Abstract
Study design
Retrospective analysis of prospectively collected data.
Objective
Central cord syndrome (CCS) is reported to have better outcomes than other cervical lesions, especially for ambulation and bladder recovery. However, a formal comparison between patients with CCS and other incomplete cervical spinal cord injuries (iCSCI) is lacking. Aim of the study is to investigate the neurological and functional outcomes in patients with or without CCS.
Setting
European Multicenter Study.
Methods
Data following SCI were derived from the European Multicenter Study about Spinal Cord Injury Database. CCS was diagnosed based on a difference of at least ten points of motor score in favour of the lower extremities. Patients were evaluated at 30 days, 6 months and 1 year from injury. The neurological and functional data were collected at each time point based on the International Standards for Neurological Classification of Spinal Cord injury (ISNSCI) and Spinal Cord Independence Measure (SCIM). Patients were selected with a matching procedure based on lesion severity, neurological level of injury (NLI) and age. Evaluation of the outcomes was performed by means of two-way Anova for repeated measures.
Results
The matching produced 110 comparable dyads. At all time points, upper extremity motor scores remained lower than lower extremity motor scores in CCS compared with iCSCI. With regard to daily life independence, both cohorts achieved comparable improvements in self-care sub-scores between T0 and T2 (6.6 ± 6.5 in CCS vs 8.2 ± 6.9 in iCSCI, p = 0.15) but this sub-score was significantly lower in CCS compared with iCSCI (3.6 ± 5.2 in CCS vs 7.3 ± 7.0 in iCSCI at T0, 13.7 ± 6.2 vs 16.5 ± 5.7 at T2), while the other sub-scores were comparable.
Conclusions
In contrast to previous reports, people with CCS have poorer outcomes of self-care ability compared with iCSCI.
Similar content being viewed by others
Introduction
The epidemiology of traumatic spinal cord injury (SCI) in the western countries has been changing over the last decades.
The United States Spinal Cord Injury Statistical Center [1] reported an increase in the average age of patients, from 29 years in the 1970s to 43 years in the last report. Comparable changes are reported in recent epidemiological studies from European countries [2,3,4,5]. For example, in Italy the average age of SCI patients increased from 38.5 years in 1997–1999 [6] to 54 years in 2013–2014 [4].
The causes of SCI have also changed: 20 years ago, the most frequent cause was traffic accidents, while at present, SCI occurs more often due to falls from a low height, in particular among people over the age of 55 [4, 7].
The third epidemiological evidence is a change of the neurological level of injury (NLI) and severity of SCIs with a progressive increase in incomplete cervical spinal cord injuries (iCSCI) [1]. With regard to cervical lesions, there has been an increase in C1–C4 lesions (from 21.7% in the years 1994–1998 to 31.2% in the years 2009–2013) [8] and an increase of incomplete lesions (from 20.9% in the years 1997–1999 [6] to 43.3% in the years 2013–2014) [4].
Central cord syndrome (CCS) is considered the most common incomplete tetraplegia, accounting for about 9% of all traumatic SCIs [9, 10] with an increasing incidence [11]. CCS is characterized by a disproportion of impairment in the upper and lower limbs, with more pronounced muscle weakness and reduced function in the upper extremities, neurogenic bladder dysfunction and different degrees of sensation loss [12].
CCS has a bimodal age distribution with a cut-off around the age of 50, with a peak at a younger age (where CCS is attributable to “high-energy impact”) and a peak at an older age (where CCS is most likely due to a “low-energy impact” event) [11, 13]. In elderly patients, CCS is usually produced by a hyperextension trauma of the neck with pre-existing cervical spondylosis or stenosis of the cervical canal [13].
CCS is considered a spinal syndrome with a better prognosis in terms of neurological and functional recovery compared with other iCSCI [12,13,14,15,16]. Patients with CCS tend to show good improvement in total motor score, bladder management, daily life independence and walking [17, 18].
However, these data rely mainly on a relatively small case series of CCS patients. Also, comparisons of patients with CCS to those with other types of iCSCI typically do not take into account possible confounding factors known to influence outcome, which may be different between these two types of iCSCI. Disappointingly, a formal comparison between patients with CCS and iCSCI is lacking.
