Abstract
Study design
Retrospective, case-control study.
Objectives
In a traumatic spinal injury (TSI) cohort from Tanzania, we sought to: (1) describe potential risk factors for pressure ulcer development, (2) present an illustrative case, and (3) propose a low-cost outpatient protocol for prevention and treatment.
Setting
Tertiary referral hospital.
Methods
All patients admitted for TSI over a 33-month period were reviewed. Variables included demographics, time to hospital, injury characteristics, operative management, length of hospitalization, and mortality. Pressure ulcer development was the primary outcome. Regressions were used to report potential predictors, and international guidelines were referenced to construct a low-cost outpatient protocol.
Results
Of 267 patients that met the inclusion criteria, 51 developed a pressure ulcer. Length of stay was greater for patients with pressure ulcers compared with those without (45 vs. 30 days, p < 0.001). Potential predictors for developing pressure ulcers were: increased days from injury to hospital admission (p = 0.036), American Spinal Injury Association Impairment Scale grade A upon admission (p < 0.001), and thoracic spine injury (p = 0.037). The illustrative case described a young male presenting ~2 months after complete thoracic spinal cord injury with a grade IV sacral pressure ulcer that lead to septic shock and death. Considering the dramatic consequences of pressure ulcers in lower- and middle-income countries (LMICs), we proposed a low-cost protocol for prevention and treatment targeting support surfaces, repositioning, skin care, nutrition, follow-up, and dressing.
Conclusions
Pressure ulcers after TSI in LMICs can lead to increased hospital stays and major adverse events. High-risk patients were those with delayed presentation, complete neurologic injuries, and thoracic injuries. We recommended aggressive prevention and treatment strategies suitable for resource-constrained settings.
Similar content being viewed by others
Introduction
Traumatic spinal injury (TSI) with associated spinal cord injury (SCI) is a major public health problem in lower- and middle-income countries (LMICs) [1]. One estimated incidence of TSI in Africa is 13.6 per 100,000, almost triple that of the United States (U.S.) and Canada [2]. Patients with TSI suffer from downstream complications affecting nearly every organ system. Pressure ulcer development due to loss of sensation and lack of supportive care is particularly dangerous and can lead to rehospitalization and sometimes death [3].
Pressure ulcers are defined as localized injury to the skin and underlying tissue, most often over a bony prominence, as a result of shear forces [4]. Pressure ulcers are categorized as grade I, nonblanchable erythema; grade II, partial thickness skin loss; grade III, full thickness skin loss with subcutaneous fat usually visible; and grade IV, full thickness tissue loss with exposed muscle, tendon, or bone [4, 5]. Healthcare costs associated with pressure ulcer management in the U.S. range from $6 to $15 billion annually [6]. In Africa, one report suggested that pressure ulcer treatment accounts for 25% of all costs related to SCI care [7]. The prevalence of pressure ulcers in TSI patients across LMICs ranges from 27 to 46% [8], although it is believed this may be an underestimation [9]. Prevention and treatment strategies targeting less-resourced populations have largely been descriptive, suggesting improvements in acute care, nurse-to-patient ratios, and support surfaces, and are limited to inpatient settings [8]. Unlike more developed countries, rehabilitation centers in LMICs are limited, and patients are usually discharged home.
Despite the prevalence and financial burden of pressure ulcers in the spine trauma population of LMICs, limited resources exist to identify the most at risk patients and manage this complication in the outpatient setting, where the majority of care is delivered. In a large TSI cohort from a major referral center in Tanzania, the objectives of the current study were to: (1) describe risk factors for development of pressure ulcers, (2) present an illustrative case, and (3) propose a low-cost outpatient management protocol for prevention and treatment of pressure ulcers.
Methods
Study design and clinical setting
A retrospective, case-control study of prospectively collected data at Muhimbili Orthopaedic Institute (MOI), a joint orthopaedic and neurosurgery hospital in Dar es Salaam, Tanzania, was performed. MOI is the tertiary referral center for the country, with 120 general ward beds, 16 Intensive Care Unit (ICU) beds, 10 Emergency Department beds, and 5 operating rooms. X-ray, Computed Tomography, and Magnetic Resonance Imaging (MRI) are all readily available. The current study represents an extension of a prior series that focused exclusively on operative treatment of TSI and did not address pressure ulcer development [10].
