Abstract
Introduction
Mycobacterium tuberculosis infections remain a significant cause of morbidity and mortality worldwide. Extrapulmonary infections are less common, and skeletal tuberculosis accounts for about 5–20% of all cases. Skeletal tuberculosis cases often pose diagnostic challenges due to its insidious onset, non-specific clinical presentation and radiographic findings similar to other diseases. Multifocal skeletal tuberculosis is an extremely rare clinical entity, and is defined as an infection that affects two or more non-contiguous bony structures. This clinical entity can mimic bony metastasis and may lead to delays in diagnosis and treatment.
Case presentation
We present a case of multifocal skeletal tuberculous infection mimicking widespread bony metastasis, occurring in an immunocompetent 28-year-old male, and discuss the diagnostic challenges faced and management strategies. The patient successfully underwent instrumentation and stabilization of a pathological T11 vertebra fracture and treatment of tuberculosis infection.
Discussion
While TB infections remain less common in developed countries, they can still cause significant morbidity. Multifocal skeletal tuberculous infections can resemble spinal or bony metastasis on various imaging modalities. Care must be taken when interpreting such imaging results, with histopathology and mycobacterial cultures remaining the gold standard to determine the presence of active TB infections.
Similar content being viewed by others
Introduction
Tuberculosis (TB) remains a global health issue, endemic among developing countries and increasingly prevalent in developed ones [1]. In the World Health Organization Global Tuberculosis Report 2020, it is estimated that up to 10 million people were afflicted with the infection in 2019. Mycobacterium tuberculosis, the pathogen involved in TB, typically affects the lung. Extrapulmonary manifestations are less common, such as skeletal TB which accounts for about 5–20% of all TB infections, of which 50% of cases affect the spine [2,3,4]. Multifocal skeletal TB is defined as the involvement of two or more non-contiguous locations in the skeletal system. It is an extremely rare clinical entity and only accounts for about 5% of all skeletal TB cases [4].
TB is often labeled as the great mimicker—its varied and often non-specific presentation, as well as radiologic manifestations, can mimic various diseases across different organ systems. Diagnosis of TB in such cases remains a challenge but is imperative as treatment strategies for TB differ greatly from other conditions.
This article reports a rare case of disseminated mycobacterium tuberculous infection affecting the skeletal system, mimicking widespread bony and spinal metastasis.
Case presentation
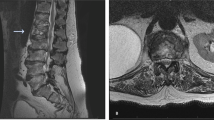
The patient was a 28-year-old male of Southeast-Asian descent, with no significant past medical history and no travel history over the past few years. He presented to the orthopedics clinic with worsening lower back pain of 6 months duration, with significant symptoms of rest pain and night pain. He denied any prior falls or trauma, and did not have any fever, cough or constitutional symptoms. Clinical examination did not show any midline or paravertebral spinal tenderness, with intact upper and lower limb neurology. Initial plain radiographs reviewed T11 vertebra fracture with patchy lucencies seen over T10 and T11 vertebra (Fig. 1). Chest radiographs were normal.
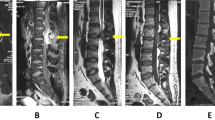
Biochemical investigations for this patient revealed normal cell counts on complete blood count, normal liver and kidney function tests, negative myeloma panel, mildly elevated erythrocyte sedimentation rate (ESR) level of 21 mm/h and negative HIV tests. Magnetic resonance imaging (MRI) of the thoracolumbar spine showed pathological T11 vertebra burst fracture with posterior retropulsion indenting the spinal cord (Fig. 2), with multiple foci of marrow infiltrative process involving thoracic vertebra (T5, T7–T11), lumbar vertebra (L1, L4, L5), sacrum and left iliac bone (Fig. 3). There was also an epidural soft tissue lesion at T10/T11 foramina surrounding the left nerve roots. Subsequent bone scintigraphy with technetium 99m-methyl diphosphonate (Tc-99m MDP) reviewed heterogeneous tracer uptake involving T7 to L5 vertebrae, manubrium, sternum, iliac bones, and sacrum (Fig. 4). These findings were consistent with bony metastasis. Computed tomography (CT) imaging of the thorax, abdomen and pelvis was then performed that revealed right upper lung lobe nodularity (Fig. 5) and enlarged lymph nodes involving the left supraclavicular station, celiac axis and porta hepatis station. There were no mass lesions or obvious tumors demonstrated in the chest, abdomen or pelvis.
The patient underwent T9–T12 posterior instrumentation, left T10/T11 facetectomy and biopsy. Surgery was conducted under general anesthesia, with the patient in the prone position. Pedicle screws were inserted for T9 and T12 segments under O-arm navigation, and biopsy of T10 and T11 vertebral bodies was performed via the left pedicular approach. Left T10/11 facetectomy was performed under microscopy, and left T10/T11 foramina tissue was sent for histology and culture. Histology came back showing necrotizing granulomatous inflammation composed of epithelioid histiocytes forming discrete granulomas with central necrosis. There was no evidence of malignancy or metastatic disease. Ziehl–Neelsen stain was negative for acid-fast bacilli. Aerobic and anaerobic cultures were negative for bacterial infection.
