Abstract
Although stem cell-based therapy has demonstrated considerable potential to manage certain diseases more successfully than conventional surgery, it nevertheless comes with inescapable drawbacks that might limit its clinical translation. Compared to stem cells, stem cell-derived exosomes possess numerous advantages, such as non-immunogenicity, non-infusion toxicity, easy access, effortless preservation, and freedom from tumorigenic potential and ethical issues. Exosomes can inherit similar therapeutic effects from their parental cells such as embryonic stem cells and adult stem cells through vertical delivery of their pluripotency or multipotency. After a thorough search and meticulous dissection of relevant literature from the last five years, we present this comprehensive, up-to-date, specialty-specific and disease-oriented review to highlight the surgical application and potential of stem cell-derived exosomes. Exosomes derived from stem cells (e.g., embryonic, induced pluripotent, hematopoietic, mesenchymal, neural, and endothelial stem cells) are capable of treating numerous diseases encountered in orthopedic surgery, neurosurgery, plastic surgery, general surgery, cardiothoracic surgery, urology, head and neck surgery, ophthalmology, and obstetrics and gynecology. The diverse therapeutic effects of stem cells-derived exosomes are a hierarchical translation through tissue-specific responses, and cell-specific molecular signaling pathways. In this review, we highlight stem cell-derived exosomes as a viable and potent alternative to stem cell-based therapy in managing various surgical conditions. We recommend that future research combines wisdoms from surgeons, nanomedicine practitioners, and stem cell researchers in this relevant and intriguing research area.
Similar content being viewed by others
Introduction
Stem cells are a population of undifferentiated cells with unique abilities to self-renew and recreate functional tissues. They are primarily classified by their differentiation potential, origin and lineage progression. According to their potency, stem cells can be totipotent, pluripotent, multipotent, oligopotent and unipotent.1 Stem cells exist both in embryos and adult cells. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are best examples of pluripotent stem cells,2 whereas adult multipotent stem cells are exemplified by hematopoietic stem cells (HSCs),3 mesenchymal stem cells (MSCs),4 neural stem cells (NSCs),5 and endothelial stem/progenitor cells (EPCs)6 (Fig. 1a). All these subtypes of stem cells have been extensively trialed for the treatment of human diseases.
Illustration of the upstream measures of exosome therapy (figure generated using Autodesk 3ds Max 2023). a production and purification of exosomes (MSCs and NSCs are used as examples for multipotent stem cells). b content of natural exosomes. c modification of exosomes. (BM bone marrow, DC dendritic cell, IAC immunoaffinity chromatography, iPSC induced pluripotent stem cell, MHC major histocompatibility complex, miRNA microRNA, MSC mesenchymal stem cell, MVB multivesicular body, NSC neural stem cell, SEC size-exclusion chromatography, UC umbilical cord)
Stem cell-based therapy, as a modality of regenerative medicine, has generated tremendous attention, as it offers new options for patients suffering from previously incurable diseases. Subsequently, thousands of related clinical trials have been registered, covering a wide spectrum of medical problems, such as musculoskeletal and neurological disorders, immune diseases, hematological dysfunctions, and degenerative conditions.7 However, some trials have failed to show any benefit in the clinic. This is likely due to the inevitable limitations of stem cell therapy, such as infusion toxicity, immunogenicity, tumorigenic potentials and ethical issues.8 Exosome, secreted by almost all cell types including stem cells (Fig. 1a), has been posited as a safer and more versatile alternative to stem cell therapy.9
Exosomes are nanoscale, spherical, and lipid bi-layered single membrane extracellular vesicles, which act as intercellular messengers.10 Exosomes have been regarded as miniature versions of their parental cells, partially because exosomes from a certain cell type provide cell-specific or unique sets of biomolecules. In addition, the stem cells have been found to function in a paracrine fashion through their soluble secretome including exosomes.11 In other words, stem cell-derived exosomes (SC-Exo) inherit similar therapeutic effects from their parental cell of origin, e.g., anti-inflammation, immunomodulation and tissue regeneration.12 Collectively, stem cell-derived exosomes are a potent surrogate for stem cell therapy without exhibiting the disadvantages their cellular counterparts present13 (Table 1).