Patient identification
All patients admitted for TSI over a 33-month period (September 2016 to May 2019) were reviewed. Patients under the age of 14 or with a concomitant moderate to severe brain injury were excluded. During the study period, operative decisions were made without an official spine trauma protocol. Both the decision to operate and operative plan were heavily dependent on the patient’s financial resources to pay for spinal implants and surgeon preference.
Clinical data collection
Independent variables included demographics, injury mechanism, fracture type/level, and insurance status, categorized as public or private. Patients with public insurance were required to pay for hospital services before receiving them, while private patients were not required to provide payment upfront. Prehospital care was described by distance from injury site to MOI (km), prior outside hospital (OSH) admission (yes/no), and days from injury to MOI admission. Fracture type was defined using the AO Classification System (A0-4, B1-3, C) [11]. Level of injury was recorded as axial cervical (occiput-C2), subaxial cervical (C3-C7), thoracic (T1-T12), or lumbar (L1-L5). If the injured levels included C7 and T1 or T12 and L1, it was classified as cervicothoracic or thoracolumbar, respectively. Neurologic status upon admission and discharge was determined according to the International Standards for Neurological Classification of Spinal Injury using the American Spinal Injury Association Impairment Scale (AIS) [12]. Improvement or decline in neurologic status was defined by change in at least one AIS grade during the hospital stay.
The primary outcome of interest was development of pressure ulcer during initial hospitalization. Location of pressure ulcer was not recorded. Additional outcomes included neurologic improvement, defined as improvement in AIS grade vs. stable or decline, length of stay (LOS), and mortality during admission. Of note, these outcomes were treated as predictor variables in the subsequent multivariate analysis for development of a pressure ulcer.
Development of outpatient pressure ulcer protocol
Though pressure ulcers could be more adequately prevented in a hospital setting, anecdotal feedback from nurses and physicians was that patients with SCI are often readmitted after being sent home. Given the paucity of outpatient pressure ulcer care, the need for an outpatient pressure ulcer protocol was realized. A comprehensive literature review of pressure ulcer management was combined with weekly, local stakeholder meetings to learn about current hospital practices. These stakeholders included: floor nurses, ICU nurses, nursing leadership, physiotherapists, nutritionists, and anesthesiologists. Meetings involved qualitative discussion of current practice and cost-effective ideas for outpatient management.
Statistical analysis
Continuous variables were reported as mean (standard deviation) and median (range). Categorical data were presented as count (%). Student’s t tests were used for comparing normally distributed, continuous data; chi-square and Wilcoxon rank sum tests were used for categorical data. Independent variables of pressure ulcer vs. non-pressure ulcer patients were compared. Predictors of pressure ulcer development were determined by univariate followed by multivariate logistic regression; potential risk factors were chosen based on prior expert knowledge of all authors to look for associations with the outcome of interest. Any variable < 0.10 on univariate analysis was entered into the multivariate regression, and results were considered significant if p < 0.05. Statistics were performed using RStudio, version 1.2.1335. The study was approved by the local institutional review board and in compliance with all ethical guidelines.
Results
Demographics
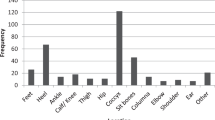
A total of 270 patients were enlisted; 3 did not have pressure ulcer data and were excluded. Fifty-one patients with pressure ulcers were compared with 216 without. The pressure ulcer group had more males (p = 0.046), lived farther from MOI (p = 0.038), had more complex fractures (p = 0.002), and suffered more injuries to the thoracic region (p < 0.001) (Table 1). In terms of neurologic status, those with pressure ulcers were more commonly AIS A (p < 0.001), and those without were more commonly AIS B (p = 0.013), AIS D (p = 0.026), and AIS E (p = 0.003). The majority of pressure ulcers occurred in AIS A patients with considerably less in AIS B–E patients (Fig. 1). No difference was seen in those who underwent operative and nonoperative treatment. Of all pressure ulcers, 49% were categorized as severe (Grades III and IV).
Predictors of pressure ulcer development
A total of six variables were found to be significant after univariate logistic regression: gender, distance from injury site to MOI, days from injury to MOI, neurologic status, and level of spinal injury (Table 2). After multivariable logistic regression, three variables emerged as independent predictors of pressure ulcer development: increased number of days from injury to hospital admission (odds ratio (OR) 1.03, p = 0.036, 95% confidence interval (CI) 1.00–1.06), AIS A neurologic exam (OR 8.33, p < 0.001, 95% CI 3.34–24.61), and injury to the thoracic spine (OR 2.16, p = 0.037, 95% CI 1.05–4.49).
