests were estimated at 68 Gg y–1 and 2–3 Gg y–1, respectively [4, 5]. Of note, the actual amount of DCM formed in these environments must be substantially higher because a sink term for DCM (i.e., consumption by microorganisms) has not been considered. The analysis of air samples collected worldwide revealed that DCM concentrations in the atmosphere have shown a rapid increase of 38–69% over the last decade [6, 7].
DCM is of concern because it is a toxic groundwater contaminant, and also contributes to stratospheric ozone destruction [8, 9]. DCM can be degraded by aerobes and facultative anaerobes harboring glutathione-dependent DCM dehalogenases [10, 11]. Compared with aerobic DCM degradation, the fate of DCM under strictly anoxic conditions remains poorly understood. Anaerobic degradation of DCM by mixed cultures was observed under methanogenic, acetogenic, and fermentation conditions, where DCM degradation was associated with methane and/or acetate production [12,13,14,15,16]. To date, Dehalobacterium formicoaceticum strain DMC of the Peptococcaceae family is the only described isolate capable of utilizing DCM as the sole energy source under anoxic conditions. During growth with DCM, this culture generates acetate and formate in a molar ratio of 1:2 consistent with a fermentation pathway (i.e., an internally balanced oxidation–reduction reaction yielding acetate as reduced and formate as oxidized products) [17, 18]. Cell-free crude extracts of D. formicoaceticum catalyzed the conversion of DCM to methylene-tetrahydrofolate (CH2=THF), and the activities of most of the Wood–Ljungdahl pathway enzymes were detected in in vitro enzyme assays [18]. Based on the physiology of D. formicoaceticum and enzymatic studies, it was proposed that DCM was dechlorinated, converted to CH2=THF, and metabolized via the Wood–Ljungdahl pathway. Disproportionation of CH2=THF with two thirds being oxidized to formate generating reducing equivalents (i.e., [H]) and one third (together with one CO2) being reduced to acetate matched the observed product pattern [18].
Culture RM was enriched from pristine freshwater sediment and degrades DCM under anoxic conditions generating biomass, inorganic chloride, methane, and acetate as end products [15]. Physiological, phylogenetic, and metagenomic analyses identified the specific DCM-degrading bacterium in culture RM as ‘Candidatus Dichloromethanomonas elyunquensis’, which represents a new genus and species within the family of Peptococcaceae [19]. The complete Wood–Ljungdahl pathway is encoded on the genomes of both DCM-degrading bacteria, and all the associated proteins have been detected in the proteome during growth with DCM [20,21,22], supporting the hypothesis that DCM is metabolized via the Wood–Ljungdahl pathway. DCM degradation by ‘Ca. Dichloromethanomonas elyunquensis’ generates H2, and exogenously amended H2 impeded DCM degradation, which suggested the dependence of this organism on H2-consuming partner populations [23]. Consistent with this hypothesis, 16S rRNA gene amplicon sequencing-based microbial community analysis revealed that, in addition to the dominant population ‘Ca. Dichloromethanomonas elyunquensis’, culture RM grown with DCM also harbored populations belonging to the genera Acetobacterium and Methanospirillum, with relative abundances of around 1.4% and 7.1%, respectively [23]. In contrast, D. formicoaceticum does not produce H2 during DCM degradation and does not rely on H2-scavenging microorganisms. Intriguingly, dual carbon (C) and chlorine (Cl) isotope fractionation analysis revealed distinct dual element C–Cl isotope correlations during anaerobic DCM degradation by D. formicoaceticum and culture RM, suggestive of mechanistically distinct steps in the DCM degradation pathways employed by the two bacterial populations [24]. A proteogenomic study revealed the expression of reductive dehalogenases exclusively during DCM metabolism by ‘Ca. Dichloromethanomonas elyunquensis’, whereas D. formicoaceticum does not possess reductive dehalogenase genes, lending further support to the hypothesis of distinct modifications that channel DCM into the Wood–Ljungdahl pathway [22]. The present study employed stable carbon isotope labeling and metabolite analyses to compare and elucidate anaerobic DCM metabolism in the axenic culture D. formicoaceticum and the mixed culture RM harboring ‘Ca. Dichloromethanomonas elyunquensis’.
Materials and methods
Chemicals
DCM (purity >99.95%) was purchased from Acros Organics (Thermo Fisher Scientific, Fair Lawn, NJ, USA). 13C-labeled DCM (99 atom % 13C) was obtained from two sources: Cambridge Isotope Laboratories Inc. (Andover, MA, USA) and Sigma-Aldrich (St. Louis, MO, USA). HPLC grade propanol, pyridine, propyl chloroformate (PCF), hexane, and [2-13C]-acetate (99 atom % 13C) were purchased from Sigma-Aldrich. All other chemicals used were analytical reagent grade or higher, unless otherwise specified.
Microorganisms and cultivation
Culture RM, harboring DCM-degrading ‘Ca. Dichloromethanomonas elyunquensis’, was enriched from freshwater sediment [15]. After establishing the enrichment culture, culture RM was maintained in the laboratory by repeated transfers to defined, completely synthetic basal salt medium with DCM as the sole electron donor. D. formicoaceticum was obtained from American Type Culture Collection (ATCC 700118). Both D. formicoaceticum and culture RM were cultivated in bicarbonate-buffered (30 mM, pH 7.3) anoxic basal salt medium reduced with Na2S (0.2 mM) and L-cysteine (0.2 mM) and the routine cultivation was performed as described [23, 25]. Briefly, cultivation occurred in 160-mL glass serum bottles containing 100 mL of medium. The vessels were sealed with black butyl rubber stoppers (Bellco Glass, Vineland, NJ, USA) under a headspace of N2/CO2 (80/20, vol/vol) and 5–10 µL neat DCM (78–156 µmol) was provided as the sole energy source prior to inoculation from a DCM-grown culture (5%, vol/vol). To inhibit methanogenesis, 2 mM 2-bromoethanesulfonate (BES) was added to the medium. All culture vessels were incubated at 30 °C in the dark without agitation. To investigate the requirement of bicarbonate/CO2, bicarbonate buffer was substituted by HEPES buffer (30 mM, pH 7.3) and the medium was flushed with N2 gas instead of a N2/CO2 gas mixture. Control incubations with the HEPES-buffered medium received 10 mM bicarbonate from an anoxic, sterilized 1 M bicarbonate stock solution.
