Abstract
Study design
A randomized, sham-controlled clinical trial.
Objective
To test the effects of tDCS, combined with robotic training, on gait disability in SCI. Our hypothesis was that participants who received active tDCS would experience greater walking gains, as indexed by the WISCI-II, than those who received sham tDCS.
Setting
University of São Paulo, Brazil.
Methods
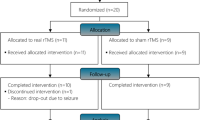
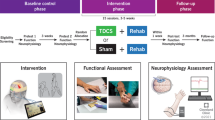
This randomized, double-blind study comprised 43 participants with incomplete SCI who underwent 30 sessions of active (n = 21) or sham (n = 22) tDCS (20 min, 2 mA) before every Lokomat session of 30 min (3 times a week over 12 weeks or 5 times a week over 6 weeks). The main outcome was the improvement in WISCI-II. Participants were assessed at baseline, after 15 and 30 sessions of Lokomat, and after three months of treatment.
Results
There was a significant difference in the percentage of participants that improved in WISCI-II at the 30-session, compared with baseline: 33.3% in the sham group and 70.0% in the active group (p = 0.046; OR: 3.7; 95% CI: 1.0–13.5). At the follow-up, the improvement compared with baseline in the sham group was 35.0% vs. 68.4% for the active group (p = 0.046; OR: 3.7; 95% CI: 1.0–13.5). There was no significant difference at the 15-session.
Conclusion
Thirty sessions of active tDCS is associated with a significant improvement in walking, compared to sham. Moreover, 15 sessions had no significant effect. The improvement in WISCI-II can be related to different aspects of motor learning, including motor recovery and compensation.
Similar content being viewed by others
Introduction
Traumatic spinal cord injury (SCI) is one of the main causes of permanent disability, primarily affecting young people, with significant personal, social, and economic implications [1]. Among its functional impairments, the inability to walk has the greatest impact on the lives of SCI participants. Consequently, it should be treated as a priority in the rehabilitation program [2].
Robotic gait therapy allows massed exercise with many repetitive movements, being demonstrated to improve gait distance, speed, leg strength, and functional level of mobility of incomplete SCI patients [3]. However, a high percentage of participants continue to experience functional deficits, with a higher risk of mortality, even with the best available treatments, underscoring the need for novel adjuvant treatments [3, 4]. Robotic-assisted gait training (RAGT) therapy is in developmental stages and its superiority in comparison to other therapies has yet to be established. Furthermore, the protocols of RAGT are variable, applied during the 4th, 8th and 12th weeks of treatment, with a frequency of treatment ranging from three to five times per week. The number of sessions also varies, having demonstrated clinical benefits with 12, 16, 24, 30, 40 and 60 sessions, with a duration ranging from 30 to 60 min [3]. There is no gold standard regarding RAGT protocols, nor have the different levels of effectiveness between protocols been scientifically demonstrated. Thus, for the present trial, we used previously established effective parameters, including 30 sessions of RAGT, that could be performed three times per week (outpatients) or five times per week (inpatients). Transcranial direct current stimulation (tDCS) appears to have a synergistic effect when coupled with robotic training. tDCS modulates cortical excitability, most likely by increasing and decreasing it through anode and cathode electrodes, respectively [5, 6]. In this context, tDCS can have a long-term effect due to synaptic mechanisms involving long-term depression (LTD)-like and long-term potentiation (LTP)-like plasticity [7]. Thus, anodal tDCS applied to the motor cortex enhances brain plasticity and potentiates functional recovery by improving the efficacy of the remaining connections in the spared descending corticospinal pathways [8, 9] or by facilitating the unmasking of related neural networks [10]. TDCS can likely potentialize motor learning by increasing acquisition and retention of different types of learning, including skills, adaptation, and use-dependent learning (i.e., repeated practice of movements) [11]. Based on this rationale, two trials (15 and 24 participants) tested the use of repetitive sessions of tDCS, combined with a robot-assisted gait [12, 13]. Whereas Raithatha et al. reported conflicting outcomes, with an improvement in strength in the active tDCS group compared to the sham tDCS group, yet, with an improvement in TUG (Time Up and Go Test) only for the sham group; Kumru et al. did not observe any improvement (as indexed by lower extremity motor score, 10MWT and Walk Index for Spinal Cord Injury) with 20 sessions of tDCS.