Illustrative case
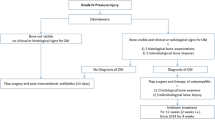
A 25-year-old male with no prior medical history was playing competitive soccer in the coastal region of Mtwara, Tanzania when he reportedly collided with another player in the air, then fell landing on his back. He immediately experienced complete loss of strength and sensation in both of his lower limbs. After several days of no improvement and ensuing urinary retention, he presented to an OSH. Due to the severity of the injury, he was transferred to a second OSH, where he stayed for ~2 months and was managed nonoperatively. Due to lack of improvement over that time period, he was then transferred 562 km to MOI for further management. Upon arrival, the patient’s neurologic exam was AIS A and his MRI revealed T7/8 burst fractures with spinal canal compromise.
The patient was admitted to MOI with a grade II sacral pressure ulcer. He was given an air mattress and treated with daily wound dressing changes, intravenous (IV) antibiotics due to positive wound cultures, and sloughectomy, where necrosed tissue is debrided. On hospital day 8, the neurosurgical team decided that the fractures were stable given the length of time from the initial injury, and surgery was not needed. The patient was discharged with advice to pursue physiotherapy and purchase a cushioned wheelchair and air mattress, and avoid direct pressure to the ulcer.
Forty-three days after initial MOI admission, the patient was readmitted due to progression of his sacral pressure ulcer to grade IV, and emergence of new ulcers on his hips, knee, and heel (Fig. 2a–c). The patient was cachectic, contracted, and severely anemic. Treatment included broad spectrum IV antibiotics again due to positive wound cultures, daily wound dressing changes with honey and vinegar, serial sloughectomies, position changes every 2 hours, 2 units of transfused blood, IV fluids, and a high protein diet. On hospital day 16 (59 days after initial MOI admission), the patient entered septic shock and died on the ward.
Development of outpatient pressure ulcer protocol
Guidelines and previous reports were used to formulate the Outpatient Pressure Ulcer Protocol (Table 3) [13,14,15,16,17]. This protocol addressed six areas of attention: (1) support surfaces, (2) repositioning, (3) skin care, (4) nutrition, (5) follow-up, and (6) dressing. A proposed daily schedule for the Outpatient Pressure Ulcer Protocol was also formulated (Fig. 3). Relevant pictures of protocol tools are shown: water-filled gloves, pads for repositioning, wheelchair use for mobilization, and over-the-counter Sudocrem (Forest Tosera Ltd., Dublin, Ireland) which contains a water-repellant base plus antibacterial and antifungal properties (Fig. 4a–d).
a Large pad for frequent repositioning; b Water-filled glove to redistribute body-weight on heels; c Cost-effective wheelchair as a repositioning option; d Sudocrem (Forest Tosera Ltd., Dublin, Ireland) to be applied on regions at high-risk for pressure ulcer development, such as the heels, sacrum, hips, back of head and other bony prominences.
Discussion
The current study described potential risk factors for developing pressure ulcers in a cohort of TSI patients in Tanzania and presented an illustrative case to underscore the dramatic consequences of this complication. Of the 267 patients included, 19% developed pressure ulcers. Eighty-eight percent of patients with pressure ulcers had complete neurologic injuries, and the majority were high-grade ulcers. Longer time from injury to admission, complete injuries, and thoracic injuries were independent predictors for pressure ulcer development. We also provided outpatient pressure ulcer guidelines to prevent and treat patients with pressure ulcers in resource-limited settings. The following analysis further discusses who is most at risk and delves into strategies for sustainable implementation of the protocol.
