Abstract
Study design
Randomized double blind, placebo-controlled trial.
Objectives
To examine the effect of early intravenous zoledronic acid (ZA) on bone markers and areal bone mineral density (aBMD) in persons with acute ASIA Impairment Scale (AIS) A traumatic spinal cord injury (SCI).
Setting
Two inpatient rehabilitation units.
Methods
Thirteen men, 2 women, aged 19–65, C4-T10 AIS A SCI, received 5 mg intravenous ZA vs. placebo 12–21 days post injury. Markers of bone formation (procollagen N-1 terminal propeptide [P1NP]), bone resorption (serum C-telopeptide [CTX]), and aBMD by dual-energy X-ray absorptiometry (DXA) for hip (femur—proximal, intertrochanteric, neck), and knee (distal femur, proximal tibia) were obtained at baseline, 2 weeks post infusion (P1NP, CTX only), 4 and 12 months post injury.
Results
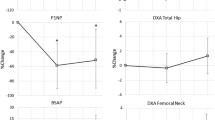
P1NP remained unchanged, while CTX decreased in ZA but increased in controls at 2 weeks (mean difference = −97%, p < 0.01), 4 months (mean difference = −54%, p < 0.05), but not 12 months (mean difference = 3%, p = 0.23). Changes in aBMD at the hip favored ZA at 4 months (mean difference 10.3–14.1%, p < 0.01) and 12 months (mean difference 10.8–13.1%, p < 0.02). At 4 months, changes in aBMD favored ZA at the distal femur (mean difference 6.0%, 95% CI: 0.7–11.2, p < 0.03) but not proximal tibia (mean difference 8.3%, 95% CI: −6.9 to 23.6, p < 0.23). Both groups declined in aBMD at 12 months, with no between group differences.
Conclusion
ZA administered ≤21 days of complete traumatic SCI maintains aBMD at the hip and distal femur at 4 months post injury. This effect is partially maintained at 12 months.
Similar content being viewed by others
Introduction
Increased bone turnover and resultant bone loss is a prevalent condition following acute traumatic spinal cord injury (SCI), affecting nearly 100% of motor complete patients examined in a chronic care setting [1, 2], 40% of whom are likely to experience fractures. In the initial weeks following SCI, a process of rapid bone remodeling occurs, with resorption exceeding formation, leading to bone loss [3]. Radiographic evidence suggests that an estimated 25% of areal bone mineral density (aBMD) below the level of injury is lost within the first 4 months following acute SCI and progresses to a 33% loss by 16 months post injury [1, 4], leaving patients at or near the fracture threshold [1]. Additional investigations extending the time from injury to 2 years estimate aBMD reduction of 30–40% at the femoral neck, 37–43% at the distal femur [5], and 50–60% at the proximal tibia [6], with the majority of this loss occurring during the first 12 months.
Zoledronic acid (ZA) is the most potent of the bisphosphonates, a class of medication that affects the ability of the osteoclast to further resorb bone, resulting in relative bone preservation. Within the osteoclast, ZA inhibits a key regulatory enzyme known as farnesyl diphosphate synthetase 17 times more effectively than alendronate and 67 times more so than pamidronate [7, 8]. Binding of ZA to hydroxyapatite in regions of high bone turnover, namely trabecular bone endosteal area, results in greater reduction of bone markers [9]. In addition, because ZA is administered intravenously, there is less concern of developing gastrointestinal (GI) irritation or erosive gastritis. Moreover, patient compliance is far superior with a once-yearly infusion than with a daily or weekly oral medication [10].
Individuals with SCI specifically develop sublesional osteoporosis, namely bone loss below the level of paralysis [5]. The hip is the area of most frequent study in terms of bone density changes following ZA administration [11,12,13]. However, regions around the knee (distal femur and proximal tibia) have the highest incidence of fracture [13, 14].
Five investigations [11,12,13, 15, 16] have examined the effect of ZA on bone preservation in SCI patients during the subacute phase of rehabilitation (8–12 weeks post injury). Reduction of bone loss was seen at the hip, but not at the knee [13, 16]. Since bone loss begins within weeks of injury, we hypothesized that earlier administration of ZA would be more effective than administration in the subacute phase of injury.