Prior to clinical applications, exosomes must be prepared and optimized in terms of production, purification, and modification (Sections 2.3 and 2.4). A wide range of medical reviews analyzing these upstream measures of exosome therapy have been published in recent years. Nevertheless, some research avenues remain under-investigated: in particular, systematic investigation dedicated to downstream clinical applications is lacking, especially from a surgical perspective. Tissues that have been damaged, whether by disease or a surgeon’s scalpel, respond by inflammatory and regenerative dynamics,14 making surgery a perfect arena for stem cell-derived exosome therapy.15 Stem cell-derived exosomes inherit similar therapeutic effects from their parental cell of origin, e.g., tissue regeneration, anti-inflammation and immunomodulation.12,16,17,18
In this work, we will dissect relevant publications from the last five years in order to present a comprehensive, up-to-date, specialty-specific and disease-oriented review (Fig. 2). Our aim is to bridge the gap that currently exists between surgeons, nanomedicine practitioners, and stem cell researchers.
Illustration of the downstream surgical applications of exosome therapy (figure generated using Adobe Photoshop 2023 and Adobe Illustrator 2023). The therapeutic effects of exosomes are a hierarchical translation through disease-specific tissue responses, tissue-specific cellular alterations, and cell-specific molecular signaling pathways
General background of exosomes and exosome therapy
Biogenesis, composition, and uptake of exosomes
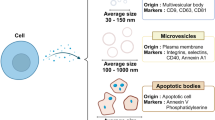
Exosomes differ from other types of primary extracellular vesicles (e.g., apoptotic bodies and microvesicles) in terms of size, content, and production mechanism.19 The most popularly accepted mechanism of exosome formation, i.e., an endosomal route, is as follows (Fig. 1a). The initial endosomes are produced by cell membrane invagination during which the bioactive substances begin to accumulate within the early sorting endosomes. The late sorting endosomes then form multivesicular bodies (MVBs) after a second indentation. Finally, the MVBs fuse with the cell membrane, releasing the carried exosomes to the outside. Non-endosomal route of exosome biogenesis, such as plasma membrane budding, has also been reported.20
As the three major exosome databases (i.e., ExoCarta, Vesiclepedia, and EVpedia) summarize, exosomes contain numerous molecules, including proteins, glycoconjugates, lipids, nucleic acids, metabolites, and other bioactive substances (Fig. 1b). The examples of each category and the corresponding functions have been thoroughly reviewed elsewhere.21,22 On the one hand, exosomes comprise a complex protein network including external proteins (e.g., tetraspanins, antigen-presenting complexes, and adhesion molecules) and internal proteins (e.g., heat shock proteins, ESCRT machinery, cytokines and chemokines, and membrane transporters).23 On the other hand, as the most abundant in human exosomal nucleic acids, microRNA (mRNA) could participate in hematopoiesis, exocytosis, and nerve and vascular regeneration through exosome-mediated cellular communication.24
There are various uptake mechanisms once exosomes reach the recipient cell, all of which can be categorized into membrane fusion, receptor interaction, and internalization21 (Fig. 1b). Finally, the exosomal cargos are released into the cytoplasm, the process of which depends on the source of the exosome, nature of the cargo, and the metabolic state of the recipient cell.25 The entire lifecycle from exosome biogenesis to uptake and intracellular signaling can be tracked using fluorescent, luminescent, and radioactive techniques.26,27
Source and classification of exosomes
Depending on whether exosomes have been artificially modified, they are broadly classified into natural exosomes and engineered exosomes (Section 2.4). Depending on the species of origin, exosomes are divided into animal-derived and plant-derived exosomes. Currently, exosomes are mainly classified according to the type of their parental cells. Almost all types of human cells can produce exosomes. These include, but are not limited to, macrophages, dendritic cells (DCs), platelets, stem cells, and even tumor cells28 (Fig. 1a).
For example, macrophage-derived exosomes contribute to disease progression (e.g., diabetes, atherosclerosis and heart failure)29 and disease treatment (e.g., cutaneous wound, inflammatory bowel disease, and fungal and viral infection).30 However, they seem to play paradoxical roles in suppressing and promoting tumors.31 Like DCs, DC-derived exosomes (Dex) could also interact with immune cells (e.g., T cells, B cells, and NK cells) through their surface proteins such as major histocompatibility complexes (MHCs).32 Some preclinical and clinical trials have demonstrated the effectiveness and safety of Dex-based immunotherapy for cancers.33 Furthermore, tumor-derived exosomes (Tex) not only are involved during tumor proliferation, invasion, metastasis, and immunity but also can be used as biomarkers for cancer diagnosis and treatment.34 Lately, Tex has been used as an anti-tumor drug and an antigen presenter for DC vaccination, serving as a promising cell-free cancer immunotherapy.35 Finally, the clinical applications of stem cell-derived exosomes will be discussed in detail in the following sections.