Consistent with prior reports, days from injury to admission and complete spinal cord injuries were independent risk factors for pressure ulcer development. Gélis et al. [18] in 2009 systematically reviewed 22 studies on pressure ulcer risk factors among SCI patients and found a positive correlation between increased time since injury and severity of neurologic injury with pressure ulcer development. One of the only LMIC studies on pressure ulcers in TSI patients was from 2011 by Idowu et al. [19], who analyzed 105 SCI patients at a single center in Nigeria. The authors found that patients with pressure ulcers had decreased nutritional status assessed by serum albumin, longer intervals before admission, and most commonly suffered AIS A injuries. In the current study, the pressure ulcer group presented to the hospital on average 3 days later than patients without pressure ulcers, and AIS A patients had eightfold increased odds of developing a pressure ulcer. It is critical that TSI patients receive immediate supportive care, and lack thereof due to delayed hospital admission may increase one’s chance of developing a pressure ulcer. AIS A patients likely were at increased odds because of complete sensation loss below the neurologic level of injury. Furthermore, patients in our study with thoracic injuries had twofold increased odds of having a pressure ulcer, which is contrary to findings from Gélis et al. [18] who stated that there was strong evidence against level of SCI being correlated with pressure ulcers. One explanation for our finding is that severe thoracic spine dislocations often cause bony prominences in the midback that increase skin pressure and friction when laying supine. Unfortunately, pressure ulcer location in this subgroup or others was not recorded.
Surgical intervention was not associated with decreased risk of pressure ulcer development, which could be a result of significant delays to surgery. Leidinger et al. reported a mean time of 33.2 days to surgery among 72 spinal trauma patients from our center, primarily due to patients’ inability to pay for implants [20]. Without insurance, patients must gather funds from extended family, which takes time. Current efforts at MOI are focusing on implementation of a “Spine Trauma Protocol” that prioritizes early decompression regardless of a patient’s ability to pay. Theoretically, early surgery increases the chance of neurologic improvement, in turn improving a patient’s sensation and decreasing the incidence of pressure ulcers. We plan to further study pressure ulcer incidence after implementation of the new “Spine Trauma Protocol.”
Due to their severity and potential for added morbidity, SCI patients would benefit from a cost-effective treatment plan, especially in the outpatient setting where acute hospital services and nursing care are no longer offered. The proposed cost-effective pressure ulcer treatment and prevention protocol was derived from evidence-based guidelines outlined by the American College of Physicians [15, 16] and others [13, 14], and emphasizes a multimodal approach. Whenever possible, outpatient providers caring for patients with pressure ulcers in resource-limited settings should address each of the six intervention targets, as lacking in even one category might increase the chance for pressure ulcer development or progression of an existing ulcer to high-grade. For instance, an excellent plan for support surfaces and skin care would be easily undermined by poor nutrition. Although the current protocol is translatable to many settings, providers should also feel comfortable using the evidence-based recommendations to construct their own plans. Sudocrem (Forest Tosera Ltd., Dublin, Ireland) is easily obtained in Tanzania, but could be replaced by one of the many affordable over-the-counter skin care creams depending on the provider’s location. Providers should also consider local natural remedies when supported by literature, such as gauze dressed with honey, which creates a negative pressure environment due to its high osmolarity, inactivates destructive proteases by acidifying the wound, and provides antibacterial activity [17]. Lastly, telemedicine should be utilized when possible, encouraging patients to send photos of pressure ulcers to their provider for close follow-up [21]. The adaptability of the proposed protocol makes it easy to initiate, but long-term adherence will require a multidisciplinary effort as discussed below.
Successful implementation of the current protocol has the potential to drastically reduce pressure ulcer burden in TSI patients within resource-limited settings. We offer the following recommendations for sustainable, long-term management:
-
1.
Target TSI patients who are most at risk. Patients who present in a delayed fashion with AIS A injuries at the thoracic spine level should be identified early as high-risk patients.
-
2.
Counsel all stakeholders and patients on the six intervention targets. Stakeholders and TSI patients should be educated on the importance of (1) support surfaces, (2) repositioning, (3) skin care, (4) nutrition, (5) follow-up, and (6) dressing for preventing and treating pressure ulcers. Complete patient understanding of the protocol before discharge from the hospital is essential for proper outpatient care.
-
3.
Reassess patients with consistent and frequent follow-up appointments. Pressure ulcers can progress rapidly, as seen in our illustrative case, and require assessment by a healthcare provider at least monthly. In addition to wound care, these visits should address the unique barriers that each patient faces to protocol adherence.
-
4.
Establish local care centers with experience in pressure ulcer treatment. TSI patients in resource-limited regions often travel long distances to receive initial care; in the current study, patients with pressure ulcers traveled on average 344 km to MOI. To ensure frequent follow-ups, local facilities should be trained in basic pressure ulcer management and understand when to escalate care to tertiary hospitals.