Therefore, the aim of the present study is to compare the neurological and functional outcome of patients with CCS and other forms of iCSCI.
Methods
Data were derived from the European Multicenter Study about Spinal Cord Injury (EMSCI) database (https://www.emsci.org, ClinicalTrials.gov Identifier: NCT01571531). EMSCI is a prospective longitudinal cohort study, involving 25 European spinal cord injury centers in the systematic collection of patients’ data during the first year after traumatic and vascular SCI. The study, started in 2001, includes a large sample of spinal cord injured patients who have been treated with state-of-the-art therapies and rehabilitation. Before entering the study, all patients gave informed consent to participate. The study conforms to the standards expressed in the Declaration of Helsinki and was approved by the ethical committee of the participating centers.
EMSCI time schedule and core set
EMSCI establishes the data collection in fixed time points from injury, i.e. within 15 days and 1, 3, 6 and 12 months after injury. The assessments of the essential core set include clinical, functional and independence evaluations.
Clinical assessment was based on sensory and motor scores derived from ISNCSCI, allowing evaluation of NLI and severity as well as upper (UEMS) and lower extremity motor scores (LEMS) [19, 20]. Within the EMSCI database, a validated EMSCI-ISNCSCI calculator (http://ais.emsci.org) [21] electronically calculates AIS and all other classification variables.
Functional assessments of ambulation include the following:
-
(1)
the 6-min walk test (6MWT) [22], which measures the distance covered by a subject walking at his/her own preferred in 6 min;
-
(2)
the 10-m walk test (10MWT) [23], which measures the time required by a subject to walk a 10-m distance;
-
(3)
the Walking Index for Spinal Cord Injury II (WISCI II) [24], which grades the ability of patients to walk a 10-m distance: the score ranges from 0 (inability to walk) to 20 (ability to walk without aids or assistance).
Independence in daily life activities is evaluated through Spinal Cord Independence Measure (SCIM) versions II and III [25, 26], comprising three domains: self-care (sub-score 0–20), respiration and sphincter management (sub-score 0–40) and mobility (sub-score 0–40), and provides a total score (range 0–100), with 100 indicating full independence.
Study design
From the EMSCI database, we extracted data of all patients who suffered iCSCI within 40 days from injury, with a date of injury between July 2001 and 2016. In order to assess incidence and range of severity of CCS, a “central myelopathy index” (CMI) was calculated in the same way as a previously published score developed to quantify Brown-Séquard-like spinal hemi-syndrome [27]. For each patient, the percent ratio of average segmental motor scores below NLI was calculated from upper and lower extremities. A CMI of 50% (100%), for example, would indicate an average segmental motor score of 2.5 (0) for all cervical segments (including T1) below NLI and an average segmental motor score of 5 in all lumbar/sacral myotomes. This allowed us to describe CCS as a continuum of patients presenting with a range of severity of CCS, rather than applying an arbitrary cut-off difference between UEMS and LEMS.
To compare the neurological and functional outcome of patients with CCS and other forms of iCSCI, CCS was defined by a difference between LEMS and UEMS of at least ten points in favour of LEMS, in line with the diagnostic criteria suggested by Middendorp [28]. Outcomes of this group were then compared with iCSCI. A preliminary analysis showed that patients with CCS were significantly older than the average patient with iCSCI (56.3 ± 16.3 vs 49.3 ± 19.5, p < 0.001). Furthermore, they had a higher incidence of NLI C1–C4 (68% vs 50%, p < 0.01) and higher percentage of AIS D lesion (82% vs 63%, p < 0.01). As with age, NLI and AIS grade are all well-known prognostic factors for SCI, so a matching procedure based on these features was used to create two comparable cohorts of patients with CCS and iCSCI. The match was exact for AIS grade and NLI, while for age an interval within ±5 years was tolerated. The patients were not matched by gender because the effect of gender on SCI outcome is questionable. The matching was performed using R package MatchIt [29].
Outcome measures
The primary outcome was the level of independence at enrolment (i.e. within 40 days), 6 and 12 months after SCI, evaluated through SCIM II/III total score and the analysis of its sub-scores: self-care, respiration and sphincter management and mobility. Furthermore, bladder and bowel independence were also assessed as the percentage of patients with a SCIM “Sphincter management-bladder” score of 15 (for bladder management) and “Sphincter management-bowel” of 10 (for bowel management) at the first and last evaluations.