Like RAGT, the parameters of tDCS are not well established, varying among several neurological and psychiatric disorders. Parameters describe the number of sessions from one to 40 sessions, with a frequency of one to five times per week [14]. For the present trial, participants received 30 sessions of tDCS three times per week (outpatients) or five times per week (inpatients).
As in previous studies, our hypothesis was that participants who receive active tDCS will experience greater walking gains, as indexed by Walk Index for Spinal Cord Injury (WISCI-II), than those who undergo sham tDCS. Thus, we tested the effects of 30 sessions of tDCS, combined with robotic training, on gait disability in incomplete spinal cord injury (SCI) using a larger sample and more sessions than the previous study with a similar hypothesis.
Methods
Participant
Participants were recruited from the rehabilitation program at the Institute of Physical Medicine and Rehabilitation, Faculty of Medicine, University of São Paulo, Brazil. This research was approved by a local research ethics committee under registration number CAAE: 43450715.7.0000.0068. The trial was also registered at clinicaltrials.gov (identifier NCT02562001). Participant recruitment started in October of 2016 and finished in May of 2018, after attaining the planned sample.
The inclusion criteria were ages between 18 and 65 years old, both sexes, clinical and radiological diagnosis of incomplete spinal cord injury of traumatic etiology (surgical intervention or external trauma), period between one to 36 months after lesion, AIS “C” or “D” (International Standards for Classification of Spinal Cord Injury Motor Score (ISNCSCI, formerly ASIA)) classification, stable clinical status, cognitive function preserved and tolerance to sit upright for at least 1 h.
The exclusion criteria were as follows: History of traumatic brain injury with loss of consciousness, stroke, epilepsy and/or any other previous or concomitant neurological conditions to spinal cord injury; Previous orthopedic problems or unhealed fracture of the bones of the lower limbs; Osteoporosis with pathological fracture risk; Irreversible muscle contractures and hypertonic limb (grade > 3 on the modified Ashworth scale, which are “considerable increase in muscle tone, passive movement difficult or affected part(s) rigid in flexion or extension”); Active/passive joint range of motion limitations (Normal values considered for a range of motion for the knees (flexion from 0 to 130 and extension from 120 to 0 degrees), ankle (plantar flexion from 0 to 50 degrees and dorsiflexion from 0 to 20 degrees), and hips (flexion from 0 to 125 degrees and extension from 115 to 0 degrees)); Lack of physical resistance for the proposed physical training (evaluated by the participant’s report of exercise tolerance); Body weight >150 Kg; Asymmetry in the lower limbs > 2 cm; Skin lesions and/or pressure ulcer in areas where the orthosis of Lokomat will press or in site of stimulation; Unchecked autonomic dysreflexia that hinders Lokomat training; Tracheostomy; Presence of electric, magnetic or mechanically activated implant (including cardiac pacemakers); Intracerebral vascular clip or any other electrically sensitive device in any part of the head; Pregnancy.
All participants included were evaluated by a multidisciplinary team, including a physiatrist, social worker, psychologist, nurse, physiotherapist, nutritionist, and occupational therapist. Participants were also evaluated by a cardiologist, urologist, and neurologist, and were administered complementary exams when necessary.
Study design
This study is a randomized double-blind parallel clinical trial in which participants were randomly allocated (ratio 1:1) to receive active or sham tDCS followed by gait training with Lokomat. Block randomization, with a block size of four, was used generate by software and was also stratified in outpatients (GO) and inpatients (GI). Since we assumed differences between GI and GO groups, we considered it methodologically important to stratify randomization to ensure balanced groups. As this approach was successful, there was no need to further discuss findings separated by GI/GO groups or control our analyses.
The random allocation sequence was generated by the principal researcher. Physicians and therapists enrolled participants, and study monitors assigned participants to interventions. The randomization list was concealed and managed by the study monitor who only became aware of participant’s allocation after opening the sealed envelopes. The envelopes were numbered so that the research monitor could not modify the randomization sequence. All participants performed 30 sessions of Lokomat training with a frequency of 3 sessions/week for 10 weeks (GO group) or 5 sessions/week for 6 weeks (GI group). Participants were assessed before the beginning of the intervention(baseline), after 15 sessions of Lokomat (intermediate), after 30 sessions of Lokomat (post-treatment) and three months after treatment (follow-up). Sample size of 42 participants were calculated estimating an effect size of 0.9, for a power of 80% and an alpha of 0.05 bicaudal.