Exosomes can be found in all body fluids such as blood, saliva, urine, plasma, tears, semen, amniotic fluid, and even breast milk.36 Body fluid-derived exosomes are a highly stable reservoir of disease biomarkers, assisting liquid biopsy in various clinical settings such as cancers, cardiovascular diseases, and perinatal disorders.37,38 However, the coexisting contents and availability of each type of body fluid might create challenges to exosome isolation.
Production, isolation and purification of exosomes
One of the major obstacles preventing exosome-based therapeutics from entering clinical practice is the low yield and efficiency of exosomes. For example, only less than 1 μg exosomal protein could be harvested from 1 ml culture medium in a laboratory setting.39 There are various methods of upscaling exosome production, which are categorized into biochemical strategies (e.g., LPS, BMP-2, HIF-1α, and IFN-γ and TNF-α), physical strategies (hypoxia, thermal stress, and starvation), mechanical strategies (shear stress and 3D culturing) and instrumental strategies (hollow-fiber bioreactors and stirred tank bioreactors).40
Exosomes are heterogeneous in terms of size, content, surface markers, and source, which makes their isolation difficult. The currently available techniques for exosome isolation and purification are based on their size, surface charge, or immunoaffinity26 (Fig. 1a). However, there is no ‘one-fits-all’ approach as these techniques all have advantages and disadvantages.
For example, ultracentrifugation is deemed the gold standard for exosome extraction. Although it requires minimal reagents and expertize, the time consumption, high cost, low efficiency, and lipoprotein co-separation have limited its large-scale use.41 Immunoaffinity chromatography is a separation technology based on the specific binding of antibodies and ligands. It is rapid and provides high purity, specificity, and yield. However, the antigen/protein coupling used needs to be expressed on the surface of exosomes.19 Size-based isolation techniques mainly refer to ultrafiltration and size-exclusion chromatography, both of which are quick and suitable for large-scale applications. But pore clogging, exosome loss, and low purity are making this method difficult to popularize.42 Although no single technique is perfect, combining the above techniques with others (e.g., precipitation-based and microfluidics-based) might be a solution to simultaneously meet multiple requirements for exosome isolation and purification.
Modification of exosomes
Exosomes can be biochemically modified to broaden, change, or improve their therapeutic effects. The modification of exosomes is classified into internal strategies (e.g., drug loading) and external strategies (e.g., surface modification). On the one hand, exosomes may be an ideal therapeutic carrier to deliver drugs, nucleic acids, and vaccines due to their advantages in stability, non-immunogenicity, and targeting recipient cells.43 There are various cargo loading techniques including pre-production loading methods (e.g., transfection, co-incubation, and electroporation) and post-production loading methods (e.g., freeze-thaw cycles, incubation, sonication, extrusion, and hypotonic dialysis) depending on whether they are applied before or after exosome biogenesis10,26,44,45,46 (Fig. 1c). For example, Tian et al. loaded doxorubicin in Dex using electroporation for the treatment of breast cancer.47 Kim et al. loaded paclitaxel in RAW 264.7-derived exosomes using incubation and sonication to overcome multidrug resistance in cancer cells.48 Ohno et al. loaded antitumor let-7a miRNA in HEK293-derived exosomes using transfection to manage breast cancer.49
On the other hand, surface modification of exosomes is exemplified by genetic engineering of exosomal membrane or parental cells, chemical connection of targeting ligands, electrostatic interaction, and magnetic nanoparticle technology.10 The main purpose of surface modification is to selectively deliver exosomes to target cells for precise treatment. For example, Alvarez-Erviti et al. modified DCs using genetic engineering to express Lamp2b and RVG peptides, thereby targeting the central nervous system (CNS).50 Zhu et al. inserted tumor-targeting peptides, c(RGDyK), into the exosome surface using a chemical reaction to target glioblastoma.51 Nakase et al. bound exosomes with a complex formed by pH-sensitive fusion peptide and cationic lipid using electrostatic interaction, thereby achieving enhanced cytosolic delivery.52
Characterization and verification of exosomes
Exosomes need to undergo characterization and verification before therapeutic applications. Current methods used for exosome characterization mainly focus on the size, morphology, and cargo profile of exosomes.43 Size-oriented verification includes nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), and tunable resistive pulse sensing (TRPS), whereas morphology-oriented analysis includes scanning electron microscopy (SEM) and transmission electron microscopy (TEM).19 In addition, cargo profiling is further subdivided into proteomic, lipidomic, and genomic analyses including western blotting, ELISA, flow cytometry, mass spectroscopy, and PCR.36 Since each of the above characterization methods has advantages and disadvantages, it is a universal practice to combine analyses from three different aspects, e.g., a package of TEM, NTA, and western blotting, to identify isolated exosomes.