DNA extraction and quantitative real-time polymerase chain reaction (qPCR)
16S rRNA gene-targeted qPCR assays were used to monitor growth of the DCM degraders, i.e., D. formicoaceticum and ‘Ca. Dichloromethanomonas elyunquensis’, in cultures grown with DCM as the sole energy source. For DNA extraction, 5 mL of culture suspension samples were collected and filtered onto 0.22 µm Durapore membranes (Millipore, Cork, Ireland). DNA was extracted using the DNeasy PowerSoil DNA isolation kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. qPCR primers (Dforq1205F, 5′-CACCACGAAAGTTGGCAACA-3′; and Dforq1265R, 5′-TTCGGCGACTGCTTCCTT-3′) and a probe (Dforq1229-MGB, 5′-FAM-AAGTCGATGAGCGAACC-MGB-3′) were used to specifically target the 16S rRNA gene of D. formicoaceticum. qPCR primers and a probe targeting 16S rRNA gene of ‘Ca. Dichloromethanomonas elyunquensis’ have been reported [19]. qPCR followed published protocols [19, 26] and was conducted using an ABI ViiA7 real-time PCR system (Life Technologies) and data processed with ViiA7 Software (Life Technologies).
Analytical procedures
DCM and methane were measured by manual headspace injections (0.1 mL) into an Agilent 7890A gas chromatograph (GC) (Santa Clara, CA, USA) equipped with a DB-624 column (60 m length, 0.32 mm i.d., 1.8 mm film thickness) and a flame ionization detector (FID). The GC inlet was maintained at 200 °C, the GC oven temperature was kept at 60 °C for 2 min followed by an increase to 200 °C at a ramping rate of 25 °C min–1, and the FID detector was operated at 300 °C. Acetate and formate were measured using an Agilent 1200 series high-performance liquid chromatography (HPLC) system equipped with an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) and a UV detector set to 210 nm. The separation occurred at a column temperature of 30 °C over a 25 min period, and the eluent (4 mM H2SO4) was delivered isocratically at a rate of 0.6 mL min–1.
For 13C labeled acetate analysis, a volume of 1000 µL culture sample was taken using a N2-flushed syringe and centrifuged to remove cells. The derivatization of acetate to propyl acetate was performed as described [27]. In brief, 800 µL sample was mixed with 500 µL of propanol/pyridine mixture solvent (3:2, vol:vol) in a glass vial and 100 µL of PCF was subsequently added. The resulting mixture was vortexed briefly and the derivatization reaction proceeded in an ultrasonic water bath (Fisher Scientific, Pittsburg, PA, USA) for 1 min. After derivatization, the organic compounds were extracted with 300 µL of hexane, and the hexane extracts were analyzed with GC-MS. GC-MS analysis was performed using an Agilent 7890A gas chromatograph (Santa Clara, CA, USA) equipped with a DB-624 column (60 m length, 0.32 mm i.d., 1.8 mm film thickness) and an Agilent 5975C inert XL MSD with a Triple-axis detector (Santa Clara, CA, USA). The GC inlet was maintained at 200 °C, and the GC oven temperature was kept at 60 °C for 2 min followed by an increase to 90 °C at a ramping rate of 5 °C min–1 and then another increase to 200 °C at a rate of 25 °C min–1.
1H and 13C NMR spectroscopy analyses
All NMR spectra were acquired using a Varian VNMRS 600 MHz NMR spectrometer equipped with a 5-mm inverse-proton (HX) triple resonance probe. The NMR tube used was a custom order from New Era Enterprises, Inc. featuring an outer 5-mm diameter tube with a septum screw tip and a separate coaxial insertion tube with a cap. For NMR analysis, 600 µL of culture sample was added into the outer NMR tube followed by insertion of the inner NMR tube containing 100 µL of D2O with 9.2 mg of internal standard 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS), and the NMR tube was sealed with a screw cap with a rubber O-ring. The experimental setup was conducted in a glove bag filled with argon gas to avoid any contact with oxygen. The culture RM sample was scanned for 27 days and scanning began once the labeled 13C-DCM had been injected into the outer tube. 1H NMR and 13C NMR spectra were taken over the time period with additional 2D spectra (1H–13C) heteronuclear single quantum coherence spectroscopy (HSQC), 1H–13C heteronuclear multiple bond correlation (HMBC), 1H–1H homonuclear correlation spectroscopy (COSY) and 13C–13C distortionless enhancement of polarization transfer (DEPT) taken for metabolite identification. The axenic culture D. formicoaceticum grown with 13C-DCM was scanned with both 1H NMR and 13C NMR, with additional 1H–13C HSQC for peak confirmation.
Results and discussion
Stoichiometry of DCM degradation by culture RM
In axenic cultures of D. formicoaceticum, DCM degradation supported growth and the organism's 16S rRNA gene copy number increased from 4.82 × 106 ± 1.30 × 105 to 6.98 × 108 ± 5.26 × 107 (Supplementary Information, Fig. S1). A yield of 3.66 × 107 ± 2.79 × 106 cells per µmol of DCM consumed was calculated for D. formicoaceticum, which is similar to the reported value of 5.25 × 107 ± 1.0 × 107 cells per µmol of DCM for ‘Ca. Dichloromethanomonas elyunquensis’ in culture RM [19]. In DCM-grown RM cultures without amendment of the methanogenesis inhibitor BES, methane and acetate were produced as metabolic end products (Fig. 1a). The amount of 155.6 ± 9.3 μmol of DCM was completely degraded over a 28-day incubation period generating 42.5 ± 7.7 μmol of methane and 28.1 ± 2.3 μmol of acetate per bottle (Fig. 1a). The addition of 2 mM BES completely abolished methane production but did not prevent DCM degradation (Fig. 1b). In BES-amended cultures, DCM (147.1 ± 4.3 μmol) was completely degraded within 40 days of incubation generating 71.4 ± 3.2 μmol of acetate per bottle (Fig. 1b). Stoichiometric calculations determined an electron recovery of approximately 97% and 91% with and without the amendment of BES, respectively, indicating that the majority of electrons released from DCM oxidation were recovered in methane and/or acetate (Table 1). Since DCM degradation occurred in BES-amended cultures without methane formation (Fig. 1b), the stepwise reductive dechlorination of DCM to chloromethane (CH3Cl) and then to methane (CH4), a pathway typically observed during reductive dechlorination of chlorinated ethenes [28], can be excluded. This conclusion is consistent with the absence of chloromethane as an intermediate in cultures grown with DCM [23].