Intervention
Rehabilitation program
Thirty thirty-minute sessions of robotic-assisted gait training (RAGT) using the Lokomat system (Hocoma AG Switzerland) were administered to participants by a trained and certified physiotherapist.
Participant body weight was supported by the Lokomat system and started at the beginning of the treatment with 50%. The patient’s body weight support decreases 10% every 10 sessions, reaching a minimum of 30%, if tolerated by the patient. The guidance force started at 100% for the first 5 min. From 5 min of training to 25 min, it varied from 100% to 50% according to the patient’s tolerance. In the last 5 min of training it returned to 100%, completing a total time of 30 min. The training speed started with 1.0 km/h and could reach the maximum 1.5 km/h, depending on the patient’s tolerance during speed variation, similar to previous studies [3]. As for safety measures, participants evaluated every session for pain, fatigue, skin lesions, cardiovascular symptoms, and other side effects that may be related to therapy. Participants continued their normal rehabilitation program during the study.
tDCS protocol
tDCS was performed using a monophasic current device (DC stimulator, NeuroCom, Germany or Soterix Medical, New York, EUA) and sponge surface electrodes (35 cm2) soaked in saline. The anode was placed over the primary motor cortex (M1) region, with the center of the sponge in coronal plane, five centimeters from “Cz” (International 10/20 system), and a cathode was placed over the supraorbital region, contralateral to the anode.
Participants received 30 sessions of 20 min tDCS per day, applied immediately before Lokomat training, with a frequency of 3 sessions/week for 10 weeks (GO group) or 5 sessions/week for 6 weeks (GI group). Each 20 min session was divided into 10 min of anode stimulation for each hemisphere, alternating the starting side daily. The intensity of current was continuous at 2 mA, with gradual current ramp-up and ramp-down of 30 s. The intensity of the current was the same for every participant in every session. For sham stimulation, the procedure was similar, but the stimulation was only administered for the initial 30 s, with the power turned off for the remaining period. Participants and the rater were blinded for tDCS stimulation. The rater did not have access to randomization list and was not in contact with the participants during tDCS stimulation.
Outcomes
The evaluations were performed by blinded raters with appropriate training (physiatrist, psychologist, physiotherapist, and occupational therapist). The primary outcome was the change in the WISCI-II after 15 sessions, after 30 sessions and at the follow-up. Secondary measures were performed to characterized population and for future exploratory analyses, including Wechsler Adult Intelligence Scale (WASI), Ashworth Modified Scale (MAS) performed for flexion and extension of hips, knees, and ankles, Berg Balance Test (BBT), 10-Meter Walking Test (10MWT) and 6-Minute Walking test (6MWT), Timed Up and Go Test (TUG); Short Form - 36 Quality of Life Test – (SF 36); Spinal Cord Independence Measure (SCIM); Lower Extremity Isokinetic Dynamometry; Visual Analogic Scale for pain (VAS); McGill Pain Questionnaire; Pressure Algometer; Conditioned Pain Modulation (CPM); Pain-Related Self-Statements Scale - Catastrophizing Subscale (PRSSS-Catastrophizing); Hospital Anxiety and Depression Scale (HAD); Beck Depression Inventory; Patient Health Questionnaire 9 (PHQ 9). The summary of these results is in supplementary file (see Supplementary Table 1). Each measurement was performed once per moment of evaluation, with an exception for the Pressure Algometer and CPM, which were performed three times and averaged. In addition, for other exploratory analyses to study the mechanisms of tDCS, we measured neuronal activity by means of transcranial magnetic stimulation, electroencephalography (EEG) and Functional Near-Infrared Spectroscopy (fNIRS) [15, 16].
Statistical analyses
Baseline characteristics were reported. The primary outcome was WISCI-II changes from baseline to after 30 sessions of RAGT. The improvement of WISIC-II was calculated by subtracting baseline data from data obtained post rehabilitation program, and a Histogram was used to assess the distribution of this data.
The main change was to categorize the data of WISC-II into two groups (improvement = zero or improvement ≥ 1) to perform a logistic regression analysis. This analysis was not initially planned (due to unexpected results that most of the participants did not improve), and thus a parametric analysis would be invalid as the assumption of normality was not met. This modification is also adequate for an exploratory study and is coherent with the study hypothesis. Moreover, one point in using WISCI-II as categorical is that it is considered clinically meaningful, and the scale has good validity and reliability to measure improvements in walking following acute and chronic SCI [17].