For example, microscopy-based methods, such as SEM and TEM, can directly visualize the surface topography and internal structure, respectively. However, TEM is not suitable for quick measurement of a large number of samples due to complicated operation and tedious sample preparation.53 NTA facilitates fast detection and real-time exosome observation while having a higher resolution than flow cytometry. The main disadvantage of NTA is its difficulty in distinguishing exosomes from contaminated proteins.54 As a mature technique, western blotting can qualitatively and quantitatively detect the expression of exosomal protein biomarkers, especially exosomes from cell culture media. However, it is time-consuming and not suitable for the detection of exosomes from biological fluids.55,56
Storage of exosomes
The currently used preservation methods for long-term storage of exosomes mainly include cryopreservation, lyophilization, and spray-drying.10 Temperature and antifreeze are the two most important ingredients for cryopreservation. Storage at 4 °C might weaken the biological activity and reduce the protein cargo of exosomes, whereas −80 °C is considered the optimal temperature causing the least impact on exosome morphology and content.57,58 Non-permeable disaccharide antifreeze, especially trehalose, represents the best choice as it prevents exosome aggregation and cryodamage.59 Heat-sensitive materials, e.g., exosomes and vaccines, treated by lyophilization of freeze-drying can be easily stored and reconstituted by simply adding water. A recent study showed that lyophilization with cryoprotectant could retain the activity of exosomal proteins and RNA for approximately 4 weeks even when stored at room temperature.60 Finally, in contrast to freeze-drying, spray-drying is a single-step process, thereby reducing the need for expensive equipment and lengthy multi-step milling. However, core parameters of spray-drying such as exosome feeding rate, atomization pressure, and outlet temperature, can all affect exosome stability and cargo integrity.61
Orthopedic and trauma surgery and SC-Exo therapy
Fracture
Fractures are the most common traumatic large-organ injuries, and approximately 10% heal improperly.62 Fracture healing involves an anabolic tissue-bulking phase and a catabolic tissue-remodeling phase, which are controlled by various factors such as stem cells, innate and adaptive immune functions, and stability.63 Biopharmacological treatment for fractures can be given locally (e.g., bone morphogenetic protein, BMP) or systemically (e.g., parathyroid hormone, PTH). As a promising alternative, exosome therapy for fracture healing mostly utilizes bone marrow-derived MSCs as a cellular supplier (Table 2).
The presumed mechanism of how MSC-derived exosomes promote fracture healing is as follows. Firstly, the progression of bone repair needs a variety of cells, e.g., inflammatory cells in the inflammation stage, endothelial and mesenchymal progenitor cells in the fibrovascular stage, osteoblasts and chondrocytes during bone formation, and osteoclasts during callus remodeling.62 Secondly, most of these cells can uptake exosomes, especially osteoblasts and vascular endothelial cells,64 which are most related to fracture healing. Lastly, upon exosome absorption, the gene expression of the recipient cells is modified, thereby activating various signaling pathways (Fig. 3a), causing various cellular and tissue responses (Fig. 3b) and ultimately leading to improved fracture healing.