The first and primary aim of this investigation was to determine the short- and long-term effects of ZA administered within 21 days of injury on aBMD values of the femur (proximal, intertrochanteric, and neck), distal femur, and proximal tibia in patients with complete traumatic SCI. Our primary hypothesis was that participants treated with ZA would have no more than half the decline in aBMD than controls in all areas evaluated at 4 and 12 months post injury. Related hypotheses were that those receiving ZA would maintain original aBMD values in three hip regions, and decline ≤5% at the distal femur and proximal tibia at 4 months and ≤10% at 12 months.
A second aim of the investigation was to measure and compare levels of pro-osteoclastogenic factor interleukin 1-β (IL-1β), bone formation marker procollagen N-1 terminal propeptide (P1NP), and bone resorption marker serum C-terminal telopeptide of type I collagen (CTX) in SCI participants receiving ZA vs. controls. Our hypotheses were that (1) levels of IL-1β and P1NP in ZA participants would be equal to or slightly above controls at 4 months; (2) those administered ZA would have lower levels of CTX at 2 weeks post infusion, 4 and 12 months post injury and be within the reference range specific to an individual’s age and gender; (3) controls would demonstrate CTX levels significantly above the reference range at all time points.
Our third aim was to determine the tolerability of early administration of intravenous ZA in traumatic AIS (American Spinal Injury Association Impairment Scale) A SCI. We hypothesized that individuals given ZA (1) will have an increased incidence of myalgias, fever, and flu-like symptoms in the 3 days following infusion of ZA; (2) will be less likely to participate in physical and occupational therapy during the first week after infusion; and (3) not desire ZA at any future time.
Methods
Participant screening process
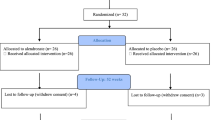
Between July 2012 and July 2017, 16 participants of C4-T10 complete traumatic SCI, and were aged 18–65, were invited to participate within the first 18 days of acute traumatic SCI. Prior to the first participant being enrolled, the study was registered on clinicaltrials.gov (NCT01642901) and subsequently received University IRB approval. This oversight committee authorized study initiation based on compliance with ethical standards of care and patient protection guidelines. A separate Data Safety Monitoring Board evaluated safety of the protocol, study conduct, and integrity of the trial and circumstances leading to any severe adverse event.
All participants were drawn from two acute inpatient rehabilitation units in a large urban area. Inclusion criteria and exclusion criteria are given in Table 1. A caudal limit of T10 was established to avoid inclusion of participants with cauda equina syndrome. Once consented, participants were interviewed for presence of any hereditary or acquired metabolic bone disorder, conditions that would interfere with vitamin D intake or absorption, personal medical history, prior medication use, menstrual status (pre or post menopausal), and social or lifestyle factors that could alter ability to participate.
After informed consent was given, but prior to day 10 post injury, investigators obtained serum calcium, intact parathyroid hormone (iPTH) and serum vitamin D25OH levels to determine further eligibility. Participants with vitamin D deficiency were allowed to receive supplementation to raise their level above 13 ng/mL in order to participate. Additional screening labs were performed, and participant interviews conducted. Detailed protocols for screening labs and assessments are outlined in Supplementary Appendices 1 and 2.
Baseline laboratory indexes
Before infusion of the study drug, baseline levels of IL-1β, creatinine phosphokinase (CPK), P1NP, and fasting serum CTX were obtained. These laboratory tests were used for monitoring indexes of bone metabolism, kidney function, and muscle enzymes. Serum CPK was added after participant 6 demonstrated an elevated level when drawn for an unrelated purpose. Thus, it was calculated only for the remaining nine participants. Infusion procedure, lab, and imaging timing are outlined in Supplementary Appendix 2. There was a range of 3–10 days between enrollment and study drug administration, with all participants receiving infusion of the study drug by day 21 post injury.
Randomization method
Participants were assigned to treatment versus placebo group in a 2:1 ratio through the method of random permuted blocks of sizes 3 and 6, generated by the University Division of Biostatistics. Since bone loss is well established following SCI, investigators felt the control group did not need to be as large. Researchers also believed the 2:1 ratio would help with recruitment in an environment of many competing trials. The Division of Biostatistics also prepared a set of sealed envelopes with participant assignment, kept by a blinded administrative assistant. The block size was not disclosed to the investigators or research assistants, all of whom were blinded to assignment of each participant’s intervention. Only the investigational pharmacist was not blinded.