For the total study´s participants, 51% showed no improvement for the main outcome WISC-II. The score of improvement was calculated as post minus baseline, so most participants scored zero, resulting in a skewed distribution of the data. Since the dependent variable distribution is abnormal, it was not possible to treat the data as continuous with the real WISCI-II scores and perform linear regression analyses.
We used univariate logistic regression for the binary improvement of WISCI-II as the dependent outcome and the intervention group as the independent variable. We also built three independent multivariate logistic regression models to test the three different periods of evaluation (intermediate, post-treatment and follow-up) considering tDCS intervention (Active or Sham tDCS) as the main independent variable and adjusting for the stratified randomization (GI vs. GO), as recommended by previous authors [18] and also selected demographic and clinical characteristics. The initial step was to perform a univariate analysis for each covariate, including “Age”, “Sex” (as a continuous and binary variable, respectively). Clinical characteristics at the baseline measured by the scales WISCI-II, MAS, BBT and HAD were tested as continuous covariates. The level of lesion (divided in tetraplegic or paraplegic), the scale AIS (divided in “C” or “D”) and presence of “chronic pain” (divided in present or absent), performing intensive rehabilitation (yes/no) and treatment with botulinum toxin (yes/no) were treated as binary covariates. The time since injury was tested as a continuous covariate (months) and as binary (chronic vs. acute) considering chronic as the time superior to 6 months of lesion. All analyses were based on two-tailed tests. A variable was considered a confounder if it changed the OR of the variable tDCS intervention by > 10%. We used a significance level of 0.05. As a measure of variability, 95% Confidence Interval (95% CI) were used. The area under the curve (AUC) was used to test for model accuracy. The last observation, carryforward, was used to address missing WISCI-II data.
For secondary outcomes, Wilcoxon signed-rank test was used to compare the baseline with different moments of evaluation (intermediate, post and follow-up). To compare the two groups (active tDCS Vs. placebo tDCS), the improvement (post minus baseline) was first calculated, followed by the Wilcoxon rank-sum test. Participants that could not perform the test (i.e., TUG, 6MWT and 10MWT) were excluded from the analyses.
Results
Forty-three participants were included in this study (21 anodal tDCS + 22 sham tDCS). There were four dropouts, two participants (one active and one sham) before the interim evaluation, and two participants (one active and one sham) before the follow-up evaluation (Fig. 1). The reasons for dropouts are described in flow diagram (Fig. 1) and are likely not related to tDCS assignment. For baseline characteristics, there was no difference between groups of age, and time since injury, AIS scale, BBT and WISCI-II classification, and sex. There was a difference in the level of the lesion. Those aspects were controlled in multivariate models. Demographic and clinical characteristics are summarized in Table 1.
The mean value of WISCI-II for the sham group was 8.5 (SD ± 7.7) at baseline, 10.0 (SD ± 7.4) at intermediate evaluation, 10.8 (SD ± 6.7) post-treatment and 10.6 (SD ± 6.7) at three months follow-up. For the active group, it was 9.9 (SD ± 7.4) at baseline, 12.2 (SD ± 6.8) at intermediate evaluation, 13.1 (SD ± 6.3) post treatment and 13.7 (SD ± 6.2) at three months follow-up. These results are summarized in Fig. 2. All the participants tolerated tDCS sessions, without unexpected adverse effects. The most common side effects reported were tingling and itching. One participant did not tolerate Lokomat training due to pain related to the straps of the body weight-support mechanism and was excluded from the study.
WISCI-II analysis
In our main endpoint, post-treatment, the percentage of participants that improved in WISCI-II was 33.3% (7 of 21 participants) in the sham group and 70.0% (14 of 20 participants) in the active group. This difference was statistically significant (p = 0.046; OR: 3.7; 95% CI: 1.0–13.5). In the follow-up, there was also statistically significant difference in the changes for the two groups (p = 0.046; OR: 3.7; 95% CI: 1.0–13.5). However, at 15 days there was no significant difference between groups (p = 0.45; OR: 1.6, 95% CI: 0.5–5.8).