Early research has employed various animal models of fracture healing. In a transverse femoral shaft fracture model, exosomes were found to not only promote osteogenesis in wild-type mice, but also rescue retardation of fracture healing in CD9−/− mice, a strain known to have a lower bone union rate.65 In a femoral nonunion model, exosomes enhanced fracture healing by promoting osteogenesis and angiogenesis possibly via the BMP-2/Smad1/RUNX2 pathway.66 In a tibial distraction osteogenesis model, exosomes secreted by young MSCs promoted osteogenic capacity of older MSCs and enhanced new bone formation in older rats.67 In addition, EPC-derived exosomes accelerated bone regeneration during distraction osteogenesis by stimulating angiogenesis.68
As a major cargo of exosomes (Section 2.1), RNA can alter recipient cell gene expression and phenotypic function, with microRNA (miRNA) and long non-coding RNA (lncRNA) being the most widely studied.69 From the perspective of an exosomal miRNA, one group discovered that miR-136-5p from bone marrow MSC-derived exosomes promoted osteoblast proliferation and differentiation in vitro, thereby promoting fracture healing in vivo.70 This was achieved by inhibiting the downstream target gene of miR-163-5p, low-density lipoprotein receptor-related protein 4 (LRP4), through the Wnt/β-catenin pathway. The other group found that MSC-derived exosomal miR-25 could regulate the ubiquitination and degradation of Runt-related transcription factor 2 (Runx2) by Smad ubiquitination regulatory factor 1 (SMURF1) to promote fracture healing in mice.71 From a lncRNA perspective, especially the bone-specific lncRNA H19, a Chinese group revealed that although a high-fat diet reduced osteogenic differentiation and weakened fracture healing, this could be reversed by MSC-derived exosomal lncRNA H19 via miR-467/HoxA10 axis in an obesity-induced fracture model.72 In addition, an American group demonstrated that exosomal lncRNA H19 not only improved osteogenesis but also angiogenesis through the angiopoietin 1/Tie2-NO signaling pathway in an immunocompromised nude mouse model.73
Instead of using naturally derived exosomes from MSCs, some researchers have conducted pre-isolation modification of exosomes to achieve better results. Liang et al. preconditioned MSCs with low doses of dimethyloxaloylglycine (DMOG), a small angiogenic molecule, to prepare the exosomes for an enhanced angiogenesis and bone regeneration in a critical-sized calvarial defect model by targeting the protein kinase B/mechanistic target of rapamycin (Akt/mTOR) pathway.74 Alternatively, Lu et al. loaded MSC-derived exosomes with miR-29a, which showed a robust ability in promoting angiogenesis and osteogenesis by targeting vasohibin 1.75 Furthermore, umbilical cord MSC-derived exosomes demonstrated comparable results to their bone marrow counterparts during fracture healing.76 In addition, exosomes derived from MSCs under hypoxia exhibited better effects on bone fracture healing than those under normoxia. Mechanistically, hypoxia preconditioning enhanced the production of exosomal miR-126 through the activation of hypoxia-inducible factor 1 (HIF-1α). Various studies have shown that hypoxia preconditioning represents an effective and promising optimization of the therapeutic effects of MSC-derived exosomes for bone fracture healing.
Osteoarthritis
Osteoarthritis (OA) is the most common joint disease and most frequent reason for activity limitation in adults, affecting approximately 240 million patients globally.77 The pathology of OA has evolved from being viewed as cartilage-only to a multi-tissue disease that affects all components of the whole joint, including bone, synovium, muscle, ligament, and periarticular fat.78 Clinical trials have successfully revealed systemic compounds that arrest structural progression (e.g., cathepsin K and Wnt inhibitors) or reduce OA pain (e.g., nerve growth factor inhibitors). As a potential treatment option for OA, most MSC-derived exosome therapy used chondrocytes as a target in in vitro models. These MSCs could originate from various tissues, such as bone marrow, synovium, gingiva, and infrapatellar fat pads (IPFPs).
Some studies focusing on chondrogenesis demonstrated a particular interest in the role of miRNA. Wu et al. found that IPFP MSC-derived exosomes protect articular cartilage from damage and ameliorate gait abnormality in OA mice by miR100-5p-regulated inhibition of mTOR-autophagy pathway.79 Since it is easy to retrieve human IPFP from OA patients by arthroscopic operation within a clinic, this type of exosome therapy might simplify and accelerate the process from bench to bedside. Liu et al. discovered that MSC-derived exosomes could promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in OA.80 The cellular work conducted by Kong et al. showed that synovial MSC-derived exosomal miR-320c could enhance chondrogenesis by targeting ADAM19.81 In addition, Mao et al. suggested that exosomal miR-92a-3p from chondrogenic MSCs could enhance chondrogenesis and suppress cartilage degradation via targeting Wnt5a.82 In contrast to these studies using original exosomes, few groups modified exosomes prior to their systemic administration. Tao et al. modified exosomes by transfecting synovial MSCs with miR-140-5p and found that exosomal miR-140-5p-overexpression could enhance cartilage tissue regeneration and prevent OA of the knee in a rat model.83 Meanwhile, Wang et al. used TGF-β1 to stimulate MSCs, and the resultant exosomal miR-135b increased chondrocyte proliferation by regulating specificity protein-1.84 In a comparative study, Zhu et al. demonstrated that exosomes from iPSC-derived MSCs could provide a stronger therapeutic effect on OA than synovial membrane MSC-derived exosomes.85
Other studies have focused on not only chondrogenesis but also anti-inflammation and immune modulation during OA treatment. For example, MSC-derived exosomes inhibited inflammatory factors, glutamine metabolic activity-related proteins, glutamine, and GSH/GSSG ratio in vitro, while improving mice’s chondrocyte function, tissue inflammation, and exercise capacity in vivo, thereby alleviating OA progression.86 Using a holistic approach, recent studies have shifted the attention away from cartilage towards other tissues (e.g., bone) in a diarthrodial joint. Firstly, bone marrow MSC-derived exosomal miR-206 promoted proliferation and osteogenic differentiation of osteoblasts in OA by reducing E74-like factor 3 (Elf 3), and ameliorated inflammation and increased expression of osteocalcin and BMP2 in mouse femoral tissues.87 Secondly, MSC exosome-treated osteochondral defects demonstrated a regenerative immune phenotype, characterized by a higher infiltration of CD163+ M2 macrophages over CD86+ M1 macrophages, with a concomitant reduction in pro-inflammatory synovial cytokines IL-1β and TNF-α.88 Lastly, gingival MSC-derived exosomes proved to be immunosuppressive in preventing collagen-induced arthritis.89 Compared with parental cells, these exosomes had the same or stronger effects in inhibiting IL-17A and promoting IL-10, reducing incidences and bone erosion by arthritis, via inhibiting the IL-17RA-Act1-TRAF6-NF-κB signaling pathway.
