Abstract
Study design
Systematic review.
Objective
To determine the effectiveness of physiotherapy interventions for the treatment of spasticity in people with spinal cord injuries.
Setting
Not applicable.
Methods
A comprehensive search was undertaken to identify all randomised controlled trials of physiotherapy interventions that included an assessor-reported (objective) or participant-reported (subjective) measure of spasticity. Only trials that provided a physiotherapy intervention on more than one occasion were included. The susceptibility to bias of each trial was rated on the PEDro scale. Data were extracted to derive mean between-group differences (95% CI) for each trial.
Results
Twenty-eight trials were identified but only 17 provided useable data. Seven trials compared a physiotherapy intervention to no intervention (or a sham intervention) and 10 trials compared one physiotherapy intervention to another physiotherapy intervention. The median (IQR) PEDro score of the 17 trials was 6/10 (6–8). The most commonly used assessor- and participant-reported measures of spasticity were the Ashworth scale and Spinal Cord Injury Spasticity Evaluation Tool, respectively. Only one trial demonstrated a treatment effect. This trial compared continuous passive motion of the ankle to no treatment on the Ashworth scale. The remaining 16 trials were either inconclusive or indicated that the treatment was ineffective for reducing spasticity.
Conclusions
There is no high-quality evidence to indicate that physiotherapy interventions decrease spasticity but this may reflect a lack of research on the topic. Future trials should focus on participant-reported measures of spasticity that distinguish between the immediate, short-term and long-term effects of any physiotherapy intervention.
Similar content being viewed by others
Introduction
Spasticity can pose a major problem for people with spinal cord injuries (SCI) limiting their ability to move and perform activities of daily living [1, 2]. It can also cause pain, insomnia, pressure ulcers and contractures. Various physiotherapy interventions are advocated for the management of spasticity. These include passive stretching, transcutaneous electric nerve stimulation (TENS), electromyographic biofeedback, heat, and various types of exercise [3, 4]. However, it is unclear whether any or all of these interventions are effective.
A notable previous review, conducted as part of the Spinal Cord Injury Rehabilitation Evidence (SCIRE) initiative, analysed the effectiveness of surgical, non-pharmaceutical and pharmaceutical interventions for the management of spasticity. This review was originally conducted in 2007 [5] and then updated in 2016 [4]. The review is comprehensive capturing all types of studies (including case series) related to physiotherapy interventions. However, it rates the evidence according to the methodology proposed by Sackett without consideration of the size and precision of point estimates [6]. For this reason, it is difficult to judge if the treatments are clinically worthwhile and if the conclusions of the authors are justified.
In addition to the SCIRE review, there is a recent 2019 overview of systematic reviews [3]. This included systematic reviews of studies involving people with all types of neurological conditions. It however, only identified one scoping review involving people with SCI that was completed in 2014. The Scoping Review had a very specific focus on vibration [7]. It only identified one clinical trial looking at an intervention not typically administered by physiotherapists (penile vibratory stimulation) [8]. Since this time, there have been two other systematic reviews. Both have analysed specifically electrical stimulation (with [9] and without cycling [10]); neither found evidence of a reduction in spasticity. There remains considerable uncertainty about the evidence base for the many different physiotherapy interventions administered for the reduction of spasticity in people with SCI.
The aim therefore of this systematic review was to determine the effectiveness of physiotherapy interventions for the treatment of spasticity in people with SCI. Specifically, the two objectives were to determine:
-
The effectiveness of any physiotherapy intervention versus no physiotherapy intervention for the management of spasticity in people with SCI.
-
The effectiveness of one physiotherapy intervention versus another physiotherapy intervention for the management of spasticity in people with SCI.
The term, spasticity, is being used throughout this review, in a broader sense than originally articulated by Lance [11]. That is, the term is being used to reflect the many different presentations of involuntary muscle contractions (intermittent or sustained) that are associated with upper motor neuron lesions including spasms and clonus [12]. We have justified this approach on the basis that physiotherapy interventions are advocated and used for the management of spasticity without consideration of its various manifestations. Similarly, outcome measures such as the Ashworth Scale, Tardieu Scale, the Penn Spasm Frequency Scale, the Spinal Cord Assessment Tool for Spastic Reflexes (SCATS) and the Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET), do not clearly distinguish one symptom from another. We have taken the same approach.
Methods
Inclusion criteria
The inclusion criteria were as follows:
Types of studies
Only full reports of randomised controlled trials or randomised cross-over trials published in English were included. Abstracts from conference proceedings were not included.
Types of participants
Only trials involving adults with either traumatic or non-traumatic SCI were included. Trials that included a mix of adults and children, were only included if at least 75% of participants were over 16 years of age. Trials that included people with SCI and other conditions (e.g. spina bifida) were included provided at least 75% of participants had a SCI or the individual patient data could be retrieved. Trials were included regardless of the time post SCI.