We created three models (Intermediate, post-treatment and follow-up), combining the main variable “tDCS intervention” with the other covariates (adding one by one). For the “Intermediate” model (after 15 days of intervention) the effect of treatment remains insignificant in adjusting for covariables.
For the main model (post-treatment), the effect of treatment remains significant (OR = 13.87; p = 0.013; 95% CI: 1.7–111.3) when adjusting for baseline HAD (OR = 0.80; p = 0.045; 95% CI: 0.64–0.99) and baseline WISCI-II (OR = 0.84; p = 0.007; 95% CI:0.74–0.95), with AUC = 0.86. For the follow-up model, the effect of treatment also remains significant (OR = 13.38; p = 0.014; 95% CI: 1.7–105.6) when adjusting for baseline HAD (OR = 0.79; p = 0.042; 95% CI: 0.63–0.99) and baseline WISCI-II (OR = 0.85; p = 0.010; 95% CI: 0.74–0.96), with AUC = 0.86.
Secondary analysis
Regardless of intervention group, statistical difference exist between baseline and the other periods (intermediate, post and follow-up) as measured by BBT (p = 0.001, p = 0.001, p = 0.001 and, respectively), 10MWT (p = 0.010, p = 0.001 and p = 0.001, respectively), 6MWT (p = 0.008, p = 0.001 and p = 0.001, respectively), TUG (p = 0.023, p = 0.001 and p = 0.002, respectively). There was also a significant improvement between baseline and the periods post and follow-up for HAD, (p = 0.035 and p = 0.001, respectively), and SCIM (p = 0.049 and p = 0.021, respectively). There was no statistical difference between baseline and intermediate evaluation for SCIM (p = 0.075). There was no statistical difference between baseline and the other periods (intermediate, post and follow-up) measured by MAS (p = 0.61, p = 0.33 and p = 0.90, respectively).
Regarding the improvement in the secondary outcomes (BBT, 10MWT, 6MWT, TUG, HAD and SCIM), there was no statistical difference between groups for the tested periods (intermediate, post and follow-up).
Discussion
In this study, we found that tDCS, combined with Lokomat training, potentiates gait improvement after 30 sessions and at the three months follow-up. At the intermediate evaluation, after 15 sessions, tDCS was not superior to sham, suggesting that the effect is dose-dependent.
Despite the advances in rehabilitation therapy, including robot-assisted gait training, motor improvements continue be limited in SCI [3, 19]. This pattern is consistent with the results of our sham group, in which 33.3% (post-treatment) and 35.0% (at the follow-up) improved their WISICI-II scores. Conversely, 70.0% (OR: 4.7) and 68.4% (OR: 4.0) of the active group improved post-treatment and at the follow-up, respectively. Moreover, the effects of tDCS remained significant even when several possible confounders were tested in a multivariate model, including stratification group (GI and GO), confirming the robust effect of the intervention. Although this trial was a phase 2 study, our results suggest that the inclusion of tDCS is a paradigm shift in the rehabilitation of SCI.
At baseline, the proportion of tetraplegic participants in the sham group was higher than in the active group. Previous studies have suggested that people with paraplegia have a better prognosis in regards to walking than people with tetraplegia; thus, this difference could be an important confounder in the trial [20]. However, the variable “level of lesion” (paraplegic vs. tetraplegic) was not significant in the model and did not alter the results.
Our findings are consistent with part of the results from Raithatha and colleagues, who showed that anodal tDCS is superior to sham in improving motor strength in lower limb of SCI. In contrast, Kumru and colleagues did not observe any superiority of active tDCS over sham. Our study differed from that of Kumru et al. by having a larger sample size (which minimized the possibility of type II errors), less variability in SCI etiology, and especially by the difference in tDCS montage. In Kumru et al., the anode was placed over the vertex (leg motor cortex), with the cathode over the nondominant supraorbital area. It is possible that this montage had a smaller effect over the cortex due to the shunting of the current over the sagittal sinus, skin, and cerebrospinal fluid [21, 22]. Finally, Kumru et al. used only 20 sessions of tDCS, which should be considered insufficient. Our analysis of 15 sessions failed to show a significant difference. Thus, the number of sessions is a critical factor, consistent with what other studies in chronic pain found [23, 24].
Two previous trials, one in SCI and the in stroke, have carried out a follow-up after completing the intervention of tDCS combined with gait training, showing that the improvement lasted up to one month [25]. Our study shows the effect lasting for three months, suggesting that tDCS may help consolidate improvement.