The current study is not without limitation. Time of pressure ulcer onset was not collected, and thus we were unable to differentiate patients who came to the hospital with pressure ulcers from those who developed pressure ulcers after admission. In addition, follow-up data were not gathered, and therefore the clinical outcomes of patients with pressure ulcers was unknown. Lastly, while the pressure ulcer treatment and prevention regimen herein was designed to work in most resource-constrained settings, it will need to be catered to each center’s unique set of available resources.
Conclusion
Pressure ulcers in TSI patients are difficult to manage in LMICs and lead to increased lengths of stay and major adverse events. TSI patients at high-risk for development of pressure ulcers are those with delayed hospital presentation, complete neurologic deficits (eightfold increased odds), and thoracic spine injuries (twofold increased odds). We recommended aggressive prevention and treatment strategies suitable for patients and providers in resource-constrained settings. Future work should prospectively describe incidence and outcomes among TSI patients with pressure ulcers who adhere to the proposed protocol in a resource-limited setting.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Injury GBDTB, Spinal Cord Injury C . Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87.
Kumar R, Lim J, Mekary RA, Rattani A, Dewan MC, Sharif SY, et al. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018;113:e345–e63.
Sezer N, Akkus S, Ugurlu FG. Chronic complications of spinal cord injury. World J Orthop 2015;6:24–33.
National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Emily Haesler (Ed.). Cambridge Media: Perth, Australia; 2014.
Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised national pressure ulcer advisory panel pressure injury staging system: revised pressure injury staging system. J Wound Ostomy Cont Nurs 2016;43:585–97.
Markova A, Mostow EN. US skin disease assessment: ulcer and wound care. Dermatol Clin.2012;30:107–11.
Iyun AO, Malomo AO, Oluwatosin OM, Ademola SA, Shokunbi MT. Pattern of presentation of pressure ulcers in traumatic spinal cord injured patients in University College Hospital, Ibadan. Int Wound J. 2012;9:206–13.
Zakrasek EC, Creasey G, Crew JD. Pressure ulcers in people with spinal cord injury in developing nations. Spinal Cord 2015;53:17–7.
Burns AS, O’Connell C. The challenge of spinal cord injury care in the developing world. J Spinal Cord Med. 2012;35:3–8.
Magogo J LA, Mango M, Zuckerman SL, Leidinger A, Msuya S, Rutabasibwa N, et al. Operative treatment of traumatic spinal injuries in Tanzania: surgical management, neurologic outcomes, and time to surgery. Global Spine J. 2020;1–10. https://doi.org/10.1177/2192568219894956.
Schnake KJ, Schroeder GD, Vaccaro AR, Oner C. AOSpine classification systems (subaxial, thoracolumbar). J Orthop Trauma. 2017;31:S14–23.
Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011;34:547–54.
Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA. 2008;300:2647–62.
Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296:974–84.
Qaseem A, Humphrey LL, Forciea MA, Starkey M, Denberg TD. Clinical Guidelines Committee of the American College of P. Treatment of pressure ulcers: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2015;162:370–9.
Qaseem A, Mir TP, Starkey M, Denberg TD. Clinical Guidelines Committee of the American College of P. Risk assessment and prevention of pressure ulcers: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2015;162:359–69.
Molan P, Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27:141–51.
Gelis A, Dupeyron A, Legros P, Benaim C, Pelissier J, Fattal C. Pressure ulcer risk factors in persons with spinal cord injury part 2: the chronic stage. Spinal Cord. 2009;47:651–61.
Idowu OK, Yinusa W, Gbadegesin SA, Adebule GT. Risk factors for pressure ulceration in a resource constrained spinal injury service. Spinal Cord. 2011;49:643–7.
Leidinger A, Kim EE, Navarro-Ramirez R, Rutabasibwa N, Msuya SR, Askin G. et al. Spinal trauma in Tanzania: current management and outcomes. J Neurosurg Spine. 2019;31:103–11.
Irgens I, Rekand T, Arora M, Liu N, Marshall R, Biering-Sorensen F, et al. Telehealth for people with spinal cord injury: a narrative review. Spinal Cord. 2018;56:643–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed, including institutional review board approval. Patient information in the dataset and the illustrative case were deidentified prior to review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lessing, N.L., Mwesige, S., Lazaro, A. et al. Pressure ulcers after traumatic spinal injury in East Africa: risk factors, illustrative case, and low-cost protocol for prevention and treatment. Spinal Cord Ser Cases 6, 48 (2020). https://doi.org/10.1038/s41394-020-0294-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-020-0294-5