Types of interventions
Trials were included if they involved the administration of any type of physiotherapy intervention that is typically used in the clinical setting to manage spasticity. For example:
-
Passive interventions This included passive movements, stretches, tilt table standing and splinting.
-
Gait-related therapies This included assisted standing, robotic-assisted gait training, and treadmill training with or without overhead suspension and body-weight support, electrical stimulation, somatosensory stimulation or biofeedback.
-
Generic exercises and/or rehabilitation This included strengthening, balance and fitness exercises with or without electrical stimulation, somatosensory stimulation or biofeedback.
-
Electrotherapy This included electrical stimulation (ES), somatosensory stimulation, transcutaneous electric nerve stimulation (TENS), ultrasound and other types of electrotherapy.
-
Other This included treatments such as vibration therapy, heat therapy, hydrotherapy and massage.
Interventions such as transcranial magnetic stimulation and epidural spinal stimulation were excluded. In addition, trials examining the effectiveness of surgical or pharmaceutical interventions were excluded unless these interventions were provided as co-interventions to both groups to enable the isolation of the effectiveness of the physiotherapy intervention. For example, a trial comparing robotic gait training and transcranial magnetic stimulation versus transcranial magnetic stimulation would have been included, but a trial comparing robotic gait training versus transcranial magnetic stimulation would have been excluded.
Trials were only included if the intervention was applied on more than one occasion unless the intervention was applied for many hours. Consequently, experimental studies that only analysed the effects of one-off treatments were not included.
Types of comparisons
Trials were included if they compared:
-
Any physiotherapy intervention to no physiotherapy intervention, or a placebo or sham physiotherapy intervention.
-
Any physiotherapy intervention to another physiotherapy intervention. In these trials, we needed to assign one group as experimental and the other as control for the purpose of the analyses and interpretation. We considered relying on the authors’ hypotheses to assign the groups but not all trials had clearly stated hypotheses. That is, it was not always clear which group the authors believed would be superior to the other. We therefore adopted a convention whereby the intervention that decreased spasticity was labelled as the experimental group. In all but two trials [13, 14] this aligned with the authors’ stated (or assumed) hypothesis. We acknowledge that this approach has the potential to bias our findings in favour of reporting a treatment effect but as it turned out, in one trial the two interventions were ineffective regardless of how we defined the experimental condition [13], and in the other trial the conclusion moved between inconclusive and ineffective depending on how we defined the experimental condition [14].
Types of outcome measures
Trials were included if they contained an:
-
Assessor-reported measure of spasticity This included any measure of spasticity taken by an assessor. For example the Tardieu Scale, the Wartenberg Pendulum Test, the Penn Spasm Frequency Scale, the Ashworth Scale, the Modified Ashworth Scale and Surface Electromyography.
-
Participant-reported measure of spasticity This included any measure of spasticity (or the implications of spasticity) that were self-reported by participants. For example, the Spinal Cord Assessment Tool for Spastic Reflexes (SCATS), Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET) and The Spasticity-related Quality of Life Tool (SQoL-6D).
To avoid problems of multiplicity we adopted the following strategies [15]. In trials that had multiple different measures of spasticity, only one outcome measure that captured an assessor- and/or participant-reported measure of spasticity were extracted. Priority was given to outcomes that were fully reported and commonly used in the identified trials and clinical practice. In trials that measured spasticity in more than one muscle or joint, only one set of results were extracted. Priority were given to the results most likely to reflect the effectiveness of the treatment (e.g. if a trial involved treatment to the quadriceps muscle and the trial measured spasticity in the quadriceps and plantarflexor muscles, data for the quadriceps muscles were extracted). Similarly if data were provided for both sides of the body, a decision was made a priori to only extract data from the right side of the body. In trials that included more than one endpoint (e.g. 1 h after the last treatment, one day after the last treatment and 1 month after the last treatment), only the data collected as soon as possible after the last treatment were extracted.
Search strategy
The following databases were searched from inception to August 2020: Embase (via the Ovid search engine): Medline (via the Ovid search engine) and the Cochrane Central register of controlled trials (via the Ovid search engine). The Ovid search strategy for randomised controlled trials was combined with a combination of search terms to capture two themes, namely, spinal cord injuries and spasticity (see Supplementary File 1 for the search strategy). The search strategies were adjusted for the various databases and lines were added to exclude animal studies. The results of this search were combined with the results of a search of PEDro using the filters for neurotrauma and clinical trials. We also scanned the 207 papers we had identified from our own previous search of randomised controlled trials of physiotherapy interventions for people with spinal cord injuries. The search strategy and results have been described elsewhere and were last updated in August 2020 [16].
Titles and abstracts were examined independently by two review authors (PB, LH). They each compiled a list of potentially eligible studies. The full papers on both lists were retrieved and both review authors independently culled on the basis of the full text articles. Any disagreements were resolved through discussion and when necessary a third author was asked to arbitrate.