(a) DCM degradation and methane and acetate formation. (b) In presence of the methanogenesis inhibitor BES, methane production was abolished, and acetate was the sole product of DCM metabolism by RM cultures. Data shown represent means ± standard deviation (n = 3), and error bars represent standard deviations.
Refuting acetate as a direct product of anaerobic DCM degradation by culture RM
DCM degradation by the axenic culture D. formicoaceticum yielded acetate and formate as terminal products in a molar ratio of approximately 1:2 [17, 18]. Therefore, it was proposed that DCM was fermented to acetate and formate using the Wood–Ljungdahl pathway according to Eq. (1) [17, 18].
Based on the observation that H2 is an intermediate and acetate is an end product of DCM metabolism in culture RM, it was proposed that ‘Ca. Dichloromethanomonas elyunquensis’ fermented DCM to acetate, CO2, and H2 according to Eq. (2) [23].
In both of the hypothetical DCM fermentation pathways (Eqs. (1) and (2)), acetate is assumed to be a direct product of DCM metabolism, and the molar ratio of acetate versus DCM should be 1:3. Acetate was a dead-end product in axenic cultures of D. formicoaceticum and also in mixed culture RM, which did not produce methane from acetate.
16S rRNA gene amplicon sequencing applied to DCM-grown culture RM revealed the presence of Acetobacterium spp. [23]. These homoacetogens exclusively produce acetyl-CoA (and from that acetate as the end product) from reducing two molecules of CO2 with H2 as an electron donor through the reactions of the Wood–Ljungdahl pathway [29]. According to Eq. (2), DCM undergoes a fermentation reaction leading to the formation of H2, which is consumed in H2/CO2 reductive acetogenesis by Acetobacterium populations. Assuming ‘Ca. Dichloromethanomonas elyunquensis’ would catalyze DCM fermentation according to Eq. (2) and Acetobacterium spp. in culture RM utilize the H2 generated during DCM degradation and available CO2 to produce acetate, the measured amount of acetate should be more than one third of the initially amended amount of DCM. However, RM cultures that received 155.6 ± 9.3 μmol of DCM without BES amendment only produced 28.1 ± 2.3 μmol per bottle of acetate (Fig. 1a, and Table 1). This amount was far less than the theoretical amount of acetate expected in culture RM based on Eq. (2) (viz., at least one third of DCM should be recovered as acetate if DCM is metabolized according to Eq. (2)), thus raising the question whether acetate was a direct product of DCM metabolism by ‘Ca. Dichloromethanomonas elyunquensis’. Careful monitoring of DCM consumption and acetate formation in RM cultures demonstrated that acetate production started after DCM had been depleted (Fig. 1a). These observations imply that acetate detected in culture RM may not be a direct product of DCM metabolism but the result of homoacetogenic bacteria (e.g., Acetobacterium) reducing CO2 with H2 as an electron donor.
To further explore the products directly derived from DCM degradation by ‘Ca. Dichloromethanomonas elyunquensis’, stable carbon isotope labeled DCM (13CH2Cl2) was employed to trace product formation in culture RM. 13C-labeled DCM (99 atom % 13C) was initially obtained from Cambridge Isotope Laboratories Inc.; however, several attempts to grow culture RM and D. formicoaceticum with this product failed. Careful examination of this commercial product using gas chromatography detected impurities, one of which co-eluted with chloroform. A prior study demonstrated that culture RM is sensitive to chloroform [30]. Inhibition of dechlorinators by impurities is not unprecedented and the presence of 0.4% (mol/mol) of chloroform in commercial cis-1,2-dichloroethene prevented reductive dechlorination by Dehalococcoides mccartyi strain 195 [31]. We then switched to a different supplier and both DCM-degrading cultures grew with 13C-labeled DCM (99 atom % 13C) obtained from Sigma-Aldrich with similar lag times and degradation rates observed with unlabeled DCM.
Gas chromatography-mass spectrometry (GC-MS) analysis of an unlabeled acetate standard (measured as propyl acetate after derivatization) revealed three major characteristic ion fragments with mass-to-charge ratio (m/z) values of 43, 61, and 73 (Fig. 2a). RM cultures that received 13C-DCM or unlabeled DCM as the sole electron donor and 2 mM BES to inhibit methanogenesis produced acetate, but no methane, as metabolic end product. GC-MS analysis of the products formed in culture RM grown with 13C-DCM revealed that the three major ion fragments of propyl acetate had the same m/z values as those of the unlabeled standard (i.e., m/z values of 43, 61, and 73) indicating the acetate pool generated in culture RM was not 13C labeled (Fig. 2b). This finding is consistent with the hypothesis that acetate in culture RM was not directly derived from DCM metabolism by ‘Ca. Dichloromethanomonas elyunquensis’ but is a product of H2/CO2 reductive acetogenesis mediated by homoacetogens present in the mixed culture. In contrast, 13C-labeled acetate was detected in growth experiments with axenic culture D. formicoaceticum that received 13C-DCM as the sole substrate (Fig. 2c). When grown with 13C-DCM, the m/z values of the three dominant ion fragments of derivatized acetate (i.e., propyl acetate) were one mass unit greater (i.e., m/z values of 44, 62, and 74) than those of the unlabeled standard (Fig. 2c). The label incorporation demonstrated that acetate was a direct product of DCM metabolism by D. formicoaceticum, which is consistent with the proposed fermentation pathway (Eq. (1)). In a previous study, 13C-labeled acetate was also detected in a DCM-fermenting mixed culture dominated by Dehalobacter populations during growth with 13C-DCM, suggestive of fermentative DCM metabolism [32].