Imaging technique
Dual-energy X-ray absorptiometry (DXA) (Delphi W; Hologic, Inc, Bedford, MA) was used to assess aBMD in the proximal femur by standard procedures for Hologic densitometers. The distal femur and proximal tibia were evaluated using separate high-resolution scans that were conducted with the lumbar spine scan mode. The distal femur scan began just below the femoral condyles and extended 239 mm proximally. The proximal tibia scan began just above the tibial plateau and extended 239 mm distally. The analyses were conducted using a modified version of the procedure described by Shields et al. [17] and Global Region of Interest software. The analyses occurred in the distal femur and proximal tibia scans within region of interest boxes that were a height 7% of femur length and a width greater than the width of the bone. In the distal femur, the distal edge of the region of interest box was above the terminus of the lateral distal femur at a distance 13% of femur length. In the proximal tibia, the proximal edge of the region of interest box was at the distal meeting point of the tibia and fibula. This region selection of the proximal tibia was slightly different from that described by Shields et al. [17], which began at the proximal meeting point of the tibia and fibula, because it avoided overlap in the tibia and fibula. See Supplementary Appendix 3 and Fig. S-3.
Statistical analysis
Continuous outcomes were summarized using means, standard deviations, medians, and ranges. Categorical outcomes were summarized using category frequencies, and percentages. Groups were compared using independent sample t-tests for continuous outcomes and Fisher’s exact test for categorical outcomes. Percent changes of CTX and P1NP were not normally distributed, and were examined with nonparametric statistics.
The effect size was calculated as the difference in mean percent change (ZA-control). SAS/STAT software, version 14.2, SAS System for Windows (SAS Institute, Cary, NC), was used for all analyses.
Results
Demographic composition
A consort flow diagram of participant enrollment is given in Fig. 1. One ZA participant lacked valid DXA imaging for distal femur and proximal tibia at 4 and 12 months, due to imaging challenges from spastic flexion contractures. His remaining data were included. Another ZA participant withdrew at 10 months (moved 3000 miles away). Demographics at baseline are noted in Table 2. There were no clinically meaningful differences between the groups in age, gender, race, or neurological status.
Laboratory studies
At baseline, no differences between groups in serum creatinine, calcium, phosphate, magnesium, iPTH, vitamin D25OH, or IL-1β were found (Table 2). In addition, P1NP did not differ between groups, but CTX was higher in the participants who received ZA.
In the 3 days after infusion, there was significantly lower serum calcium in the ZA group relative to controls. Calcium was aggressively supplemented in oral or IV form. Several participants required supplementation with 50,000 units ergocalciferol, in order to reach the 13 ng/ml threshold of vitamin D25OH for participation. Those participants also required continued weekly doses of the 50,000 units of ergocalciferol until therapeutic levels were reached, after which time they continued with a maintenance dose of 2000 units daily. In accordance with institutional standards of care, Endocrinology consultants followed those with low initial levels of vitamin D25OH. No participants were in secondary hyperparathyroidism at any point in the study. For IL-1β, no values for either ZA or control participants were outside of the normal limits at any assessment point.
Despite a concern that ZA might decrease ability to heal bone, since there exists a coupling of bone formation and bone resorption [18], no participants, control or ZA, had levels of P1NP below normal at either the 1- or 4-month follow-up. Thus, no evidence of slowed bone formation was evident after giving ZA (see Supplementary Appendix 4). All spine fractures in ZA participants healed within or earlier than the anticipated timeframe.
The ZA group showed significant percent reductions of CTX levels at 2 weeks post infusion (mean difference = −97%; 95% CI −130% to −64%; Kruskal–Wallis p = 0.002) and 4 months post injury (mean difference = −54%; 95% CI −116 to 7%; Kruskal–Wallis p = 0.048), relative to changes seen in controls, noting that the ZA group had higher baseline values (Table 2, Supplementary Appendix 4, Fig. 2). By month 12, differences in percent change between groups were no longer substantial on average, though they were highly variable (mean difference = 3%; 95% CI −80 to 86%).
DXA findings
No participants were osteoporotic at baseline; however, two participants, one in each group, had osteopenia in the −1.6 to −1.7 range by adjusted Z scores. Explanations for osteopenia in these participants appear to be low baseline vitamin D and chronic lactose intolerance.