Assessment of bias
The PEDro Scale was used to assess the susceptibility to bias of included trials. Assessments were carried out by two authors independently (PB, LH). Any disagreements were resolved through discussion and when necessary a third author was asked to arbitrate.
Data extraction and analysis
The following data were extracted by one author (PB) and checked by others (LH, JG): the design of the trial, the characteristics of the participants, interventions, and outcomes, and the timing of the assessments with respect to the last treatment. Means (SD) of the outcome measures were extracted or derived by two authors (PB, LH) to enable the calculation of mean (95% CI) between-group differences. Preference was given to co-variate adjusted data, followed by change data followed by post data. If trials did not report SDs, these were imputed from other measures such as standard error (SE), interquartile ranges, p values or confidence intervals. All data were expressed so that a negative between-group difference indicated a reduction in spasticity in the experimental group compared to the control group. We planned to extract and analyse dichotomised data if necessary but no trial reported data in this way.
We planned to conduct meta-analyses if there were a sufficient number of trials looking at a similar question and using similar outcomes provided there was no clinical or statistical heterogeneity. However, this was not the case, so the details of how we planned to pool data are not provided.
Interpretation of trial results
Minimal clinically important differences (MCID) were set at 10% of the scale of each outcome measure because there is as yet no consensus on the MCID for the outcomes of the retrieved trials. For example, the MCID of the Modified Ashworth Scale (scale: 0–5 points) was deemed to be 0.5 points, and the MCID of the Patient Reported Impact of Spasticity Measures (scale: 0–164) was deemed to be 16.4 points. Results were then categorised as:
Effective: if the 95% CI of the mean between-group difference was above the MCID.
Inconclusive: if the 95% CI of the mean between-group difference spanned the MCID.
Ineffective: if the 95% CI of the mean between-group difference was below the MCID.
Results
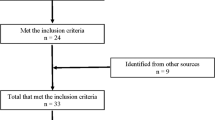
The search retrieved 2169 papers with 1621 after duplicates were removed (see Fig. 1). Twenty-four papers met the inclusion criteria. Another four papers were identified from our own database of randomised controlled trials involving physiotherapy interventions for people with SCI. In total, 28 trials met the inclusion criteria of which only 17 provided useable data and are included in this review [13, 14, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] (See Supplementary File 2 for the 11 trials that were eligible for inclusion but did not provide useable data).
The 17 trials had a median (IQR) sample size of 15 participants (11–20). The median (interquartile range: IQR) PEDro score was 6/10 (6–8) (see Figs. 2–5 for details about the PEDro score for each trial). The most common sources of bias were failure to blind participants, therapists and assessors.
The results are expressed as mean differences (95% CI) with negative values indicating a reduction in spasticity of the experimental group compared to the control group. The results are interpreted as effective if the 95% CI is below the minimal clinically important difference (MCID), inconclusive if the 95% CI spans the MCID and ineffective if the 95% CI is above the MCID (the MCID is set at 10% of the total possible score). The risk of bias according to PEDro is also indicated. The risk of bias indicates whether each of the PEDro items were satisfied (green) or not (red) where: A: random allocation; B: concealed allocation; C: baseline compatibility; D: participant blinding; E: therapist blinding; F: assessor blinding; G: adequate follow-up; H: intention-to-treat analysis; I: between-group differences; J: point estimates. Abbreviations: CPM continuous passive motion, TENS transcutaneous electrical nerve stimulation, FES functional electrical stimulation, PMs passive movements, PRT progressive resistance training, PFs plantarflexor muscles, Pts points. The study Chang et al. [18] is change data derived from participant level data provided in Table 1; The study by Oo [21] is back converted from mean (99% CI) between-group difference of 2.13 (0.59–3.66); The study by Ralston et al. [22] is back converted from a mean (95% CI) between-group difference of −1.9 (−4.9 to 1.2) with slight differences in upper end of CI due to rounding; The study by Tamburella et al. [23] is post data; The study by Harvey et al. [19] is back converted from a median (95% CI) between-group difference of 0 (0 to 1) with slight differences in 95% CI due to statistical methodology used to derive a 95% CI for the median difference. The study by Bye et al. [17] is back converted from a mean (95% CI) between-group difference of 0.03 (−0.25 to 0.32).
The results are expressed as mean differences (95% CI) with negative values indicating a reduction in spasticity of the experimental group compared to the control group. The results are interpreted as effective if the 95% CI is below the MCID, inconclusive if the 95% CI spans the MCID and ineffective if the 95% CI is above the MCID (the MCID is set at 10% of the total possible score). The risk of bias according to PEDro is also indicated. The risk of bias indicates whether each of the PEDro items were satisfied (green) or not (red) (see legend for Fig. 2 for details). Abbreviations: FES functional electrical stimulation, Pts points. Means (SD) of the outcome measures were extracted or derived to calculate mean (95% CI) between-group differences. Preference was given to co-variate adjusted data, followed by change data followed by post data. If trials did not report SDs, these were imputed from other measures such as standard error (SE), interquartile ranges, P values or confidence intervals. The study by Tamburella et al. [23] is post data; The study by Ralston et al. [22] is back converted from a mean (95% CI) between-group difference of −5 (−13 to 2) (the slight difference in the 95% CI is due to rounding); The study by Kwok et al. [20] is back converted from a mean (95% CI) between-group difference of −0.1 (−0.3 to 0.2) with numbers flipped to reflect the direction of the effect as reported here.