Mass spectra of propyl ester derivatives of an acetate standard (a). Panels B and C show the mass spectra of acetate propyl ester derivatives extracted from culture RM and from D. formicoaceticum supplemented with 13C-DCM as the sole electron donor, respectively. The identification of the acetate propyl ester in (b) and (c) is based on comparison of the GC retention time of 9.58 min of the derivatized acetate propyl ester standard.
Metabolite analysis of cultures grown with 13C-DCM using NMR spectroscopy
1H and 13C NMR spectroscopy were employed for in vivo monitoring of metabolite formation in both cultures, i.e., in culture RM harboring ‘Ca. Dichloromethanomonas elyunquensis’ and in axenic culture D. formicoaceticum, both grown with 13C-labeled DCM as the sole electron donor. In culture RM, 1H NMR spectroscopy revealed two distinct peaks at chemical shift of δ 5.29 ppm and δ 5.59 ppm, which corresponded to the two hydrogen (H) atoms of 13C-DCM. In the 13C NMR spectra, 13C-DCM showed a single peak at a chemical shift of δ 53 ppm. As shown in both 1H and 13C NMR spectra, the amended 13C-DCM was completely degraded by culture RM over a span of 11 days in the NMR tube (Fig. 3a). Concomitant with the degradation of 13C-DCM, 13C-labeled CO2 was formed in culture RM as revealed in the 13C NMR spectra with a peak at a chemical shift of δ 125 ppm, suggesting DCM was oxidized to CO2 (Fig. 3a). Acetate and methane were detected by 1H NMR with peaks at chemical shifts of δ 1.90 ppm and δ 0.16 ppm, respectively; however, neither methane nor acetate was labeled with 13C as evidenced by the absence of splitting and corresponding peaks in the 13C NMR spectra (Fig. 3a).
The axenic culture D. formicoaceticum produced 13C-labeled acetate when grown with 13C-DCM, and the 13C-label was found exclusively in the methyl group of acetate (i.e., [2-13C]-acetate) as revealed in the 13C NMR spectra with a peak at a chemical shift of δ 23 ppm (Fig. 3b). There was no peak in 13C NMR spectra at a chemical shift of δ 181 ppm, which would correspond to 13C-acetate with 13C-label in the carboxyl carbon (i.e., [1-13C]-acetate) (Fig. 3b). 1H NMR analysis revealed that in addition to [2-13C]-acetate (viz., the 1H of [2-13C]-acetate had two splitting peaks at chemical shifts of δ 2.01 ppm and δ 1.80 ppm), a peak with a chemical shift at δ 1.90 ppm in the 1H NMR spectrum indicative of unlabeled acetate was also detected (Fig. 3b). The 1H and 13C NMR spectra of [2-13C]-acetate and unlabeled acetate standards are provided in the Supplementary Information (SI, Fig. S2). The formation of unlabeled acetate might be attributed to carbon isotope exchange with the ambient unlabeled CO2 because of the reversibility of Wood–Ljungdahl pathway [33, 34]. In addition to [2-13C]-acetate, 13C-formate was also formed in cultures of D. formicoaceticum when grown on 13C-DCM. 13C-formate showed a peak at the chemical shift of δ 171 ppm on 13C NMR and two splitting peaks at δ 8.59 ppm and δ 8.27 ppm in the 1H NMR spectrum. Similar as acetate, unlabeled formate was also detected on 1H NMR, which had a peak at a chemical shift of δ 8.44 ppm. In addition, 13C-labeled methanol and glycine were detected by 1H and 13C NMR spectroscopy and confirmed by 1H-13C HSQC (Fig. 3b; and SI, Fig. S3), suggesting these compounds are formed during DCM metabolism by D. formicoaceticum. Similar to the 1H NMR spectra of 13C-DCM, 13C-labeled acetate, formate, methanol, and glycine were all detected with two large side peaks on 1H NMR caused by splitting due to the presence of a labeled 13C atom in the molecules (Fig. 3b).
The detection of methanol in D. formicoaceticum cultures was consistent with a previous study suggesting methanol might be a byproduct of DCM metabolism by D. formicoaceticum [18]. Glycine can be synthesized from CH2=THF in a reaction catalyzed by glycine synthase (i.e., glycine cleavage enzyme) [35], which is encoded on the genome of D. formicoaceticum [21]. Thus, the formation of 13C-labeled glycine from CH2=THF can be explained in D. formicoaceticum cultures grown with 13C-DCM. The detection of the 13C-label in glycine provides additional evidence that DCM was metabolized via the Wood–Ljungdahl pathway. The 1H and 13C NMR analyses of 13C-DCM-grown culture RM and D. formicoaceticum agree with the GC-MS analysis (Figs. 2 and 3), and corroborate that ‘Ca. Dichloromethanomonas elyunquensis’ and D. formicoaceticum metabolize DCM differently. This conclusion is supported by distinct dual carbon and chlorine isotope fractionation associated with DCM degradation in culture RM and D. formicoaceticum [24].