Hip region outcomes
The mean aBMD changes in placebo and ZA groups for total proximal femur, intertrochanteric femur, and femoral neck at 4 months post injury are given in Table 3. Independent sample t-tests indicate that the ZA group had significantly higher aBMD than controls at 4 months in all 3 locations. At 12 months, the total proximal femur and intertrochanteric femur had declined by 8.4% while the femoral neck declined 4.8%. This degree of loss was between 60 and 77% less than the loss observed in the control group. Results for aBMD indicate a large effect size for all outcomes involving the hip (Table 3).
Knee regional outcomes: distal femur and proximal tibia
Table 4 summarizes findings for aBMD in distal femur and proximal tibia. The number of participants in the ZA group was decreased by 1 participant at 4 months and 12 months for both regions near the knee due to imaging difficulties from spastic flexion contractures. One participant from the control group was over 1 month late for the 4-month assessment. Complete results for aBMD for hip and knee regions appear in Supplementary Appendix 5.
At 4 months post injury there was a significant difference in change in aBMD between groups, with a mean difference of 6.0% (95% CI: 0.7–11.2; p = 0.029) favoring the ZA group. At 12 months post injury, the distal femur had lost 8% original BMD in ZA participants versus 10% loss among controls in the study, a difference that was not statistically significant (Mean difference = 2.0%; 95% CI = −6.5 to 10.3; p = 0.62). The above findings indicate a large effect size at 4 months but a small effect size at 1 year.
For the proximal tibia variability within groups resulting in large standard deviations for change in aBMD rendered the difference between the 2 groups nonsignificant at 4 and 12 months, despite large mean differences. Effect size at 4 months was moderate, but at 12 months was small.
Participation outcomes
No time in weekly therapy was missed, but for some patients, less time was scheduled on the day after infusion and shifted later in the week. All participants agreed they would enroll again in the study when questioned at 7 days and 1 year after study drug. Those experiencing postdrug fevers and fatigue attributed symptoms to factors other than the drug interestingly. Participants were not dropped if they converted to incomplete injuries. Recent reports in the literature give annual conversion rates from complete to incomplete as high as 34.7% for cervical SCI and 16.4–31.5% for thoracic SCI [19, 20]. At 4 months, 1 control participant had converted to AIS B (deep anal pressure only), another to AIS C, (1/5 strength in long toe flexor and deep anal pressure). At 12 months, 3/15 were now AIS B (2 ZA, 1 control) and 2 AIS C (1 from each group, both with motor scores of 3 or less).
Telephone conferences after hospital discharge
Questions regarding vitamin D and calcium compliance, postdischarge unplanned hospital visits and reasons for visits, along with any falls and fractures was obtained in the phone questionnaires. These interviews revealed compliance with calcium and vitamin D tablets for all participants. Interviews also revealed that 2 ZA participants had fallen from wheelchairs, but no fractures or other injuries occurred from these events or any others reported by ZA or control participants.
Adverse events
Those in the ZA group did have statistically higher occurrences of fever (p = 0.041) of 100.6–103.2 that presented 24–48 h post infusion and lasted from 2 h to 2 days. Of the 8 participants experiencing temperatures greater than 100.5, 7 received ZA and 1 received saline placebo (later attributed to UTI). One acute kidney injury (AKI) in the ZA group was observed 6 days after receiving the study drug. Creatinine rise of 1.2 points from baseline (1.1–1.3) to 2.3–2.5, occurred in the setting of elevated vancomycin trough the night prior to the lab. This participant had developed an acute fever with pneumonia and was given vancomycin and zosyn. The patient was aggressively hydrated, antibiotics changed by infection disease consultants, and creatinine returned to baseline 5 days after onset of AKI.
One participant experienced noncardiac chest pain and elevated CPK. After thorough review of labs from the referring trauma center, CPK levels had been even higher in acute care. The noncardiac chest pain resolved in 45 min with an antispasticity agent.
Discussion
To our knowledge, this investigation is the first to administer ZA in a double blind, placebo-controlled protocol, to acute SCI participants who are within the first 21 days of injury. Due to the duration following SCI of just 10–21 days, our patients were prone to fever, infections, and other early medical issues following SCI. However, all were medically stable for participation. Earlier investigations on use of ZA following SCI involved persons with AIS B or C SCI. Despite the fact that one-third of our patients had converted to incomplete injuries, neurologic improvement made little to no difference in aBMD values, perhaps because recovery was minimal.