The secondary outcomes were as follows:
-
(1)
The neurological status at enrolment, 6 and 12 months after SCI, evaluated through AIS grade, total motor score, UEMS and LEMS. Neurological improvement was assessed also in terms of AIS grade change in the matched cohorts.
-
(2)
The walking capacity at enrolment, 6 and 12 months after SCI, evaluated through WISCI II, 6MWT and 10 MWT. Walking capacity was also assessed, based on WISCI scale, as the percentage of patients unable to walk (WISCI II levels 0–3), those needing physical assistance to walk (WISCI II levels 4, 6–8, 10, 11, 14 and 17) and those walking without assistance (all the remaining WISCI levels) both at the first and last evaluations.
Statistical analysis
Data are reported as mean (standard deviation) or median (range) if continuous, as percentage if categorical. Variables from the two samples (patients with CCS and iCSCI) were compared over the three time points with two-way ANOVA for repeated measures (“between” factor: group, two levels, (CCS or TI); “within” factor: time, three levels (T0–T2) and “dependent” variables: total motor score, UEMS, LEMS, SCIM 2/3 and WISCI II, 10MWT and 6MWT). We also calculated the improvement of each outcome measure between T0–T1, T1–T2 and T0–T2, and compared them with the same statistics. Chi square test was used to evaluate AIS grade improvement, independence in bladder and bowel management and walking with/without physical assistance. Statistical analyses were performed with SPSS for windows (version 21.0, Chicago, IL).
Results
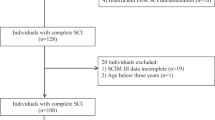
From the EMSCI database we extracted data of 1033 patients with incomplete tetraplegia. Of these, 866 could be rated with a CMI and 546 presented with the complete dataset over the first year after incidence (Fig. 1). From this sample, the matching procedure selected 110 dyads comparable for age distribution, NLI and severity (Table 1).
Baseline comparison
At T0 (i.e. within 40 days from injury), the two groups were comparable in terms of ISNCSCI total motor score. Based on the definition of CCS, these patients displayed lower UEMS, but higher LEMS, than iCSCI patients (Table 2). The distribution of matched dyads with respect to NLI was comparable and thus deemed representative for the entire sample (Fig. 2a). Calculation of CMI indicated that asymmetry of motor scores with UEMS < LEMS is a continuum with decreasing likelihood for increasing CMI (Fig. 2b). A majority of cervical SCI patients has no relevant CCS. Of 866 patients, 621 (73%) had a CMI of 20% or less, which was about equivalent to a motor score difference less than ten points between UEMS and LEMS. A CMI of more than 60% was very rare (eight cases, <1%). Figure 2b shows some overlap between the matched groups with and without CCS, which is due to defining CCS by an absolute ten-point difference UEMS < LEMS irrespective of NLI, thereby including segments which were actually intact, or underestimating proportionate UEMS–LEMS differences in cases with low NLI. The mean CMI of the matched groups was 37% (CCS) and 8% (iCSCI), respectively. The majority of patients with a CMI > 0 were AIS D, whereas few AIS C patients had high CMI (Fig. 3b/lower plot).
a Bars represent absolute number of SCI cases (horizontal) shown with respect to NLI (vertical) for a comparison of distribution of matched (CSS, blue, iCSCI, red) and unmatched cases (green) indicating that these were comparable with respect to NLI. b Bars represent absolute number of SCI cases (vertical) as distributed with respect to CMI. CMI indicates a rating of the extent of CCS, i.e., how much motor weakness was more pronounced in the arms compared to the legs. CMI was calculated for each patient individually as a percentage difference of average segmental motor scores from upper subtracted from that of the lower extremities considering all segments below NLI (segmental motor scores could range from 0 to 5; a maximum segmental difference of 5 was assumed as 100%; if average arm segment motor score was lower than that of the leg segments this resulted in positive numbers, otherwise CMI was assumed to be 0%). CMI allows to describe CCS as a continuum with a range of discrepancy between arm and leg strength rather than with a fix score difference between arms and legs irrespective of NLI. CMI can be calculated for all cervical SCI and shows that majority has no CSS pattern (0% to 20% CMI, including most of the matched iCSCI, red) and unmatched cases (green). Most of matched CCS patients showed CMI of 20% or more. SCI spinal cord injury, NLI neurological level of injury, CCS central cord syndrome, iCSCI incomplete cervical spinal cord injury, CMI central myelopathy index.