Dependence of DCM degradation on CO2
The anaerobic degradation of DCM by culture RM and D. formicoaceticum required the presence of CO2 in the medium (Fig. 4). No growth with DCM occurred in medium without CO2, and when CO2 was removed from growing cultures, DCM degradation stopped in both cultures (Fig. 4). The dependence of CO2 for DCM degradation by culture RM was observed in prior studies and attributed to a CO2 requirement of the DCM degrader ‘Ca. Dichloromethanomonas elyunquensis’ [15, 30]. Another possible explanation would be the strict CO2 requirement of the H2-consuming partner populations (i.e., hydrogenotrophic methanogens and homoacetogens) in culture RM. After removal of CO2, methanogenesis and acetogenesis activities in culture RM ceased and methane and acetate formation did not occur. The requirement for CO2 by the axenic culture D. formicoaceticum was explained by CO2-carbon assimilation into acetate via the Wood–Ljungdahl pathway, which agrees with the proposed fermentation model (viz., the carboxy group of acetate is derived from CO2) [18]. Thus, D. formicoaceticum is a mixotrophic bacterium and requires both the organic compound DCM and inorganic carbon (i.e., CO2) for growth. Mixotrophic growth lifestyles that depend on an inorganic carbon source (i.e., CO2) are common among methylotrophic homoacetogens and methanogens that utilize one-carbon (C1) compounds [29, 36, 37].
In culture RM (a) and axenic D. formicoaceticum (b), DCM degradation only occurred in the presence of bicarbonate/CO2. Experiments were performed in CO2-free HEPES-buffered mineral salt medium with N2 in the headspace. The positive control incubations used the same medium but received 10 mM bicarbonate. Data shown represent the means ± standard deviations (n = 3), and error bars represent standard deviations.
DCM mineralization versus fermentation
Taken together, the information obtained from the labeling experiments suggests that ‘Ca. Dichloromethanomonas elyunquensis’ mineralized DCM completely to CO2 and H2 according to Eq. (3), and acetate in culture RM was exclusively derived from hydrogenotrophic partner populations performing H2/CO2 reductive acetogenesis (Fig. 5a). Mineralization is a biological process, in which organic compounds (e.g., DCM) are converted exclusively to inorganic end products, e.g., in this case to CO2, H2, and inorganic chloride. DCM mineralization to CO2 and H2 (Eq. (3)) is thermodynamically favorable with a Gibbs free energy change (∆G\(^{\circ \prime}\)) of –188 kJ mol–1 of DCM under standard conditions (H2 = 1 atm, pH = 7.0).
(a) Complete mineralization of DCM to CO2 by ‘Ca. Dichloromethanomonas elyunquensis’ associated with the generation of H2, which supported growth of Acetobacterium spp. and Methanospirillum spp. to produce acetate and methane, respectively. (b) DCM fermentation to acetate and formate in axenic cultures of D. formicoaceticum. The initial dehalogenation reactions remain elusive, but DCM metabolism occurs via the Wood–Ljungdahl pathway.
Although thermodynamically feasible at high H2 partial pressures, DCM mineralization by ‘Ca. Dichloromethanomonas elyunquensis’ required the presence of H2-consuming partner populations to consume H2. Growth experiments with exogenously added H2 demonstrated a strong inhibitory effect of H2 on DCM degradation [23, 32]. A possible explanation for the negative impact of H2 are the deleterious effects of H2 on the H2-evolving hydrogenase(s). Such product inhibition on H2-evolving hydrogenase(s) has been observed in previous studies [38,39,40] and exemplifies a situation where specific enzyme features supersede thermodynamics.
The 13CO2 (about 1 mM) generated during 13C-DCM mineralization according to Eq. (3) was significantly diluted by the bulk bicarbonate buffer (30 mM) in the medium, indicating that the 13C-labeled acetate produced from hydrogenotrophic 13CO2 reduction by homoacetogens would be negligible. Consistently, no 13C-acetate was detected in RM cultures amended with 13C-DCM as a growth substrate by both GC-MS and NMR analyses. In contrast, 13C-labeled acetate and formate were detected in D. formicoaceticum cultures grown with 13C-DCM, consistent with the fermentation pathway according to Eq. (1), and DCM was fermented to acetate and formate (Fig. 5b). Although the initial dechlorination mechanism(s) remains elusive, DCM metabolism by both ‘Ca. Dichloromethanomonas elyunquensis’ and D. formicoaceticum occurs via the Wood-Ljungdahl pathway.
In culture RM, methanogenesis and H2/CO2 reductive acetogenesis are two processes competing for reducing equivalents (i.e., H2) (Fig. 5a). From a thermodynamic perspective, hydrogenotrophic methanogenesis (Eq. (4)) is more favorable than H2/CO2 reductive acetogenesis (Eq. (5)) under standard conditions.
Consistent with energetic considerations, more reducing equivalents were directed toward methane than acetate formation in DCM-grown RM cultures when methanogenesis was not inhibited by BES (Fig. 1a, and Table 1). Hydrogenotrophic methanogenesis did not completely outcompete H2/CO2 reductive acetogenesis and both processes co-occurred in culture RM over many generations in consecutive transfer cultures. Hydrogenotrophic CO2 reduction to methane and acetate is commonly observed in anoxic natural environments indicating that the thermodynamic advantage of hydrogenotrophic methanogenesis does not preclude H2/CO2 reductive acetogenesis from co-occurring [41, 42].
The Wood–Ljungdahl pathway is common in obligate anaerobic bacteria, but only specialized species can metabolize DCM via this pathway, indicating specific pathway modifications bestow the capability to grow with DCM. The experiments with 13C-labeled DCM conclusively demonstrated that D. formicoaceticum and ‘Ca. Dichloromethanomonas elyunquensis’ metabolize DCM differently, albeit both bacteria rely on the Wood–Ljungdahl pathway. D. formicoaceticum ferments DCM to formate and acetate whereas ‘Ca. Dichloromethanomonas elyunquensis’ completely oxidizes DCM to CO2 with concomitant formation of H2 (Fig. 5). These findings indicate that specific modifications of the Wood–Ljungdahl pathway determine oxidative versus fermentative metabolism of DCM. The exact pathway differences in both bacteria are currently unknown but the observations highlight the flexibility of the Wood–Ljungdahl pathway, which is used in both the oxidative and reductive directions by various microbial groups [33]. The finding that nature has evolved at least two modifications of the Wood–Ljungdahl pathway for DCM metabolism can be seen as evidence that microbes adapted to DCM as a resource long before the anthropogenic introduction of DCM into the environment. Many chlorinated compounds, including DCM, occur naturally [3] and have been part of the biosphere for a long time.