Pathophysiology of bone loss after SCI
In the early weeks following SCI, the rate of bone resorption greatly exceeds that of bone formation due primarily to upregulation of the osteoclast [21]. Hypercalcemia and hypercalciuria, observed in the weeks following paralysis, arise in parallel with elevation of resorption indices such as urine and serum telopeptides, hydroxyproline, and urine pyridinoline [21, 22] leading to overall bone loss. Trabecular bone is more commonly affected than cortical bone in the first 4 months following SCI due to its faster turnover rate [23, 24] Elevations in bone resorption markers begin at one-week post injury, peak at 16–20 weeks post- injury, and then gradually decline. At their highest value, some markers measure ten times the upper limit of normal [24].
Markers of bone formation vary from mildly depressed to slightly elevated during the first 6 months [14], but temporary elevation of formation is not sufficient to counteract the significant bone resorption during this same interval. Evidence of the above process is seen in the findings of our P1NP and CTX outcomes. Based on the work of Davies [25], IL-1β may be one of the earlier factors elevated after SCI [26] but the duration of high levels is unclear. Our study examined levels of IL-1β only up to 4 months post injury and in this time no participants, control or experimental, had elevations in IL-1β at baseline or subsequently.
aBMD outcomes at the hip
Our outcomes support the primary hypothesis for the hip in that control participants lost more than two times the aBMD of ZA participants (17.0–21.3% vs. 4.0–8.4%). Nevertheless, the ZA group did show some bone loss at 12 months, refuting our hypothesis that aBMD would be maintained. Our findings reaffirm the conclusions of prior investigations that one dose of 5 mg IV ZA preserves bone at the hip and femoral neck if given in the acute phase of SCI. The other investigations did differ in specifics of the results and the sites investigated, as well as timing of follow-ups. Two groups [11, 12] showed bone preservation at several sites in the hip, but not the femoral neck at 1 year. Bauman et al. [13] showed results at the knee paradoxically favored the control group but had similar outcomes at the hip to previous reports [11,12,13,14,15].
Since hypercalciuria and elevated CTX are seen as early as 10–14 days post injury, the other investigations that enrolled participants up to 3 months post injury, theoretically included participants whom had already been losing bone for anywhere from 2 weeks to 2 months prior to receiving intervention. In contrast, most of our patients enrolled by day 14–16, still had CTX values in the normal range. Lab values did show that those who enrolled closer to the 21-day deadline had already begun experiencing osteoclastic upregulation, suggested by elevated CTX. Although bone resorption markers may continue to be elevated for up to 3 years post injury [27, 28], a single dose of ZA is expected to last a year at most. In our study CTX markers had returned to baseline levels or nearly so by one year and had begun migrating upward even after 4 months. The timing and value of a second dose of ZA for continued marker suppression is yet to be determined in those experiencing acute SCI. Although group differences were maintained at the hip for one year, disparities lessened between months 4 and 12.
aBMD outcomes at the knee
Findings at the distal femur in our study were significant at 4 months but not by 1 year. Still, a loss of just 8% of BMD in a year is noteworthy and far better than many historical controls [1, 14]. However, this finding needs validation with larger investigations of similar participants given the differences in outcome from prior investigations. Proximal tibia outcomes did not differ between groups at both 4 and 12 months, although on average no bone was lost in the ZA group at 4 months and just 4% decline was seen at 1 year. Earlier administration of ZA in this investigation may have led to superior bone preservation in this study relative to others studying aBMD at the knee.
Compared with results of earlier investigations [13, 16], our results in the distal femur suggest a benefit in giving ZA for bone preservation after SCI, paralleling outcomes at the hip but to a much lesser effect. The benefit of ZA for preserving bone at the proximal tibia is unclear. Future directions may include giving a subsequent dose of ZA or comparable antiosteoclastogenic agent before the end of the first year after SCI, or supplementing the medication with physiatric interventions including functional electrical stimulation or vibratory techniques.