An infection disease specialist was consulted and a subsequent CT-guided biopsy of the sacrum was performed to obtain more tissue samples for testing to guide treatment. Histology again showed florid necrotizing granulomatous inflammation, this time positive for acid-fast bacilli (rare) on the Ziehl–Neelsen stain (Fig. 6). Acid-fast bacilli cultures subsequently came back positive for drug-susceptible M. tuberculosis (Fig. 7).
The patient was diagnosed with multifocal skeletal M. tuberculosis infection and was treated with rifampicin, ethambutol, pyrazinamide and isoniazid for 2 months, followed by rifampicin and isoniazid for 10 months. Follow-up after treatment completion showed full clinical recovery with stable implant positions on check radiographs (Fig. 8).
Discussion
TB remains a major worldwide healthcare burden with increasing incidence due to immigration, immunosuppression due to HIV infections and diabetes, and the emergence of multidrug-resistant TB. Skeletal TB is an uncommon manifestation but can result in significant bone destruction, deformities and neurological compromise [2,3,4,5]. Multifocal skeletal TB is an extremely rare clinical entity, accounting for approximately 5% of skeletal TB cases [4]. The diagnosis is often delayed due to its rarity and non-specific insidious symptoms [4, 6, 7]. In particular, it is difficult to distinguish between multifocal skeletal TB against bony metastasis as both entities can have similar presentations and radiological findings. Biochemical tests such as ESR, C-reactive protein, T-SPOT tests are not useful for diagnosis [3].
Typical features for plain radiographs in skeletal TB affecting the spine can show rarefaction of vertebral endplates, anterior vertebral wedging, loss of vertebral height, bony destruction and development of paraspinal soft tissue abscess [4, 8]. In our case, there was a pathological fracture at T11 with bony destruction and osteolytic lesions seen in the T10/T11 vertebral bodies.
Typical MRI features of skeletal spine TB infections include marrow edema, endplate erosions, paravertebral collections with subligamentous or epidural extension, contiguous vertebra body involvement and intraosseous abscess [8]. Interesting MRI findings noted for our patient included the involvement of posterior spinal elements, non-contiguous lesions and sacral involvement—all of which are rare findings of skeletal spine TB [9,10,11].
There have been a few reported cases of multifocal skeletal TB mimicking widespread bony metastasis in literature till date [2,3,4, 12,13,14,15]. These cases were shown to demonstrate radiographic features on various imaging modalities such as MRI, bone scintigraphy (Tc-99m MDP) and fluorodeoxyglucose (FDG) positron emission tomography (PET)–CT strongly suggestive of bony metastasis initially before the diagnosis of multifocal skeletal TB was ultimately established after analyzing tissue biopsy results.
While bone scans are a useful and common tool used for staging cancers and surveillance of bony metastasis, there are limitations. It has low specificity and has increased sensitivity towards increased bone remodeling from both benign and malignant causes. Benign osteolytic lesions such as TB infections can have a similar appearance to bony metastasis [16]. Similarly, TB infections can resemble bone metastasis in FDG PET–CT scans. FDG tracer accumulates in tumor tissue, but active TB lesions can also exhibit increased glucose metabolism and intense FDF uptake [2, 3]. Liu et al. reported a case of disseminated TB infection showing up on PET–CT scan as multiple FDG avid lymph nodes, with hypermetabolic destruction involving rib, scapula and L5 vertebral bones, mimicking bony metastasis [2]. Meiping et al. similarly reported a case of multifocal skeletal TB presenting with MRI and PET–CT scan findings strongly suggestive of bony metastasis leading to delayed TB diagnosis. For cases with an already established diagnosis of multifocal skeletal TB, it is proposed that PET–CT can be a useful follow-up tool to decide the endpoint of treatment [4, 8]. Shankar et al. suggest that CT perfusion scans can potentially help with the differentiation of the two conditions. They reported that TB infected tissues had low relative blood flow and volume while neoplastic lesions had high relative blood flow and volume which potentially allows for differentiation [17].
Tissue biopsy for histopathology and microbiology is the cornerstone for diagnosis. Key histological features for tissues with TB infection include a granulomatous reaction with epithelioid histiocytes, giant cells, and caseous necrosis. M. tuberculosis bacteria can be found inside the granuloma with Ziehl–Neelsen staining. There is however low bacterial loads in spine TB cases, and the TB bacteria can only be identified in less than 50% of cases [18]. This was seen in the first biopsy report of our patient from the spine surgery that came back negative for TB, and only came back positive on repeat CT-guided sacrum biopsy.
Treatment of multifocal skeletal TB involves a culture-directed anti-TB drug regimen with consideration of surgical interventions. Surgery may be indicated in cases of severe joint or bone deformities, unstable pathological fractures, neurological compromise, cold abscess or necrotic debris [4, 19]. Outcomes are generally good, with multiple authors reporting resolution of TB infection and symptoms following treatment [4, 14, 15, 19].