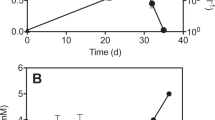
Direct competition experiments between D. formicoaceticum and ‘Ca. Dichloromethanomonas elyunquensis’ have not been performed, and it is unclear if DCM fermentation and mineralization processes co-exist. Based on thermodynamic considerations, DCM fermentation (Eq. (1)) yields slightly more energy than DCM mineralization (Eq. (3)) under standard conditions (pH = 7.0). DCM mineralization is a syntrophic process and relies on metabolic interactions between mutually dependent microbial partner populations. Elevated H2 partial pressures inhibit DCM mineralization and the H2 produced during DCM mineralization must be removed [23, 32]. If the H2 produced during DCM mineralization is consumed in hydrogenotrophic processes, the energy yield is substantially higher, and DCM mineralization becomes more favorable than DCM fermentation at H2 concentrations below 100 ppmv (~78 nM H2) (Fig. 6).
Calculations were performed assuming [CH2Cl2] = 1 mM; [CO2] = 0.2 atm; [CH3COO–] = 0.33 mM; [HCOO–] = 0.67 mM; [Cl–] = 33 mM; pH = 7.0. Both of the processes are thermodynamically favorable with ∆G < 0. DCM mineralization becomes more favorable than DCM fermentation at H2 partial pressures below 100 ppmv.
The H2 generated during DCM metabolism in culture RM must be consumed by hydrogenotrophic partner populations performing H2/CO2 reductive acetogenesis or methanogenesis. Reported H2 consumption threshold concentrations for H2/CO2 reductive acetogenesis and hydrogenotrophic methanogenesis range from 430–4660 ppmv and 6–120 ppmv, respectively [43]. At H2 partial pressures near the reported lower H2 consumption threshold concentrations for H2/CO2 reductive acetogenesis and methanogenesis, the free energy change (∆G´) associated with DCM mineralization (Eq. (3)) increases to approximately –228 and –246 kJ mol–1, respectively. Hydrogenotrophic organohalide-respiring bacteria consume H2 to even lower threshold concentrations of <0.4 ppmv, resulting in even greater free energy change (∆G\(^{\prime}\)) of about –273 kJ mol–1 associated with DCM mineralization.
Environmental implications
The finding that bacteria metabolize DCM via distinct pathways has implications for carbon and electron flow in anoxic environments and for bioremediation. Fermentation of 1 mol DCM yields 0.66 mol of formate, equivalent of 0.66 mol of H2 as formate and H2 pools can be considered energetically equivalent in anoxic environments [44]. More H2 is generated during complete oxidation of DCM by ‘Ca. Dichloromethanomonas elyunquensis’, and 2 mol of H2 per mol of DCM are produced. The H2 produced can fuel different hydrogenotrophic processes, including methanogenesis, H2/CO2 reductive acetogenesis, and organohalide respiration. Reductive dechlorination of many priority groundwater contaminants such as chlorinated ethenes relies on H2 as electron donor [45, 46], and in mixed contaminant plumes, DCM mineralization and associated H2 formation could sustain reductive dechlorination processes. Hydrogenotrophic organohalide-respiring bacteria would be ideal partner populations for syntrophic DCM mineralization because they consume H2 to very low partial pressures [43]. Obviously important is the amount of H2 generated during DCM degradation and tools to distinguish fermentation versus mineralization would be desirable, but tractable biomarkers have yet to be identified. Zero valent iron walls have been applied for in situ treatment of contaminants including carbon tetrachloride and chloroform [47, 48]. Such treatment leads to the formation of H2 and DCM. Based on the outcomes of this study, the prediction would be that bacteria employing the fermentative pathway would dominate as long as the H2 partial pressures exceed 100 ppmv.
Acetate is a central intermediate during the degradation of organic matter in the anoxic environments [49, 50]. For example, the fermentation of C1 compounds, including DCM, chloromethane, methanol, and carbon monoxide (CO), via the Wood–Ljungdahl pathway leads to acetate formation [51,52,53]. Acetate can be oxidized to CO2 if hydrogenotrophic partner populations consume the H2 generated in the oxidation reaction (i.e., syntrophic acetate oxidation), or acetate is fermented to CO2 and methane by acetoclastic methanogens; however, both of the pathways leading to acetate mineralization are thermodynamically challenging reactions and require specialized microorganisms [49, 54, 55]. Our study demonstrates that complete mineralization of DCM to inorganic products, and possibly of other C1 compounds (e.g., methanol [56]), by a single taxon can occur in cooperative interactions with hydrogenotrophic community members. The H2 partial pressure is a controlling parameter over C1 compound mineralization versus fermentation pathways with consequences for microbial community structure and carbon and electron flow in anoxic environments where DCM is introduced through natural production processes or anthropogenic activities.
Culture RM grows with DCM as the sole energy source in defined, completely synthetic, bicarbonate-buffered basal salt medium, and has undergone at least 80 consecutive transfers under these conditions over the past 7 years. Microbial community analysis based on 16S rRNA gene amplicon sequencing and metagenome analysis revealed that culture RM still harbors a very diverse community [19, 23]. It is astonishing that a simple chlorinated C1 compound (i.e., DCM) can sustain such a diverse community under strictly anoxic conditions without any external electron acceptors. A question of interest to microbial ecologist are the intricate organismal relationships that allow the sharing of energy contained in a chlorinated C1 compound (i.e., DCM) among community members. Unraveling how DCM sustains a diverse community could reveal new insights into the mechanisms that drive interdependent, syntrophic community development.
References
McCulloch A, Midgley PM. The production and global distribution of emissions of trichloroethene, tetrachloroethene and dichloromethane over the period 1988–1992. Atmos Environ. 1996;30:601–8.
Keene WC, Khalil MAK, Erickson DJ III, McCulloch A, Graedel TE, Lobert JM, et al. Composite global emissions of reactive chlorine from anthropogenic and natural sources: Reactive chlorine emissions inventory. J Geophys Res. 1999;104:8429–40.