The results are expressed as mean differences (95% CI) with negative values indicating a reduction in spasticity of the experimental group compared to the control group. The results are interpreted as effective if the 95% CI is below the MCID, inconclusive if the 95% CI spans the MCID and ineffective if the 95% CI is above the MCID (the MCID is set at 10% of the total possible score). The risk of bias according to PEDro is also indicated. The risk of bias indicates whether each of the PEDro items were satisfied (green) or not (red) (see legend for Fig. 2 for details). Abbreviations: BWSTT body-weight supported treadmill training, FES functional electrical stimulation, Pts points. The study by Lechner et al. [13] is based on individual participant change data; The study by Wu et al. [14] is based on post data; The study by Jayaraman et al. [28] is based on post data; The study by Giangregorio et al. [27] is based on post data only available in a second publication of the same study [44]; The study by Wirz et al. [30] is based on post median (IQR) data; The study by Adams and Hicks [24] is based on post data; The study by Martinez et al. [29] is derived from participant level post data provided by the authors in a Supplementary file; The study by Wu et al. [31] is based on post data; The study by Alcobendas-Maestro et al. [25] is based on post median (IQR) data. *The authors state that they used the modified Ashworth Scale with a range from 0 to 4 points but it is assumed that this includes the additional score of 1+ thus effectively resulting in a 0 to 5 point scale with 6 options.
The results are expressed as mean differences (95% CI) with negative values indicating a reduction in spasticity of the experimental group compared to the control group. The results are interpreted as effective if the 95% CI is below the MCID, inconclusive if the 95% CI spans the MCID and ineffective if the 95% CI is above the MCID (the MCID is set at 10% of the total possible score). The risk of bias according to PEDro is also indicated. The risk of bias indicates whether each of the PEDro items were satisfied (green) or not (red) (see legend for Fig. 2 for details). Abbreviations: VAS visual analogue scale, BWSTT body-weight supported treadmill training, Pts points. The study by Lechner et al. [13] is based on individual participant change data; The study by Galea et al. [26] is back converted from a mean (95% CI) between-group difference of −0.25 (−0.61 to 0.11); The study by Adams and Hicks [24] is post data; The study by Martinez et al. [29] is derived from participant level post data provided in Supplementary file; The study by Wirz et al. [30] is based on post median (IQR) data.
Fifteen trials provided an assessor-reported measure of spasticity. They used either the Ashworth or modified Ashworth scale. The Ashworth scores were presented in various ways included scores for individual muscles, and scores averaged or tallied across many muscles. Eight trials provided a participant-reported measure of spasticity. These included Visual Analogue Scales, Penn Spasm Frequency Score, SCATS, Patient Reported Impact of Spasticity Measures and the SCI-SET.
One physiotherapy intervention versus no or sham intervention
Seven trials compared a physiotherapy intervention to no or sham intervention (see Table 1A). The physiotherapy interventions were active exercise (1 trial involving progressive resistance training [17]), electrotherapy (2 trials involving TENS [21] and FES cycling [22]) and passive interventions (4 trials involving CPM [18], passive movements [19], passive standing [20] and kinesiotaping [23]). Six of these trials included an assessor-reported measure of spasticity (see Fig. 2) and three included a participant-reported measure of spasticity (see Fig. 3). Only one trial demonstrated a treatment effect (see Figs. 2 and 3) [18]. This trial compared Continuous Passive Motion (CPM) of the ankle to no treatment and used the Modified Ashworth Scale (an assessor-reported measures of spasticity). It demonstrated a mean (95% CI) between-group difference of −3.1 points (−3.9 to −2.4) indicating a decrease in spasticity in the CPM group compared to the no treatment group (the MCID was −0.5 points). The results of all the other trials were either inconclusive or indicated that the treatment was ineffective.
One physiotherapy intervention versus another physiotherapy intervention
Ten trials compared one physiotherapy intervention to another physiotherapy intervention (see Table 1B). Eight of the trials compared one type of gait training with another type of gait training or with a general exercise program [14, 24,25,26,27, 29,30,31]. The other two trials compared (i) two types of strength training programs [28] and (ii) a Bobath seated balance training technique with hippotherapy [13]. Nine trials included an assessor-reported measure of spasticity and five trials included a participant-reported measure of spasticity (see Table 1B). None of the trials indicated a decrease in spasticity in the experimental groups compared to the control groups. Instead, the results of all the trials were either inconclusive or ineffective (see Figs. 4 and 5).