Study limitations
Enrollment fell significantly below that planned at the start of the investigation. The presence of a number of competing trials on acute traumatic SCI in our hospital, each of which recruited patients between days 0 and 1 of acute SCI and were geared toward neurorecovery, left only a select number of participants available for enrollment in our clinical trial. Our investigators were unable to approach possible participants for enrollment until at least day 3 post injury, after these SCI individuals had either declined involvement in other clinical trials or were determined to be ineligible for them.
DXA imaging, particularly at the knee was limited by positioning on the table in some individuals with evolving contractures in either knee flexion or hip rotation. Another potential limitation of the study is the approach used to assess aBMD in the distal femur and proximal tibia. Although different DXA protocols have been proposed, we chose the procedure of Shields et al. [17] because it has been shown to yield reliable estimates in these regions. The assessment of the proximal tibia was modified by moving the region of interest distally such to avoid overlap in the tibia and fibula that occurs with the standard Shields procedure. Moreover, the modified region of interest captures the metaphysis, which is the region assessed in the distal femur. Although, using computed tomography, Edwards et al. [29] found that reductions in integral, cortical, and trabecular volumetric BMC and bone mineral content following SCI tended to progressively decrease moving away from the epiphysis and toward the diaphysis, the reductions they reported in the metaphysis remained notable. Eventual development of a specific imaging protocol best suited for persons with sublesional osteoporosis would enhance the interpretation of findings in future investigations.
Conclusion
A single dose of 5 mg IV ZA maintains bone at the hip and knee at 4 months, significantly reduces bone loss at the hip, and partially reduces loss at the knee at 1 year post injury. Combined interventions will likely be needed to fully maintain bone density beyond the first several months of SCI.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Data of individual participants were kept in locked files within the Spinal Cord Injury Center of Thomas Jefferson University. The University retains ownership of these records following study completion
References
Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–8.
Frey-Rindova P, de Bruin ED, Stüssi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32.
Chantraine A, Heynen G, Franchimont P. Bone metabolism, parathyroid hormone, and calcitonin in paraplegia. Calcif Tissue Int. 1979;27:199–204.
Minaire P, Neunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: comparison with biological data. Calcif Tissue Res. 1974;17:57–73.
Craven BC, Robertson LA, McGillivray CF, Adachi JD. Detection and treatment of sublesional osteoporosis among patients with chronic spinal cord injury: proposed paradigms. Top Spinal Cord Inj Rehabil. 2009;14:1–22.
Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Investig. 1990;20:330–5.
Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharm Exp Ther. 2001;296:235–42.
Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Min Res. 2000;15:1467–76.
Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Peiper CP, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N. Engl J Med. 2007;357:1799–809.
Saag K, Lindsay R, Kriegman A, Beamer E, Zhou W. A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone. 2007;40:1238–43.
Shapiro J, Smith B, Beck T, Ballard P, Dapthary M, BrintzenhofeSzoc K, et al. Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif Tissue Int. 2007;80:316–22.
Bubbear JS, Gall A, Middleton FR, Ferguson-Pell M, Swaminathan R, Keen RW. Early treatment with zoledronic acid prevents bone loss at the hip following spinal cord injury. Osteoporos Int. 2011;22:271–9.
Bauman WA, Cirnigliaro CM, La Fountaine MF, Martinez L, Kirshblum SC, Spungen AM. Zoledronic acid administration failed to prevent bone loss at the knee in person with acute spinal cord injury an observational cohort study. J Bone Min Metab. 2015;33:410–21.
Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86:498–504.
Goenka S, Sethi S, Panday N, Joshi M, Jindal R. Effect of early treatment with zoledronic acid on prevention of bone loss in patients with acute spinal cord injury: a randomized controlled trial. Spinal Cord. 2018;56:1207–11.
Schnitzer TJ, Kim K, Marks J, Yeasted R, Simonian N, Chen D. Zoledronic acid treatment after acute spinal cord injury: results of a randomized, placebo-controlled pilot trial. Phys Med Rehabil. 2016;8:833–43.
Shields RK, Schlechte J, Dudley-Javoroski S, Zwart BD, Clark SD, Grant SA, et al. Bone mineral density after spinal cord injury: a reliable method for knee measurement. Arch Phys Med Rehabil. 2005;86:1969–73.
Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, et al. Coupling of bone resorption and formation by RANKL reverse signaling. Nature. 2018;561:195–200.