Gribble GW Naturally occurring organohalogen compounds—a comprehensive update. In: Kinghorn AD, Falk H, Kobayashi J (eds). Progress in the Chemistry of Organic Natural Products. Springer Verlag, Vienna, 2010, Vol. 91, pp 12–13.
Kolusu SR, Schlünzen KH, Grawe D, Seifert R. Determination of chloromethane and dichloromethane in a tropical terrestrial mangrove forest in Brazil by measurements and modelling. Atmos Environ. 2018;173:185–97.
Kolusu SR, Schlünzen KH, Grawe D, Seifert R. Chloromethane and dichloromethane in the tropical Atlantic Ocean. Atmos Environ. 2017;150:417–24.
Leedham Elvidge EC, Oram DE, Laube JC, Baker AK, Montzka SA, Humphrey S, et al. Increasing concentrations of dichloromethane, CH2Cl2, inferred from CARIBIC air samples collected 1998–2012. Atmos Chem Phys. 2015;15:1939–58.
Hossaini R, Chipperfield MP, Saiz-Lopez A, Harrison JJ, von Glasow R, Sommariva R, et al. Growth in stratospheric chlorine from short-lived chemicals not controlled by the Montreal Protocol. Geophys Res Lett. 2015;42:4573–80.
Moran MJ, Zogorski JS, Squillace PJ. Chlorinated solvents in groundwater of the United States. Environ Sci Technol. 2007;41:74–81.
Hossaini R, Chipperfield MP, Montzka SA, Leeson AA, Dhomse SS, Pyle JA. The increasing threat to stratospheric ozone from dichloromethane. Nat Commun. 2017;8:15962.
Leisinger T, Bader R, Hermann R, Schmid-Appert M, Vuilleumier S. Microbes, enzymes and genes involved in dichloromethane utilization. Biodegradation. 1994;5:237–48.
Vuilleumier S, Pagni M. The elusive roles of bacterial glutathione S-transferases: new lessons from genomes. Appl Microbiol Biotechnol. 2002;58:138–46.
Freedman DL, Gossett JM. Biodegradation of dichloromethane and its utilization as a growth substrate under methanogenic conditions. Appl Environ Microbiol. 1991;57:2847–57.
Stromeyer SA, Winkelbauer W, Kohler H, Cook AM, Leisinger T. Dichloromethane utilized by an anaerobic mixed culture: acetogenesis and methanogenesis. Biodegradation. 1991;2:129–37.
Mägli A, Rainey FA, Leisinger T. Acetogenesis from dichloromethane by a two-component mixed culture comprising a novel bacterium. Appl Environ Microbiol. 1995;61:2943–9.
Justicia-Leon SD, Ritalahti KM, Mack EE, Löffler FE. Dichloromethane fermentation by a Dehalobacter sp. in an enrichment culture derived from pristine river sediment. Appl Environ Microbiol. 2012;78:1288–91.
Trueba-Santiso A, Parlade E, Rosell M, Lliros M, Mortan SH, Martinez-Alonso M, et al. Molecular and carbon isotopic characterization of an anaerobic stable enrichment culture containing Dehalobacterium sp. during dichloromethane fermentation. Sci Total Environ. 2017;581-582:640–8.
Mägli A, Wendt M, Leisinger T. Isolation and characterization of Dehalobacterium formicoaceticum gen. nov. sp. nov., a strictly anaerobic bacterium utilizing dichloromethane as source of carbon and energy. Arch Microbiol. 1996;166:101–8.
Mägli A, Messmer M, Leisinger T. Metabolism of dichloromethane by the strict anaerobe Dehalobacterium formicoaceticum. Appl Environ Microbiol. 1998;64:646–50.
Kleindienst S, Higgins SA, Tsementzi D, Chen G, Konstantinidis KT, Mack EE, et al. ‘Candidatus Dichloromethanomonas elyunquensis’ gen. nov., sp. nov., a dichloromethane-degrading anaerobe of the Peptococcaceae family. Syst Appl Microbiol. 2017;40:150–9.
Kleindienst S, Higgins SA, Tsementzi D, Konstantinidis KT, Mack EE, Löffler FE. Draft genome sequence of a strictly anaerobic dichloromethane-degrading bacterium. Genome Announc. 2016;4:e00037–16.
Chen G, Murdoch RW, Mack EE, Seger ES, Löffler FE. Complete genome sequence of Dehalobacterium formicoaceticum strain DMC, a strictly anaerobic dichloromethane-degrading bacterium. Genome Announc. 2017;5:e00897–17.
Kleindienst S, Chourey K, Chen G, Murdoch RW, Higgins SA, Iyer R, et al. Proteogenomics reveals novel reductive dehalogenases and methyltransferases expressed during anaerobic dichloromethane metabolism. Appl Environ Microbiol. 2019;85:e02768–18.
Chen G, Kleindienst S, Griffiths DR, Mack EE, Seger ES, Löffler FE. Mutualistic interaction between dichloromethane- and chloromethane-degrading bacteria in an anaerobic mixed culture. Environ Microbiol. 2017;19:4784–96.
Chen G, Shouakar-Stash O, Phillips E, Justicia-Leon SD, Gilevska T, Sherwood Lollar B, et al. Dual carbon–chlorine isotope analysis indicates distinct anaerobic dichloromethane degradation pathways in two members of Peptococcaceae. Environ Sci Technol. 2018;52:8607–16.
Löffler FE, Sanford RA, Ritalahti KM. Enrichment, cultivation, and detection of reductively dechlorinating bacteria. Methods Enzymol. 2005;397:77–111.
Ritalahti KM, Amos BK, Sung Y, Wu Q, Koenigsberg SS, Löffler FE. Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains. Appl Environ Microbiol. 2006;72:2765–74.
Zheng X, Qiu Y, Zhong W, Baxter S, Su M, Li Q, et al. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics. 2013;9:818–27.