Discussion
This systematic review summarises the current state of evidence about the effectiveness of physiotherapy interventions for the treatment of spasticity. While we identified 17 trials, none of the interventions or comparison were sufficiently similar to enable pooling of results. Of the 17 trials, only one [18] indicated a treatment effect and this trial’s PEDro score was only 6/10 suggesting vulnerability to bias. The trial examined CPM for the ankle compared to no treatment. Interestingly, these findings were not replicated in a trial looking at a very similar intervention, namely passive movements on the ankle [19]. Most trials were inconclusive due to small sample size and heterogeneity.
Our categorisation of studies into effective, inconclusive and ineffective was based on our definition of a MCID (made prior to examining the results). In the absence of any research that defines the amount of change in spasticity that people with SCI value after taking into account the time, cost and potential for harm of the various physiotherapy interventions [32], we defined the MCID as 10% of the range of each scale. Readers may disagree with this definition or opt to interpret the results on the basis of whether the results are statistically significant or not. However many researchers (including members of our team) have argued that we need to move past relying on p values and consider the size (and precision) of treatment effects [33]. Consideration of the size of treatment effects enables the distinction between trials that are inconclusive and trials that indicate that treatments are ineffective. For example, the very narrow 95% CI in a trial comparing passive standing and usual care to usual care ruled out a treatment effect on spasticity unless one values a possible effect as small as 0.15 points on a 7 point scale [20]. In addition, the use of a MCID ensures that one does not assume that statistically significant effects are interpreted as clinically meaningful. For example, the 95% CI associated with the mean between-group difference for one trial [21] was −3.14 to −1.12 on a scale spanning 1 to 16 points. The trial was classified as inconclusive because a possible treatment effect as small as 1.12/16 points was not considered clinically meaningful. Another trial had a similarly very small treatment effect [13, 21]. Nonetheless, if readers prefer to rely on p values then the interpretation of the results will be largely unchanged because in all but three trials the results are not statistically significant [13, 18, 21] and one of the three trials has been classified as “effective” anyway [18] thereby only potentially changing the interpretation of two trials: one about TENS [13] and one about sitting astride a Bobath roll [13].
There are similarities and differences between our findings and those of others. For example, we, like others [4], have concluded that it is not clear whether robotic gait training, hippotherapy, passive movements, passive standing and kinesiotaping decrease spasticity. However, where others say that these interventions “may reduce spasticity” [4], we say the evidence is “inconclusive” (hippotherapy was inconclusive for the participant-reported outcome but ineffective for the assessor-reported outcome). In so far as the phrase, “may reduce spasticity”, is equivalent to “inconclusive”, our findings about these interventions broadly align. We would however argue that “may reduce spasticity” should be interpreted as “may increase or may decrease spasticity” which is a subtle but important difference.
A notable difference between our conclusions and those of others relate to the effectiveness of ES (with or without cycling). We (and others [9]) have concluded that it is not clear whether ES has an effect on spasticity, while some have concluded that ES cycling decreases spasticity [9] (and others have concluded that ES is ineffective [10]. There are similar contradictions about the effectiveness of some of the other interventions which can often be explained by differences in the types of studies that have been included. This is understandable. However, some differences are due to misinterpretation of data. For example, sometimes within-group decreases in spasticity from clinical trials [34, 35] are used to support claims of Level 1B and Level 2 evidence of treatment effectiveness. This categorisation of these types of studies is misleading because it implies the evidence is coming from randomised controlled trials whereas in actual fact the evidence is coming from the equivalent of a pre-post study (any benefit of the randomisation being lost with the analysis). Other contradictions in the interpretation of evidence are harder to explain. For example, one review [4] concluded from one trial [21] that TENS decreases spasticity stating that “Ongoing (TENS) transcutaneous electrical nerve stimulation programs result in short-term reductions in spasticity which may last for up to 24 h” (pg 56 [4]). We concluded on the basis of the same trial that it is not clear whether TENS decreases spasticity. The differences between our conclusions for this intervention (and other interventions) and the conclusions of other systematic reviews point to the difficulties and nuances of interpreting evidence. It should sound a warning about the dangers of accepting the results of systematic reviews and clinical practice guidelines without careful scrutiny.