This case highlights the importance for clinicians to consider multifocal skeletal TB as a differential diagnosis for patients who present with multiple bony lesions picked up on radiographic modalities. Whilst TB infections remain less common in developed countries, they can still cause significant morbidity.
Conclusion
Multifocal skeletal tuberculous infections can resemble spinal or bony metastasis on various imaging modalities. Care must be taken when interpreting such imaging results, with histopathology and mycobacterial cultures remaining the gold standard to determine the presence of active TB infections.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Garg B, Mehta N, Mukherjee RN, Swamy AM, Siamwala BS, Malik G. Epidemiological insights from 1,652 patients with spinal tuberculosis managed at a single center: a retrospective review of 5-year data. Asian Spine J. 2021. http://www.asianspinejournal.org/journal/view.php?doi=10.31616/asj.2021.0137. online ahead of printed.
Liu B, Dong L, Wang X, Han T, Lin Q, Liu M. Tuberculosis mimicking metastases by malignancy in FDG PET/CT. QJM. 2017;110:173–4.
Ye M, Huang J, Wang J, Ren J, Tu J, You W, et al. Multifocal musculoskeletal tuberculosis mimicking multiple bone metastases: a case report. BMC Infect Dis. 2016;16:1–5. https://doi.org/10.1186/s12879-016-1376-7.
Go SW, Lee HY, Lim CH, Jee WH, Wang YP, Yoo IR, et al. Atypical disseminated skeletal tuberculosis mimicking metastasis on PET-CT and MRI. Intern Med. 2012;51:2961–5.
Mulleman D, Mammou S, Griffoul I, Avimadje A, Goupille P, Valat JP. Characteristics of patients with spinal tuberculosis in a French teaching hospital. Joint Bone Spine. 2006;73:424–7.
Singh A, Chatterjee P, Pai MC, Chacko RT. Tuberculous osteomyelitis of the scapula masquerading as metastasis. J Radio Case Rep. 2009;3:27–31.
Ozdemir M, Ozdemir HG. Evaluation of patients admitted with musculoskeletal tuberculosis: sixteen years’ experience from a single center in Turkey. BMC Musculoskelet Disord. 2021;22:1–6.
Agashe VM, Johari AN, Shah M, Anjum R, Romano C, Drago L, et al. Diagnosis of osteoarticular tuberculosis: perceptions, protocols, practices, and priorities in the endemic and non-endemic areas of the world—a waiot view. Microorganisms. 2020;8:1–19.
Ur-Rahman N, El-Bakry A, Jamjoom A, Jamjoom ZAA, Kolawole TM. Atypical forms, of spinal tuberculosis: case report and review of the literature. Surg Neurol. 1999;51:602–7.
Al-Khudairi N, Meir A. Isolated tuberculosis of the posterior spinal elements: case report and discussion of management. JRSM Short Rep. 2014;5:1–6.
Lazrak F, Abourazzak FE, Elouzzani FE, Benzagmout M, Harzy T. A rare location of sacral tuberculosis: a report of three cases. Eur J Rheumatol. 2014;49:78–80.
Miller AG, Spicer PJ. Extrapulmonary tuberculosis: a case report involving the spine and soft tissues. Radio Case Rep. 2021;16:2236–9. https://doi.org/10.1016/j.radcr.2021.05.049.
Wierzba-Bobrowicz T, Michalak E, Michalik R, Stȩpień T. Cervical spinal tuberculosis. Folia Neuropathol. 2010;48:300–4.
Johnstone RH, Ardern DW, Bartle DR. Multifocal skeletal tuberculosis masquerading as metastatic disease. ANZ J Surg. 2011;81:731–3.
Lebowitz D, Wolter L, Zenklusen C, Chouiter A, Malinverni R. TB determined: tuberculous osteomyelitis. Am J Med. 2014;127:198–201. https://doi.org/10.1016/j.amjmed.2013.12.001.
Zhang Y, Zhao C, Liu H, Hou H, Zhang H. Multiple metastasis-like bone lesions in scintigraphic imaging. J Biomed Biotechnol. 2012;2012(Figure 4):1–9.
Shankar J, Jayakumar P, Vasudev M, Ravishankar S, Sinha N. The usefulness of CT perfusion in differentiation between neoplastic and tuberculous disease of the spine. J Neuroimaging. 2009;19:132–8.
Luk KDK. Tuberculosis of the spine in the new millennium. Eur Spine J. 1999;8:338–45.
Hong L, Wu JG, Ding JG, Wang XY, Zheng MH, Fu RQ, et al. Multifocal skeletal tuberculosis: experience in diagnosis and treatment. Med Mal Infect. 2010;40:6–11.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work and were involved with the literature review, cases analysis and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yuen, W.L.P., Loo, W.L. Multifocal tuberculous osteomyelitis mimicking widespread bony metastases: review of literature and case report. Spinal Cord Ser Cases 8, 23 (2022). https://doi.org/10.1038/s41394-022-00496-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00496-9