Currently, there is no single ‘one size fits all’ drug that may be suitable for all OA patients. Disease-modifying OA drugs (DMOADs) might become the next-generation OA treatment.90 It is very valuable and relevant that MSC-derived exosome therapy for OA coincides with DMOADs: both are capable of targeting inflammatory cytokines, matrix-degrading enzymes, and the Wnt pathway. Thus, emerging approaches for DMOAD development, such as miRNA-based modality and targeting cellular senescence, might also be used to refine MSC-based exosome therapy for OA.
Spinal cord injury
Traumatic spinal cord injury (SCI) is a devastating global health issue that poses a significant functional and economic burden both on the patient and society.91 The pathophysiology of SCI includes primary injuries caused by mechanical trauma and secondary injury cascade characterized by apoptosis, edema, ischemia, inflammatory cell infiltration, and excitotoxicity.92 Despite surgical intervention, clinical studies involving pharmacotherapy can be broadly classified as either neuroprotective or neuroregenerative.93 Targeting each event of the above mechanistic chain, both MSC- and NSC-derived exosome therapy could exert a beneficial influence on spinal cord protection and regeneration.
Some groups have targeted neuronal cell death. Ma et al. revealed that insulin-like growth factor 1 (IGF-1)-stimulated NSC-derived exosomes could inhibit neuronal apoptosis while promoting functional recovery after SCI through a miR-219a-2-3p/YY1 pathway.94 Alternatively, Zhang et al. discovered that subarachnoid injection of NSC-derived exosomes could suppress neuronal cell apoptosis by activating autophagy via miR-374-5p/STK-4 axis for enhanced functional recovery in SCI.95 Shao et al. explored other forms of cell death (e.g., ferroptosis) using MSC-derived exosomes, and found that exosomal lncGm36569 could inhibit neuronal cell ferroptosis via miR-5627-5p/FSP1 axis, thereby decreasing neuronal dysfunction.96
Some groups have targeted anti-inflammation and immunomodulation. Nakazaki et al. discovered that fractionated intravenous infusion of MSC-derived exosomes could target M2 macrophages and upregulate TGF-β, thereby stabilizing microvessels and improving functional recovery.97 Similarly, Liu et al. demonstrated that in addition to hypoxia increasing exosome production from bone marrow MSCs, preconditioned exosomal miR-216a-5p could also repair traumatic SCI by shifting microglial M1/M2 polarization via the TLR4/NF-κB/PI3K/Akt pathway.98 Huang et al. valuably proved that epidural fat MSC-derived exosomes could attenuate NLRP3 inflammasome and improve functional recovery in SCI.99 Compared to MSC-derived exosomes, exosomes derived from EPCs could provide comparable anti-inflammatory effect. Yuan et al. showed that the exosomal miR-222-3p from EPCs could promote anti-inflammatory macrophages via the SOC3/JAK2/STAT3 pathway and improve mouse functional repair after SCI.100
Some groups have targeted angiogenesis and blood-spinal cord barrier (BSCB) integrity. For example, Zhong et al. used unmodified NSC-derived exosomes, found that they were highly enriched in VEGF-A, and could therefore enhance the angiogenic activity of spinal cord microvascular endothelial cells (SCMECs).101 In comparison, Chen and co-workers modified NSC-derived exosomes with FTY720, an immune modulator and microvascular regulator, to protect the barrier function of SCMECs via the PTEN/Akt pathway, thereby ameliorating hindlimb function.102 It is well-known that the connection between the microvascular endothelium of the spinal cord and the pericyte is crucial in maintaining the structural integrity of BSCB.103 Thus, Zhou’s team attempted to verify the role of exosome therapy in pericyte homeostasis.104 They proved that bone marrow MSC-derived exosomes could reduce pericyte pyroptosis and increase pericyte survival rate in vitro, while improving BSCB integrity and locomotor recovery in vivo.