Kirshblum KC, Botticello AL, Dyston-Hudson TA, Byrne R, Marino RJ, Lammertse DP. Patterns of sacral sparing components on neurologic recovery in newly injured persons with traumatic spinal cord injury. Arch Phys Med Rehabil. 2016;97:1647–55.
Aimetti AA, Kirshblum S, Curt A, Mobley J, Grossman RG, Guest JD. Natural history of neurological improvement following complete (AIS A) thoracic spinal cord injury across three registries to guide acute clinical trial design and interpretation. Spinal Cord. 2019;57:753–62.
Reiter AL, Volk A, Vollmar J, Fromm B, Gerner HJ. Changes of basic bone turnover parameters in short-term and long-term patients with spinal cord injury. Eur Spine J. 2007;16:771–6.
Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11:109–40.
Frisbie JH. Fractures after myelopathy: the risk quantified. J Spinal Cord Med. 1997;20:66–69.
Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab. 1998;83:415–22.
Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1384–93.
Garland DE, Adkins RH, Kushwaha V, Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27:202–6.
Pietschmann P, Pils P, Woloszczik W, Maerk R, Lessan D, Stipicic J. Increased serum osteocalcin levels in patients with paraplegia. Paraplegia. 1992;30:204–9.
Dauty M, Perrouin VB, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord injured patients. Bone. 2000;27:305–9.
Edwards WB, Schnitzer TJ, Troy KL. Bone mineral stiffness loss at the distal femur and proximal tibia in acute spinal cord injury. Osteoporos Int. 2014;25:1005–15.
Acknowledgements
The authors wish to thank the following individuals for their contribution to this work: Annie S. Kim, BS, Thomas Jefferson University, for assistance with manuscript preparation, table and figure construction. Justin A. Smith, MD, Case Western Reserve University, for assistance with table and figure creation. Marilyn P. Owens, RN, Thomas Jefferson University, for assistance with recruitment, obtaining signed consent forms, blood draws, and follow-up phone calls. Brittany Hayes, BSN, Thomas Jefferson University, for assistance with recruitment, obtaining signed consent forms, data archiving in secured patient files, and follow-up phone calls. Amanda B. Morina, PT, DPT, Thomas Jefferson University, for performing leg length measurements for DXA readings and performing ISNCSCI examinations. Chuan Zhang, Ph.D., University of Georgia, for assistance with image construction and reformatting of supplementary figures.
Funding
This research was supported by the U.S. Department of Health & Human Services | ACL | National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) (90SI5012). Cost of brand name Zoledronic acid and all supplies were funded by the grant and purchased through Jefferson pharmacy. Investigators did not shift to generic drugs mid-study, although they became available on study year 3.
Author information
Authors and Affiliations
Contributions
CVO was responsible for project concept and design, pre and post infusion questionnaires, assessing post infusion side effects of participants, and writing the manuscript. RJM was responsible for guiding project construction, assessing study feasibility, and reviewing the written manuscript. CSF was responsible for patient recruitment, study administration, data acquisition, and post infusion evaluation of side effects at the Magee Rehabilitation Hospital. CMM was responsible for interpreting the DXA scans and for creating Fig. S-3. BEL was responsible for randomization method and statistical analysis of results.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Oleson, C.V., Marino, R.J., Formal, C.S. et al. The effect of zoledronic acid on attenuation of bone loss at the hip and knee following acute traumatic spinal cord injury: a randomized-controlled study. Spinal Cord 58, 921–929 (2020). https://doi.org/10.1038/s41393-020-0431-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-0431-9
This article is cited by
-
Loss of lower extremity bone mineral density 1 year after denosumab is discontinued in persons with subacute spinal cord injury
Osteoporosis International (2023)
-
Zoledronic acid after spinal cord injury mitigates losses in proximal femoral strength independent of ambulation ability
Osteoporosis International (2023)
-
Preventive treatment with alendronate of loss of bone mineral density in acute traumatic spinal cord injury. Randomized controlled clinical trial
Spinal Cord (2022)
-
Factors influencing providers’ decisions on management of bone health in people with spinal cord injury
Spinal Cord (2021)
-
The efficacy and safety of bisphosphonate analogs for treatment of osteoporosis after spinal cord injury: a systematic review and meta-analysis of randomized controlled trials
Osteoporosis International (2021)