The montage used in our trial is the most extensively studied composition for motor function [14]. The effects of tDCS varies depending on the intensity of the current. The most studied and well known in regards to effects is the 2 mA, and was therefore selected for this clinical trial [26]. Although it is placed primarily over the hand area of the motor cortex, it is likely that current flowed to other areas of the motor cortex and somatosensorial cortex. Furthermore, it is likely that it indirectly effects and modulates others cortical areas and structures related to motor learning, such as cerebellum, basal ganglia, and the primary motor cortex [27].
The effect of the anode in motor learning might be related to the effect of local cortical excitability, based on the modulation of sodium and calcium-dependent channels, inhibitory interneurons, and NMDA receptor activity-promoting a long-term potentiation-like mechanism. Besides, tDCS may improve adaptive changes, such as the increased intracortical GABAergic inhibition levels, facilitating motor learning. This hypothesis is supported by the secondary analyses of this trial (published elsewhere), where we showed that the decreases in EEG high-beta power in the sensorimotor were associated with gait recovery [16]. Our primary outcome, WISCI-II, measures the level of functional mobility and physical assistance and the devices that are needed to walk. Thus, it is likely that tDCS improves various aspects of motor learning, including functional recovery and the ability to adapt to assistive technology and compensate for the motor impairment. Therefore, the improvement in WISCI-II is not only related to motor recovery, but also to motor compensation.
Moreover, the EEG analyses from the previous study showed that participants with larger high-beta power in the sensorimotor most likely to improve, suggesting that this EEG finding is a biomarker for a maladaptativa changes, which impairs the motor improvement. Therefore, a hypothesis is that RAGT can induce an improvement of this maladaptativa activity, allowing the neuronal network to reorganize in a more effective manner resulting in motor improvement. Moreover, the EEG analysis suggested tDCS potentialize this biological mechanism increasing the odds of motor learning. Another interpretation is that participants, that have already reached the full potential of motor improvement do not have the increase of high-beta power in the sensorimotor. So, the large percentage of participant that improved may be related to celling effect or failure to overcome the maladaptativa cerebral changes.
There are multiple motor learning processes related to improvement of gait, including acquiring new behavior (such as using a crutch) and recalibration of a previously learned movement [28], which are related to LTD-like and LTP-like plasticity. Therefore, we hypothesize that tDCS may induce a disruptive change in a previously consolidated neuronal network, potentializing the processes of recalibration and acquisition. Furthermore, it may potentialize the variability of motor execution, which, coupled with the reinforcement of RAGT, may improve motor learning [29]. It is important to highlight that the improvement reported is considered a noticeable change for clinical context, since the minimal detectable change (MDC) of WISCI-II can be considered one point [17].
Transcranial magnetic stimulation with respective pulses (rTMS) improves motor learning in SCI. Although it is a different method from tDCS, the mechanism of action is likely to be similar, as high-frequency rTMS also increases cortical excitability. In randomized sham-controlled trials, Kumru and colleagues (2016) and Benito and colleagues (2012) showed that high-frequency rTMS improves gait function in SCI [30, 31]. Theses previous findings from rTMS help support that the modulation of M1 may enhance motor learning in SCI. Moreover, Kumru and colleagues (2010) and Nardone (2014) reported that high-frequency rTMS over the motor cortex improves spasticity in SCI [32,33,34].
In our study, spasticity was not a confounder regarding the effect of tDCS, but participants with high spasticity were not included, and those with moderate spasticity were treated with botulinum toxin and phenolization. Thus, it is possible that tDCS improves spasticity, but a different study design is needed to test this hypothesis.
Recent metanalyses suggested that participants treated with RAGT have significantly greater improvements in gait than conventional over-ground training (OGT) groups, particularly during the acute stage. However, RAGT is not superior compared to treadmill training with body weight-support, and future studies are needed to evaluate its cost-effectiveness. In our trial, we choose RAGT since it helps to standardize the gait therapy and better evaluate the effect of tDCS. Also, improvement of a point in WISC-II is considered clinically significant; however, cost-effectiveness studies need to be performed. Moreover, as only a percentage of patients have improved, studies for the development of biomarkers that help identify participants with potential for recovery (as discussed above) are of great importance to increase resource use efficiency.