a Boxplots represent absolute total motor score recovery (0–75; vertical, right ordinate) shown with respect to CMI (horizontal, common abscissa for both graphs) in comparison for SCI with more (AIS C: hatched) and less severe lesion (AIS D: dark) indicating less motor score recovery for AIS D than AIS C irrespective of CMI, i.e., SCI lesion severity but not extent of CCS has effect on motor recovery. b Bars represent absolute number of SCI cases (vertical) shown with respect to CMI (horizontal) comparing SCI lesion severity (AIS C: hatched, AIS D: dark). This indicates that within the group of patients without signs of CCS (CMI of 0%) there is an equal distribution of SCI lesion severity (AIS C = AIS D). On the contrary, most patients with a CMI of > 0% had less severe SCI (AIS D) SCI spinal cord injury. CCS central cord syndrome. AIS American Spinal Injury Association Impairment Scale. CMI (central myelopathy index, for explanation see Fig. 2).
The CCS cohort showed a lower level of global independence (as evaluated by the total SCIM), and of independence in self-care and mobility (Table 2). With regard to bladder control, at T0, 15 (14%) CCS patients and 27 (25%) iCSCI ones had voluntary bladder control with a SCIM “Sphincter management-bladder” score of 15 (p < 0.05). With regard to bowel management, at T0, 13 (12%) CCS patients and 27 (25%) iCSCI ones had good bowel control with a SCIM “Sphincter management-bowel” score of 10 (p < 0.05).
The evaluation of walking capacity with WISCI II, 6MWT and 10MWT showed no difference in the two groups. In particular, at first evaluation, 68 (61.8%) patients in the CCS group and 67 (61%) in the iCSCI were unable to walk; 22 (20%) patients in both groups walked unassisted (p > 0.05).
Recovery over time
Both populations showed a significant improvement of all the neurological, functional and walking measures between T0 and T1. Between T1 and T2, we observed a further tendency for improvement, but this was not significant (Figs. 3–5).
When comparing the two populations, CCS patients showed a higher, but not significantly, incidence of AIS grade improvement one year after SCI: an improvement between T0 and T2 was observed in 22/110 patients with CCS and 15/110 with iCSCI (p > 0.05). At all times CCS patients showed significant lower UEMS and higher LEMS compared with iCSCI. Total motor score always were comparable in the two populations (Table 2, Fig. 3) and total motor score recovery between T0 and T2 was independent of CMI in both AIS C and D patients (Fig. 3a/upper graph). UEMS improvements were significantly higher in patients with CCS than iCSCI (Table 3, Fig. 4).
With regard to daily life activities, the comparison of matched cohorts showed that patients with CCS had lower SCIM “self-care” scores at all time points. At T0, patients with CCS also presented with significantly lower SCIM “mobility” (p < 0.05), “external mobility” (p < 0.05) and “total” SCIM-scores (p < 0.05), but these differences disappeared at the following assessments. SCIM “respiration and sphincter management” and “internal mobility” scores and walking tests were always comparable in the two groups (Table 2) (Fig. 5). SCIM sub-score improvements were comparable between the two populations (Table 3, Fig. 5).
At final evaluation 53 (49%) patients in both groups had voluntary bladder control. At T2, good bowel control was seen in 47 (43%) CCS patients and in 57 (52%) iCSCI patients (p > 0.05). With regard to walking capacity, WISCI, 6MWT and 10MWT scores were comparable at all time points (Fig. 6) as well as the respective improvements (Table 3). At the final evaluation, 7 (6%) patients in the CCS group and 13 (12%) in the iCSCI group did not walk; 100 (91%) patients with CCS and 95 (86%) with iCSCI walked without assistance (p > 0.05).
Discussion
Our study analyzed the neurological and functional evolution of patients with CCS, a spinal cord syndrome often described as less incapacitating compared with other forms of iCSCI.