Smidt H, De Vos WM. Anaerobic microbial dehalogenation. Annu Rev Microbiol. 2004;58:43–73.
Schuchmann K, Müller V. Energetics and application of heterotrophy in acetogenic bacteria. Appl Environ Microbiol. 2016;82:4056–69.
Justicia-Leon SD, Higgins S, Mack EE, Griffiths DR, Tang S, Edwards EA, et al. Bioaugmentation with distinct Dehalobacter strains achieves chloroform detoxification in microcosms. Environ Sci Technol. 2014;48:1851–8.
Maymó-Gatell X, Nijenhuis I, Zinder SH. Reductive dechlorination of cis-1,2-dichloroethene and vinyl chloride by “Dehalococcoides ethenogenes”. Environ Sci Technol. 2001;35:516–21.
Lee M, Low A, Zemb O, Koenig J, Michaelsen A, Manefield M. Complete chloroform dechlorination by organochlorine respiration and fermentation. Environ Microbiol. 2012;14:883–94.
Ragsdale SW, Pierce E. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim Biophys Acta. 2008;1784:1873–98.
Borrel G, Adam PS, Gribaldo S. Methanogenesis and the Wood–Ljungdahl pathway: an ancient, versatile, and fragile association. Genome Biol Evol. 2016;8:1706–11.
Gariboldi RT, Drake HL. Glycine synthase of the purinolytic bacterium, Clostridium acidiurici. purification of the glycine-CO2 exchange system. J Biol Chem. 1984;259:6085–9.
Jones SW, Fast AG, Carlson ED, Wiedel CA, Au J, Antoniewicz MR, et al. CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion. Nat Commun. 2016;7:12800.
Yin X, Wu W, Maeke M, Richter-Heitmann T, Kulkarni AC, Oni OE, et al. CO2 conversion to methane and biomass in obligate methylotrophic methanogens in marine sediments. ISME J. 2019;13:2107–19.
Fourmond V, Baffert C, Sybirna K, Dementin S, Abou-Hamdan A, Meynial-Salles I, et al. The mechanism of inhibition by H2 of H2-evolution by hydrogenases. Chem Commun. 2013;49:6840–2.
Shafaat HS, Rüdiger O, Ogata H, Lubitz W. [NiFe] hydrogenases: a common active site for hydrogen metabolism under diverse conditions. Biochim Biophys Acta. 2013;1827:986–1002.
Lubitz W, Ogata H, Rudiger O, Reijerse E. Hydrogenases. Chem Rev. 2014;114:4081–148.
Kotelnikova S, Pedersen K. Evidence for methanogenic Archaea and homoacetogenic Bacteria in deep granitic rock aquifers. FEMS Microbiol Rev. 1997;20:339–49.
Lever M. Acetogenesis in the energy-starved deep biosphere—a paradox? Front Microbiol. 2012;2:284.
Löffler FE, Tiedje JM, Sanford RA. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl Environ Microbiol. 1999;65:4049–56.
Schink B, Montag D, Keller A, Müller N. Hydrogen or formate: Alternative key players in methanogenic degradation. Environ Microbiol Rep. 2017;9:189–202.
Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, et al. Dehalococcoides mccartyi gen. nov., sp nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol. 2013;63:625–35.
Atashgahi S, Lu Y, Smidt H. Overview of known organohalide-respiring bacteria—phylogenetic diversity and environmental distribution. In: Adrian, L; Löffler, FE, (eds). Organohalide-respiring bacteria. Springer Verlag, Berlin, Heidelberg, 2016, p. 63–105.
Lee M, Wells E, Wong YK, Koenig J, Adrian L, Richnow HH, et al. Relative contributions of Dehalobacter and zerovalent iron in the degradation of chlorinated methanes. Environ Sci Technol. 2015;49:4481–9.
Wang S, Chen S, Wang Y, Low A, Lu Q, Qiu R. Integration of organohalide-respiring bacteria and nanoscale zero-valent iron (Bio-nZVI-RD): a perfect marriage for the remediation of organohalide pollutants? Biotechnol Adv. 2016;34:1384–95.
Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–80.
Beulig F, Røy H, Glombitza C, Jørgensen BB. Control on rate and pathway of anaerobic organic carbon degradation in the seabed. Proc Natl Acad Sci USA. 2018;115:367–72.
Meßmer M, Wohlfarth G, Diekert G. Methyl chloride metabolism of the strictly anaerobic, methyl chloride-utilizing homoacetogen Strain MC. Arch Microbiol. 1993;160:383–7.
Ragsdale SW. Life with carbon monoxide. Crit Rev Biochem Mol Biol. 2004;39:165–95.
Kremp F, Poehlein A, Daniel R, Müller V. Methanol metabolism in the acetogenic bacterium Acetobacterium woodii. Environ Microbiol. 2018;20:4369–84.
Morris BE, Henneberger R, Huber H, Moissl-Eichinger C. Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev. 2013;37:384–406.
Westerholm M, Dolfing J, Schnürer A. Growth characteristics and thermodynamics of syntrophic acetate oxidizers. Environ Sci Technol. 2019;53:5512–20.
Weijma J, Stams AJ. Methanol conversion in high-rate anaerobic reactors. Water Sci Technol. 2001;44:7–14.
Acknowledgements
This work was supported by The Chemours Company and the Strategic Environmental Research and Development Program (SERDP) under project ER-2312.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, G., Fisch, A.R., Gibson, C.M. et al. Mineralization versus fermentation: evidence for two distinct anaerobic bacterial degradation pathways for dichloromethane. ISME J 14, 959–970 (2020). https://doi.org/10.1038/s41396-019-0579-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-019-0579-5
This article is cited by
-
Proteogenomics of the novel Dehalobacterium formicoaceticum strain EZ94 highlights a key role of methyltransferases during anaerobic dichloromethane degradation
Environmental Science and Pollution Research (2023)
-
Microbial adaptation and impact into the pesticide’s degradation
Archives of Microbiology (2022)
-
Novel dichloromethane-fermenting bacteria in the Peptococcaceae family
The ISME Journal (2021)