The results of this systematic review need to be interpreted with caution for three main reasons. Firstly, there were no two studies that were similar enough to consider pooling results in meta-analyses. Yet, meta-analyses of many high-quality trials provide the best evidence of treatment effectiveness (or ineffectiveness). So there is clearly a need for more trials. Secondly, most trials relied on the Ashworth or modified Ashworth scale. The ordinal nature of this scale is very problematic for statistical analyses. Ideally, it would have been better to use ordinal methods (proportional odds) or binary methods (binary logistic regression) to determine the treatment effects [36]. However, both methods rely on authors providing the count data for each possible score. These were never provided. This problem is not unique to the Ashworth scale. For example, there are the same analytic problems with the modified Rankin Scale in studies involving survivors of stroke [37]. The solution is for researchers to either not use outcomes that rely on short ordinal scales or provide individual participant-level data as advocated by Spinal Cord [38] and other journals. The third reason why the findings of the included trials need to be interpreted with caution is because spasticity was measured in different ways in the studies and may be capturing different phenomena. For example, some argue that the Ashworth and modified Ashworth scales do not capture spasticity per se [39, 40] because according to the original definition of Lance [11], spasticity is a velocity-dependent response to movement yet neither scale manipulates velocity [41]. In addition, Ashworth scores can be influenced by the presence of contractures; a non-neurological phenomena [41]. Some argue against the use of either the Ashworth or Modified Ashworth scales as measures of spasticity, and instead advocate for the use of the Tardieu Scale [41]. This issue in part reflects ambiguities around the definitions of spasms, spasticity, tone and various other terms used to capture the different neurological features of an upper motor neurone syndrome. We have taken a pragmatic approach and used the term in its broader sense to capture the many different presentations of involuntary muscle contractions (intermittent or sustained) associated with upper motor neuron lesions. However, given we have not pooled results in meta-analyses, the only implication is that the results of the studies only reflect the effect of the intervention on the type of spasticity captured in the outcome measure. This may narrow the implications of our findings if physiotherapy interventions affect specific manifestations of spasticity that are not captured in the outcome measures. But this would seem unlikely.
We included both assessor-reported and participant-reported measures of spasticity. In two trials, the results were conflicting with participants reporting reductions in spasticity that were not mirrored by reductions in the corresponding assessor-reported outcome [13, 30]. Of course, participants’ perceptions of spasticity and treatment effectiveness are what matter most [12, 42, 43]. So arguably, these should be the focus of trials. These types of outcomes are however problematic if sham treatments can not be used. Without a convincing sham treatment it is not possible to blind participants and therefore their perceptions may at least in part reflect their own biases and expectations of treatment benefit. There are however some interventions in which it would be possible to provide a sham treatment. For example, TENS and perhaps ES. For these interventions it should be relatively simple to conduct high quality clinical trials to determine treatment effectiveness. This approach would also by-pass the problems of the Ashworth and other assessor-reported measures of spasticity.
Some of the included studies compared one treatment to another treatment. These comparisons do not tell us about the effectiveness of the treatment per se. However, we included these comparisons because if one treatment is superior to another, this provides indirect evidence that the treatment is superior to no treatment.
The trials included in this systematic review analysed the effects of physiotherapy interventions at varying times after the last treatment ranging from a few minutes (the authors only stated “immediately” after the last treatment) up until 2 months. It may be unreasonable to expect the effects of a treatment on spasticity to last more than a few hours after the last intervention. Future trials may therefore be well advised to distinguish between the immediate effects (within a few hours of the last treatment) and the lasting effects (more than 1 week after the last treatment). But of course, we also need to be open to the possibility that no physiotherapy intervention reduces spasticity. It may not be possible to influence the excitability of the lower motor neurone with the sorts of interventions physiotherapists typically administer. Regardless, physiotherapists play an important role in the overall management of spasticity. They treat impairments that can both contribute to, and result from, spasticity including contractures and pain. They also provide advice and support to patients about strategies to minimise the deleterious implications of spasticity and they refer patients on to others for pharmaceutical or surgical treatments.
This systematic review may not have captured all relevant trials but it nonetheless provides a snap shot of the state of the evidence. For example, we did not include experimental studies that only provided a one-off physiotherapy treatment to examine the immediate effects on spasticity. We reasoned that examining the effects of one treatment does not mimic clinical practice and the response to just one treatment may not reflect the response to a treatment provided on a regular basis. However, we concede that the inclusion of these studies may have provided a fuller picture. We also did not include trials that were not published in English. However, these trials are more likely than trials published in English to be negative or inconclusive so their addition may not have changed our results because publication bias tends to see trials with no effect being published in non-English journals and trials with an effect being published in English journals.
The results of this systematic review highlight how little we currently know about the effectiveness of different physiotherapy interventions for the management of spasticity with only one trial involving CPM for the ankle (compared to no treatment) suggesting a treatment effect. It is surprising that such little attention has been directed to this issue given its importance to people with SCI, and given the amount of time and effort physiotherapists devote to administering various interventions for this purpose. Admittedly there are clearly difficulties with conducting clinical trials in this area, particularly with quantifying and objectively measuring spasticity. However, perhaps some of these problems could be by-passed if researchers were more willing to rely on participant-reported measures of spasticity. Participants’ perceptions are after all what matters most and should be the focus of future trials.
Data availability
All data extracted from the original studies are available in the original publications. The authors will consider making the data extraction spreadsheets available upon reasonable request.