Finally, some groups have targeted other aspects during neuroprotection and neuroregeneration, such as neurotoxic astrocytes and endogenous NSC sustainability. Lai et al. proved that human umbilical cord MSC-derived exosomes could facilitate recovery of spinal cord function by targeting neurotoxic astrocytes.105 In addition, miR-146a-5p-modified exosomes exerted a more powerful effect than unmodified exosomes. Li et al. discovered that exosomes derived from nerve growth factor (NGF)-overexpressing bone marrow MSCs could enhance neuronal differentiation of NSCs and axonal regeneration.106 Zhou et al. demonstrated that placental MSC-derived exosomes could promote the activation of proliferating endogenous NSCs, thereby improving both locomotor activity and bladder dysfunction,107 which is a frequent sequelae that could further worsen the quality of life of SCI patients.108
Muscle and tendon tear
Muscle and tendon tears can result from either acute trauma (e.g., fractures, Section 3.1) or chronic overuse (e.g., sports injury).109 Healing of muscle strain and tendon tear follows the typical wound healing course, involving the inflammatory, proliferative, and remodeling phases. Multiple non-surgical strategies have been trialed to improve healing, including cell-based and growth factor-based therapies.110 The following proof-of-concept studies indicate that MSC-derived exosomes could become the next-generation musculoskeletal treatment.
On the one hand, some groups have focused on individual components of the muscle-tendon-bone unit. Nakamura et al. claimed that MSC-derived exosomes could improve in vitro myogenesis in C2C12 myoblasts and angiogenesis in HUVECs, while accelerating in vivo skeletal muscle regeneration in a cardiotoxin-induced muscle injury model.111 These benefits were at least in part mediated by miRNAs such as miR-494. Chen et al. discovered that exosomes from adipose MSCs could enhance the proliferation and the migration of primary tenocytes, while also improving mechanical strength of repaired tendons by upregulating decorin and biglycan in a rabbit Achilles tendon rupture model.112
On the other hand, some groups have regarded the muscle-tendon-bone unit as a single functional system and used rotator cuff tear as the disease model, which is the most common shoulder condition for which patients seek treatment.113 One group of researchers published two consecutive studies using adipose MSC-derived exosomes. In a rat model of massive rotator cuff tear, exosome therapy could prevent the atrophy, inflammation, and vascularization of muscles.114 In a rabbit model of chronic rotator cuff tear, exosome therapy could prevent fatty infiltration and improve biomechanical properties.115 Another group reported that bone marrow MSC-derived exosomes could increase the breaking load and stiffness of the rotator cuff after reconstruction, induce angiogenesis around the rotator cuff endpoint, and promote growth of the tendon-bone interface.116
Other orthopedic diseases
Osteoporosis is a metabolic bone disease characterized by low bone density and weakening of bone architecture, which increase the risk of fractures. It results from osteoclastic bone resorption undercompensated by osteoblastic bone formation.117 In a cellular study, adipose MSC-derived exosomes could antagonize hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis.118 Further animal studies revealed that umbilical cord MSC-derived exosomes could inhibit bone marrow MSC apoptosis and prevent disuse osteoporosis via miR-1263/Mob1/Hippo pathway,119 and improve tibial density and reverse estrogen-deficient osteoporosis via miR-2110 and miR-328-3p.120
Compared to SCI, damage to peripheral nerve (e.g., sciatic nerve injury) is considerably more common. The subsequent nerve regeneration is controlled by the interplay between neurons and Schwann cells, and further complicated by inflammatory cell infiltration.121 It was shown that adipose MSC-derived exosomes could target neurons by increasing neurite outgrowth in vitro and axonal regeneration and walking behavior in vivo.122 Adipose MSC-derived exosomes could target Schwann cells by promoting the proliferation, migration and secretion of neurotrophic factors in vitro and restore denervation muscle atrophy in vivo.123 LPS-preconditioned MSC-derived exosomes could target inflammatory cells by enhancing M2 macrophage polarization in vitro and accelerate peripheral nerve regeneration in vivo.124
Intervertebral disc (IVD) degeneration is a major cause of lower back pain which is the leading injury in total global years lived with disability. Its molecular processes include extracellular matrix (ECM) degeneration, inflammation, oxidative stress, apoptosis, senescence and reduced autophagy.125 The emerging avenues of exosome therapy attempt to solve some of these issues. Cheng et al. demonstrated that intradiscal injection of bone marrow MSC-derived exosomes could inhibit nucleus pulposus cell (NPC) apoptosis and alleviate IVD degeneration via exosomal miR-21.126 On the other hand, Chen et al. discovered that human ESC-derived exosomes could inhibit NLRP3 inflammasome to alleviate pyroptosis in nucleus pulposus cells by delivering miR-302c.127 In addition to cell death and mitochondrial damage, oxidative stress in NPCs was also found to be inhibited by MSC-derived exosomes.128 Since IVD degeneration and OA share a common molecular disease spectrum125 the positive results of OA treatment using MSC-derived exosomes (Section 3.2) could be used as a reference for IVD degeneration research.