For the secondary outcomes, without considering the intervention group, participants demonstrated a significant improvement in balance, measured by BBT, in locomotion (measured by 10 MWT, 6 MWT, TUG) in depression and anxiety symptoms measured by HAD, and in quality of life, measured by SCIM. However, there was no significant difference between groups for these outcomes. The absence of difference between groups for HAD is coherent since it does not expect an improvement in these aspects with M1 stimulation. The improvement in SCIM may be related to other rehabilitation that participants received during treatment and can be secondary to motor improvement, but with no difference between groups. For other measures of gait (10 MWT, 6 MWT, TUG), only about half of the participants could performed the test. Therefore, the study is underpowered for these outcomes, which can result in type II errors.
The study has some limitations. The statistical analyses were not performed as planned during trial design, as such a large number of participants were not expected to not improve with rehabilitation. Therefore, we used categorical analysis as parametric testing would be invalid. Furthermore, this is an exploratory study. For the same reason, the study was not controlled for multiple analyses for the different time points (intermediate, post and follow-up). Besides, the study was underpowered for the secondary gait outcome. Moreover, the results cannot be generalized for participants with severe impairment (AIS A and B), a higher level of spasticity, or different rehabilitation treatments.
In summary, 30 sessions of active tDCS, compared with sham tDCS, increases the likelihood of gait improvement of robot-assisted gait post-treatment. These effects lasted at least for three months after treatment ceased. Finally, 15 sessions had no significant effect on walking gains.
Data archiving
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31.
Hesse S, Werner C. Connecting research to the needs of patients and clinicians. Brain Res Bull. 2009;78:26–34.
Nam KY, Kim HJ, Kwon BS, Park JW, Lee HJ, Yoo A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14:24.
Leite VF, Souza DR, Imamura M, Battistella LR. Post-discharge mortality in patients with traumatic spinal cord injury in a Brazilian hospital: a retrospective cohort. Spinal Cord. 2019;57:134–140. https://pubmed.ncbi.nlm.nih.gov/30089892/.
Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology.2001;57:1899–901.
Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114:600–4.
Huang YZ, Lu MK, Antal A, Classen J, Nitsche M, Ziemann U, et al. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol. 2017;128:2318–29.
Martin JH. Harnessing neural activity to promote repair of the damaged corticospinal system after spinal cord injury. Neural Regen Res. 2016;11:1389–91.
Yozbatiran N, Keser Z, Davis M, Stampas A, O’Malley MK, Cooper-Hay C, et al. Transcranial direct current stimulation (tDCS) of the primary motor cortex and robot-assisted arm training in chronic incomplete cervical spinal cord injury: a proof of concept sham-randomized clinical study. NeuroRehabilitation.2016;39:401–11.
Barron HC, Vogels TP, Emir UE, Makin TR, O’Shea J, Clare S, et al. Unmasking latent inhibitory connections in human cortex to reveal dormant cortical memories. Neuron.2016;90:191–203.
Ammann C, Spampinato D, Márquez-Ruiz J. Modulating motor learning through transcranial direct-current stimulation: an integrative view. Front Psychol. 2016;7:1981.
Kumru H, Murillo N, Benito-Penalva J, Tormos JM, Vidal J. Transcranial direct current stimulation is not effective in the motor strength and gait recovery following motor incomplete spinal cord injury during Lokomat(®) gait training. Neurosci Lett. 2016;620:143–7.
Raithatha R, Carrico C, Powell ES, Westgate PM, Chelette Ii KC, Lee K, et al. Non-invasive brain stimulation and robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study. NeuroRehabilitation.2016;38:15–25.
Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation (tDCS) in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2021;24:256–313. https://pubmed.ncbi.nlm.nih.gov/32710772/.
Simis M T107. Using Functional near Infrared Spectroscopy (fNIRS) to assess brain activity of spinal cord injury patient, during robot-assisted gait. In: Santos K, Sato J, Fregni F, Battistella L, editors. Clinical Neurophysiology: Elsevier; 2018. p. e43–e4.
Simis M, Uygur-Kucukseymen E, Pacheco-Barrios K, Battistella LR, Fregni F. Beta-band oscillations as a biomarker of gait recovery in spinal cord injury patients: a quantitative electroencephalography analysis. Clin Neurophysiol. 2020;131:1806–14.