References
Tibbett J, Widerstrom-Noga EG, Thomas CK, Field-Fote EC. Impact of spasticity on transfers and activities of daily living in individuals with spinal cord injury. J Spinal Cord Med. 2019;42:318–27.
McKay WB, Sweatman WM, Field-Fote EC. The experience of spasticity after spinal cord injury: perceived characteristics and impact on daily life. Spinal Cord. 2018;56:478–86.
Khan F, Amatya B, Bensmail D, Yelnik A. Non-pharmacological interventions for spasticity in adults: an overview of systematic reviews. Ann Phys Rehabil Med. 2019;62:265–73.
Hsieh JTC, Connolly S, McIntyre A, Townson AF, Short C, Mills P et al. Spasticty following spinal cord injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, et al. editors. Spinal Cord Injury Rehabilitation Evidence, Version 6.0. 2016: Vancouver.
Hsieh JTC, Wolfe DL, Connolly S, Townson AF, Curt A, Blackmer J, et al. Spasticity after spinal cord injury: an evidence-based review of current interventions. Top Spinal Cord Inj Rehabil. 2007;13:81–97.
Sackett DL, Strauss EE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based medicine. How to practice and teach EBM. 2nd ed. Edinburgh: Churchill Livingstone; 2000.
Sadeghi M, Sawatzky B. Effects of vibration on spasticity in individuals with spinal cord injury: a scoping systematic review. Am J Phys Med Rehabil. 2014;93:995–1007.
Laessoe L, Nielsen JB, Biering-Sorensen F, Sonksen J. Antispastic effect of penile vibration in men with spinal cord lesion. Arch Phys Med Rehabil. 2004;85:919–24.
Alashram AR, Annino G, Mercuri NB. Changes in spasticity following functional electrical stimulation cycling in patients with spinal cord injury: a systematic review. J Spinal Cord Med. 2020. https://doi.org/10.1080/10790268.2020.1763713
Thomaz SR, Cipriano G Jr, Formiga MF, Fachin-Martins E, Cipriano GFB, Martins WR, et al. Effect of electrical stimulation on muscle atrophy and spasticity in patients with spinal cord injury - a systematic review with meta-analysis. Spinal Cord. 2019;57:258–66.
Lance J. The control of muscle tone, reflexes and movement: Robert Wartenberg lecture. Neurology. 1980;30:1303–13.
Bhimani RH, Gaugler JE, Skay C. Understanding symptom experiences of muscle tightness from patients’ and clinicians’ perspectives. J Clin Nurs. 2017;26:1927–38.
Lechner HE, Kakebeeke TH, Hegemann D, Baumberger M. The effect of Hippotherapy on spasticity and on mental well-being of persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1241–8.
Wu M, Kim J, Wei F. Facilitating weight shifting during treadmill training improves walking function in humans with SCI: a randomized controlled pilot study. Am J Phys Med Rehabil. 2018;97:585–92.
Lopez-Lopez JA, Page MJ, Lipsey MW, Higgins JPT. Dealing with effect size multiplicity in systematic reviews and meta-analyses. Res Synth Methods. 2018;9:336–51.
Aravind N, Harvey LA, Glinsky JV. Physiotherapy interventions for increasing muscle strength in people with spinal cord injuries: a systematic review. Spinal Cord. 2019;57:449–60.
Bye EA, Harvey LA, Gambhir A, Kataria C, Glinsky JV, Bowden JL, et al. Strength training for partially paralysed muscles in people with recent spinal cord injury: a within-participant randomised controlled trial. Spinal Cord. 2017;55:460–5.
Chang YJ, Liang JN, Hsu MJ, Lien HY, Fang CY, Lin CH. Effects of continuous passive motion on reversing the adapted spinal circuit in humans with chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94:822–8.
Harvey LA, Herbert RD, Glinsky J, Moseley AM, Bowden J. Effects of 6 months of regular passive movements on ankle joint mobility in people with spinal cord injury: a randomized controlled trial. Spinal Cord. 2009;47:62–66.
Kwok S, Harvey L, Glinsky J, Bowden JL, Coggrave M, Tussler D. Does regular standing improve bowel function in people with spinal cord injury? A randomised crossover trial. Spinal Cord. 2015;53:36–41.
Oo WM. Efficacy of addition of transcutaneous electrical nerve stimulation to standardized physical therapy in subacute spinal spasticity: a randomized controlled trial. Arch Phys Med Rehabil. 2014;95:2013–20.
Ralston K, Harvey L, Batty J, Lee B, Ben M, Cusmiani R, et al. Functional electrical stimulation cycling has no clear effect on urine output, lower limb swelling, and spasticity in people with spinal cord injury: a randomised cross-over trial. J Physiother. 2013;59:237–43.