In recent years, the epidemiology of SCI has changed, associated with increasing age and incidence of iCSCI. Cervical lesions in the elderly population will represent a unique challenge for health care systems because of the various medical co-morbidities that are associated with age and because of the more difficult recovery of daily life independence after SCI at an older age. CCS is already the most common spinal cord syndrome, accounting for about 9% of all SCI [9] and 27% of iCSCI in the present study. It is anticipated that in the near future, CCS due to falls will represent one of the main causes of SCI [7, 30, 31].
We examined prospective neurological and functional data from a large sample of patients with CCS compared with patients with iCSCI. The parameterization by CMI demonstrates that CCS is a continuum rather than a distinct subgroup of patients with iSCI. The demographic and neurological features of our data in line with the literature indicate that patients with CCS are older, and have higher NLI and a higher frequency of AIS D (Figs. 1a and 2b) [9, 12]. In order to obtain two groups of patients as homogeneous as possible, we matched a selection of patients based on age, AIS and NLI, for a representative comparison of outcomes in CCS and iCSCI. The comparison of these groups indicates that patients with CCS do have poorer outcomes than those with iCSCI. Despite better improvement of UEMS in CCS (compare Figs. 3a with 5a and see Table 3), they always remained lower compared with iCSCI. The low UEMS of patients with CCS was reflected in reduced self-care scores, due to the persistent deficit in manual ability. CCS was not characterized by a better recovery of gait, despite having higher LEMS than patients with iCSCI at all time points. The ability to walk following iSCI or CCS may be assisted or enabled by upper extremity devices, i.e. crutches, walkers, etc. Lack of upper extremity arm strength hinders grasp and/or antigravity support at the shoulder and elbow, making it difficult for patients to walk. Therefore, patients with CCS will probably require a high level of daily assistance after discharge.
Currently available literature states nearly unanimously that CCS is a syndrome characterized by a good prognosis in terms of neurological and functional recovery. However, these previous studies presented a low number of patients, used variable or diffuse definitions of CCS, employed unsuited outcome parameters, lacked a comparison of properly matched groups, or were not prospective and subject to center effects, leading to bias in outcome and prognosis [18, 32,33,34].
Mckinley et al. [9] compared the demographic characteristics of 175 patients who presented with one of the six main clinical spinal cord syndromes and used the Functional Independence Measure (FIM) and its sub-scores to compare their functional recovery. Although many demographics and in part functional data are in line with what we observed, McKinley et al.’s sample was collected retrospectively and from a single treatment center. Furthermore, few outcome measures were analyzed and FIM is less sensitive than SCIM [32]. However, CCS remained the syndrome with the lowest motor and self-care FIM scores at entry and among those with the lowest improvement at discharge. The article lacks a precise definition of CCS, but above all there is no matching between the groups: patients with NLI C2 to S2 were included, thus functional recovery trends are not reliable.
Wirz et al. [33] compared 15 patients with CCS and 15 with BSS, assessed by neurological status, walking capacity and activities of daily life independence at 1 month and 6 months from the acute event. The authors did not find any significant difference in functional recovery between the two syndromes in the first 6 months after injury. CCS patients showed higher scores in ambulatory-related assessments than BSS patients, but this difference did not reach statistical significance; with regard to daily life independence and specifically to self-care, CCS patients presented with lower values at first evaluation, but then showed comparable outcomes to those of BSS patients. Compared with the present study, Wirz included fewer patients who were examined at only two reference time points. Furthermore, CCS was only compared with another very rare spinal syndrome, thus lacking a comparison with the majority of iCSCI.
Another study [34] examined the data of 248 patients with incomplete tetraplegia, extracted from the EMSCI database, and divided into three groups: non-CCS (UEMS ≥ LEMS), intermediate-CCS (UEMS = (1–9 points) < LEMS) and CCS (UEMS = (≥9 points) < LEMS). The authors reported that good neurological and functional recovery of these patients was not correlated with CCS but rather with AIS at admission. However, the patients from the three groups were not comparable with respect to NLI, and evaluation of walking was based only on one item of SCIM; while in the present study, a more detailed evaluation was applied to assess different aspects of walking.