Burns AS, Delparte JJ, Patrick M, Marino RJ, Ditunno JF. The reproducibility and convergent validity of the walking index for spinal cord injury (WISCI) in chronic spinal cord injury. Neurorehabil Neural Repair. 2011;25:149–57.
Kahan BC, Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ.2012;345:e5840.
Gomes-Osman J, Field-Fote EC. Cortical vs. afferent stimulation as an adjunct to functional task practice training: a randomized, comparative pilot study in people with cervical spinal cord injury. Clin Rehabil. 2015;29:771–82.
Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci. 2014;8:141.
Bai S, Dokos S, Ho KA, Loo C. A computational modelling study of transcranial direct current stimulation montages used in depression. Neuroimage.2014;87:332–44.
Neuling T, Wagner S, Wolters CH, Zaehle T, Herrmann CS. Finite-element model predicts current density distribution for clinical applications of tDCS and tACS. Front Psychiatry. 2012;3:83.
Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, Brandao R, Coutinho L, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain. 2016;17:14–26.
Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2:353–61.
de Paz RH, Serrano-Muñoz D, Pérez-Nombela S, Bravo-Esteban E, Avendaño-Coy J, Gómez-Soriano J. Combining transcranial direct-current stimulation with gait training in patients with neurological disorders: a systematic review. J Neuroeng Rehabil. 2019;16:114.
Agboada D, Mosayebi Samani M, Jamil A, Kuo MF, Nitsche MA. Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci Rep. 2019;9:18185.
Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron.2011;72:469–76.
Spampinato D, Celnik P. Multiple motor learning processes in humans: defining their neurophysiological bases. Neuroscientist. 2020. https://doi.org/10.1177/1073858420939552.
Dhawale AK, Smith MA, Ölveczky BP. The role of variability in motor learning. Annu Rev Neurosci. 2017;40:479–98.
Kumru H, Benito-Penalva J, Valls-Sole J, Murillo N, Tormos JM, Flores C, et al. Placebo-controlled study of rTMS combined with Lokomat. Exp Brain Res. 2016;234:3447–55.
Benito J, Kumru H, Murillo N, Costa U, Medina J, Tormos JM, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spinal Cord Inj Rehabil. 2012;18:106–12.
Korzhova J, Sinitsyn D, Chervyakov A, Poydasheva A, Zakharova M, Suponeva N, et al. Transcranial and spinal cord magnetic stimulation in treatment of spasticity: a literature review and meta-analysis. Eur J Phys Rehabil Med. 2018;54:75–84.
Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010;24:435–41.
Nardone R, Höller Y, Thomschewski A, Brigo F, Orioli A, Höller P, et al. rTMS modulates reciprocal inhibition in patients with traumatic spinal cord injury. Spinal Cord. 2014;52:831–5.
Acknowledgements
Authors are grateful to Margarida H. Miyazaki, executive director of IMREA and Katia Lina Miyahara, Clinical Director of IMREA, for the great support of the hospital. Besides, Artur dos Santos, Antonio Migliorati, Daniel de Souza, Denise Matheus, Gerson Barbieri Filho, Karin Santos, Mariana Milazzotto, Patricia Monteiro Marchiore, Thais Terranova, Tharsila de Oliveira and Vivian da Silva for assistance with participant data collection and study monitoring.
Funding
This work was supported by grants from Ministry of Health (SIPAR: 25000.160.761/2014-54). The researchers received support from the São Paulo Research Foundation (FAPESP- SPEC, grant #2017/12943-8). The work received a support from Núcleo de Apoio a Pesquisa-Núcleo de Estudos Avançados em Reabilitação (NAP-NEAR).
Author information
Authors and Affiliations
Contributions
MS, FF, and LB conceptualized the paper. MS and LB applied the therapies. MS and FF analyzed the data. LCB and FF provided a critical review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. This study was approved by the Ethics Committee for Analysis of Research Projects of the University of São Paulo Medical School.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Simis, M., Fregni, F. & Battistella, L.R. Transcranial direct current stimulation combined with robotic training in incomplete spinal cord injury: a randomized, sham-controlled clinical trial. Spinal Cord Ser Cases 7, 87 (2021). https://doi.org/10.1038/s41394-021-00448-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00448-9
This article is cited by
-
Effects of non-invasive brain stimulation on motor function after spinal cord injury: a systematic review and meta-analysis
Journal of NeuroEngineering and Rehabilitation (2023)