Tamburella F, Scivoletto G, Molinari M. Somatosensory inputs by application of KinesioTaping: effects on spasticity, balance, and gait in chronic spinal cord injury. Front Human Neurosci. 2014;8:367. https://doi.org/10.3389/fnhum.2014.00367
Adams MM, Hicks AL. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med. 2011;34:488–94.
Alcobendas-Maestro M, Esclarin-Ruz A, Casado-Lopez RM, Munoz-Gonzalez A, Perez-Mateos G, Gonzalez-Valdizan E, et al. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: randomized controlled trial. Neurorehabil Neural Repair. 2012;26:1058–63.
Galea MP, Dunlop SA, Geraghty T, Davis GM, Nunn A, Olenko L, et al. SCIPA full-on: a randomized controlled trial comparing intensive whole-body exercise and upper body exercise after spinal cord injury. Neurorehabil Neural Repair. 2018;32:557–67.
Giangregorio L, Craven C, Richards K, Kapadia N, Hitzig SL, Masani K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on body composition. J Spinal Cord Med. 2012;35:351–60.
Jayaraman A, Thompson CK, Rymer WZ, Hornby TG. Short-term maximal-intensity resistance training increases volitional function and strength in chronic incomplete spinal cord injury: a pilot study. J Neurol Phys Ther. 2013;37:112–7.
Martinez SA, Nguyen ND, Bailey E, Doyle-Green D, Hauser HA, Handrakis JP, et al. Multimodal cortical and subcortical exercise compared with treadmill training for spinal cord injury. PloS one. 2018;13:e0202130.
Wirz M, Mach O, Maier D, Benito-Penalva J, Taylor J, Esclarin A, et al. Effectiveness of automated locomotor training in patients with acute incomplete spinal cord injury: a randomized, controlled, multicenter trial. J Neurotrauma. 2017;34:1891–6.
Wu M, Landry JM, Kim J, Schmit BD, Yen SC, McDonald J, et al. Repeat exposure to leg swing perturbations during treadmill training induces long-term retention of increased step length in human SCI: a pilot randomized controlled study. Am J Phys Med Rehabil. 2016;95:911–20.
Harvey LA. A minimally important treatment effect is a key but ellusive concept. Spinal Cord. 2019;57:83–84.
Harvey LA, Brinkhof MWG. Imagine a research world without the words “statistically significant”. Is it really possible? Spinal Cord. 2019;57:437–8.
Mirbagheri MM, Kindig MW, Niu X. Effects of robotic-locomotor training on stretch reflex function and muscular properties in individuals with spinal cord injury. Clin Neurophysiol. 2015;126:997–1006.
Manella KJ, Field-Fote EC. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci. 2013;31:633–46.
Krishan A, Weir J, Murray G, Thomas B, Sandercock P, Lewis S. Practical methods for ordinal data meta-analysis in stroke. Trials. 2013;14(Suppl 1):P120.
Churilov L, Arnup S, Johns H, Leung T, Roberts S, Campbell BC, et al. An improved method for simple, assumption-free ordinal analysis of the modified Rankin Scale using generalized odds ratios. Int J Stroke. 2014;9:999–1005.
Dijkers MP. A beginner’s guide to data stewardship and data sharing. Spinal Cord. 2019;57:169–82.
Fleuren JF, Voerman GE, Erren-Wolters CV, Snoek GJ, Rietman JS, Hermens HJ, et al. Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry. 2010;81:46–52.
Haugh AB, Pandyan AD, Johnson GR. A systematic review of the Tardieu Scale for the measurement of spasticity. Disabil Rehabil. 2006;28:899–907.
Patrick E, Ada L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin Rehabil. 2006;20:173–82.
Bhimani RH, McAlpine CP, Henly SJ. Understanding spasticity from patients’ perspectives over time. J Adv Nurs. 2012;68:2504–14.
Barnes M, Kocer S, Murie Fernandez M, Balcaitiene J, Fheodoroff K. An international survey of patients living with spasticity. Disabil Rehabil. 2017;39:1428–34.
Kapadia N, Masani K, Craven BC, Giangregorio LM, Hitzig SL, Richards K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J Spinal Cord Med. 2014;37:511–24.
Funding
LAH and JVG are funded by iCARE and associated grants from iCARE. PB and EFM are funded by CAPES from Brazilian Government.
Author information
Authors and Affiliations
Contributions
PB, LAH, JVG and EF designed the review and screening potentially eligible studies. PB, LAH, JVG and EF conducted the search. PB and LAH extracted and analysed the data. PB and LAH applied the PEDro scale and JVG and EF arbitrated the discordance. PB, LAH, JVG and EF interpreted results and wrote the report. PB, LAH, JVG and EF wrote the report and provided feedback on the report.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Barbosa, P.H.F.d.A., Glinsky, J.V., Fachin-Martins, E. et al. Physiotherapy interventions for the treatment of spasticity in people with spinal cord injury: a systematic review. Spinal Cord 59, 236–247 (2021). https://doi.org/10.1038/s41393-020-00610-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-00610-4