Compared with previous studies, our analysis has several strengths: it is a prospective study with the largest number of patients with CCS. Comparison with iCSCI patients is not confounded by varying age, severity or NLI between groups and full ISNCSCI and functional datasets were obtained by trained examiners and classified by a validated computer algorithm [21].
The study also has some limitations due to the nature of the database. Compared with previous studies, we were not able to analyse the frequency and impact of complications, the length of acute and rehabilitation stay, and the discharge dispositions, because these data were not collected. In addition, based on the data available, it is not possible to compare low-energy and high-energy impact lesions to discover possible differences in outcome. Finally, although multicentric, the EMSCI data mostly comes from European countries. It would be interesting to compare these data with data from USA or Asia with possible differences in demographics and clinical features of the patients.
Conclusions
Our results provide important findings for clinical and rehabilitation aspects of incomplete cervical SCI.
As CCS is becoming increasingly frequent, present data are important to establish the prognosis of these patients and provide resources needed during and after rehabilitation.
Due to the particular self-care deficit of CCS patients, it is important to conceive specific rehabilitation programs aimed at improving upper limb and hand recovery.
According to the health policy of some European countries, patients with minor SCI trauma (AIS D, i.e. the majority of patients of this study) do not have access to specialized SCI centers despite their evident deficits in self-care. Among them are a considerable group of CCS patients, who by this policy are denied specifically efficient rehabilitation.
Finally, it will be important to account for the different clinical presentations and recovery profiles of CSS and iCSCI to model their prognosis and thus allow inclusion of these special spinal syndromes in clinical trials.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2018. https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202018.pdf.
Nijendijk JHB, Post MWM, van Asbeck FWA. Epidemiology of traumatic spinal cord injuries in the Netherlands in 2010. Spinal Cord. 2014;52:258–63.
McCaughey EJ, Purcell M, McLean AN, Fraser MH, Bewick A, Borotkanics RJ, et al. Changing demographics of spinal cord injury over a 20-year period: a longitudinal population-based study in Scotland. Spinal Cord. 2016;54:270–76.
Ferro S, Cecconi L, Bonavita J, Pagliacci MC, Biggeri A, Franceschini M. Incidence of traumatic spinal cord injury in Italy during 2013–2014: a population-based study. Spinal Cord. 2017;55:1103–7.
Montoto-Marqués A, Ferreiro-Velasco ME, Salvador-de la Barrera S, Balboa-Barreiro V, Rodriguez-Sotillo A, Meijide-Failde R. Epidemiology of traumatic spinal cord injury in Galicia, Spain: trends over a 20-year period. Spinal Cord. 2017;55:588–94.
Pagliacci MC, Celani MG, Zampolini M, Spizzichino L, Franceschini M, Baratta S, et al. An Italian Survey of Traumatic Spinal Cord Injury. The Gruppo Italiano Studio Epidemiologico Mielolesioni Study. Arch Phys Med Rehabil. 2003;84:1266–75.
Chamberlain JD, Deriaz O, Hund-Georgiadis M, Meier S, Scheel-Sailer A, Schubert M, et al. Epidemiology and contemporary risk profile of traumatic spinal cord injury in Switzerland. Inj Epidemiol. 2015;2:28.
Garcia-Altes A, Perez K, Novoa A, Suelves JM, Bernabeu M, Vidal J, et al. Spinal cord injury and traumatic brain injury: a cost-of-illness study. Neuroepidmiology. 2012;39:103–8.
McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30:215–24.
Bosch A, Stauffer ES, Nickel VL. Incomplete traumatic quadriplegia. A ten-year review. JAMA. 1971;216:473–8.
Thompson C, Mutch J, Parent S, Mac-Thiong JM. The changing demographics of traumatic spinal cord injury: An 11-year study of 831 patients. J Spinal Cord Med. 2015;38:214–23.
Schneider RC, Cherry G, Pantek H. The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine. J Neurosurg. 1954;11:546–77.
Brooks NP. Central cord syndrome. Neurosurg Clin North Am. 2017;28:41–47.
Quencer RM, Bunge RP, Egnor M, Green BA, Puckett W, Naidich TP, et al. Acute traumatic central cord syndrome: MRI-pathological correlations. Neuroradiology. 1992;34:85–94.
Aito S, Andrea MD, Werhagen L, Farsetti L, Cappelli S, Bandini B, et al. Neurological and functional outcome in traumatic central cord syndrome. Spinal Cord. 2007;45:292–7.
Waters RL, Adkins RH, Sie IH, Yakura JS. Motor recovery following spinal cord injury associated with cervical spondylosis: a collaborativ study. Spinal Cord. 1996;34:711–5.
Marino RJ, Ditunno JF, Donovan WH, Maynard F. Neurologic recovery after traumatic spinal cord injury: data from the model spinal cord injury systems. Arch Phys Med Rehabil. 1999;80:1391–6.
Ronzi Y, Perrouin-Verbe B, Hamel O, Gross R. Spinal cord injury associated with cervical spinal canal stenosis: Outcomes and prognostic factors. Ann Phys Rehabilitation Med. 2018;61:27–32.
Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011;34:547–54.
Kirshblum S, Waring W. Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys Med Rehabil Clin N Am. 2014;25:505–17. https://doi.org/10.1016/j.pmr.2014.04.001.
Schuld C, Wiese J, Hug A, Putz C, Hedel HJ, Spiess MR, et al. Computer implementation of the international standards for neurological classification of spinal cord injury for consistent and efficient derivation of its subscores including handling of data from not testable segments. J Neurotrauma. 2012;29:453–61.
ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7.
Scivoletto G, Tamburella F, Laurenza L, Foti C, Ditunno JF, Molinari M. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord. 2011;49:736–40.
Ditunno PL, Ditunno JF. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–6.
Itzkovich M, Tripolski M, Zeilig G, Ring H, Rosentul N, Ronen J, et al. Rasch analysis of the Catz-Itzkovich spinal cord independence measure. Spinal Cord. 2002;40:396–407.
Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, et al. Multicenter International Study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:275–91.
Friedli L, Rosenzweig ES, Barraud Q, Schubert M, Dominici N, Awai L, et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci Transl Med. 2015;7:302ra134. https://doi.org/10.1126/scitranslmed.aac5811.
Middendorp JJ, Pouw MH, Hayes KC, Williams R, Chhabra C, Putz HS, et al. Diagnostic criteria of traumatic central cord syndrome. Part 2: a questionnaire survey among spine specialists. Spinal Cord. 2010;48:657–63.
Daniel E, Ho DE, Kosuke Imai K, Gary King G, Elizabeth A, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. http://www.jstatsoft.org/v42/i08/.
Fehlings MG, Tetreault L, Nater A, Choma T, Harrop J, Mroz T, et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. 2015;77:S1–5.
Kirshblum SC, Priebe MM, Ho CH, Scelza WM, Chiodo AE, Wuermser LA. Spinal Cord Injury Medicine. 3. Rehabilitation Phase Acute Spinal Cord Injury. Arch Phys Med Rehabil. 2007;88 3 Suppl 1:S62–70.
Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil. 2007;29:1926–33.
Wirz M, Zörner B, Rupp R, Dietz V. Outcome after incomplete spinal cord injury: central cord versus Brown-Sequard syndrome. Spinal Cord. 2010;48:407–14.
MH Pouw MH, JJ van Middendorp JJ, A van Kampen A, A Curt A, H van de Meent H, Hosman AJF, for the EM-SCI study group. Diagnostic criteria of traumatic central cord syndrome. Part 3: descriptive analyses of neurological and functional outcomes in a prospective cohort of traumatic motor incomplete tetraplegics. Spinal Cord. 2011;49:614–22.
Acknowledgements
The authors would like to thank all patients who were willing to contribute to the EMSCI database and acknowledge those who collected data upon which the present study is based.
Funding
The manuscript is partially supported by the European Union’s HORIZON2020 grant no. 681094 to AC and by the ERANET-NEURON grant to GS.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the study
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers/animals were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blasetti, G., Pavese, C., Maier, D.D. et al. Comparison of outcomes between people with and without central cord syndrome. Spinal Cord 58, 1263–1273 (2020). https://doi.org/10.1038/s41393-020-0491-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-0491-x
This article is cited by
-
Central cord syndrome definitions, variations and limitations
Spinal Cord (2023)
-
Is it time to redefine or rename the term “Central Cord Syndrome”?
Spinal Cord (2021)