Osteonecrosis, aka., avascular necrosis, of the femoral head (ONFH) is a disabling condition affecting a younger population, which often results in total hip arthroplasty.129 Glucocorticoid (GC)-induced osteonecrosis is one of the most common causes of ONFH, whose pathogenesis is manifested in two aspects: compromised blood supply to the femoral head and dampened osteogenic activity. Liu et al. showed that exosomes from iPSC-derived MSCs could prevent GC-induced ONFH by promoting angiogenesis and osteogenesis via the PI3K/Akt pathway.130 Zuo et al. demonstrated that miR-26a-overexpressing exosomes derived from HSCs could provide similar therapeutic effects.131
Neurosurgery and SC-Exo therapy
Ischemic stroke
Strokes are the second highest cause of death and the third leading cause of disability globally, with ischemic stroke being the most common subtype.132 The key events during the ischemic cascade include neuronal dysfunction, excitotoxicity, neurochemical injury, and neuroinflammation.133 In terms of treatment, a new generation of clinical trials is now underway, which uses cytoprotective drugs, such as immunomodulators, IL-6 receptor antagonists, Rho kinase inhibitors, and free radical scavengers.134 Targeting each event of the above pathophysiology, nearly all subtypes of SC-exo demonstrated potent therapeutic effects on stroke recovery (Table 3).
Some groups have targeted neuroprotection and neurogenesis. Firstly, SC-exo therapy could inhibit neuronal cell death. Luo et al. found that NSC-derived exosomes could inhibit apoptosis while promoting the proliferation of SH-SY5Y cells both under normal and oxygen-glucose deprivation (OGD) conditions.135 This was also tested in a middle cerebral artery occlusion (MCAO) model as a reduced infarction area and neuronal apoptosis via exosomal miR-150-3p. Other in vitro and in vivo studies showed similar anti-apoptotic effects using EPC-derived exosomes.136,137 Zhang et al. discovered that the exosomal anti-apoptotic effect could be improved by preconditioning the parental NSCs with interferon gamma (IFN-γ).138 Secondly, SC-exo therapy could protect cells of the CNS. Kang et al. revealed that exosomes derived from bone marrow MSCs could rescue OGD-induced injury in neural cells by suppressing NLRP3 inflammasome-mediated pyroptosis.139 Exosomes sourced from hypoxic cultures had a more pronounced neuroprotective effect than their counterparts from normal cultures. Similarly, Li et al. discovered that exosomes derived from human iPSC-derived neural progenitor cells exhibited a neuroprotective effect on OGD neurons and neurite outgrowth.140 This protection of neuronal function under ischemic conditions was regulated through the PTEN/Akt pathway. In addition, Sun et al. proved that NSC-derived exosomes could also protect astrocytes, which become supporting reactive astrocytes (RAs) after strokes.141 Thirdly, SC-exo therapy could improve post-stroke neurogenesis. Wei et al. suggested that Zeb2/Axin2 from bone marrow MSC-derived exosomes could improve post-stroke neurogenesis, neural plasticity, and spatial memory and nerve function, likely via the SOX10, Wnt/β-catenin, and endothelin-3/EDNRB pathways.142 Wang et al. illustrated that miR-126-modified EPC-derived exosomes could alleviate acute brain injury and promote functional recovery after stroke by enhancing neurogenesis.143
Some groups have targeted the inhibition of the neuroinflammation. Firstly, unmodified SC-exo therapy exhibited an anti-inflammatory effect through exosomal miRNAs. Dong et al. showed that bone marrow MSC-derived exosomes could induce BV2 microglia deactivation and M2 polarization in vitro, while reducing infarct size and improving neuronal function in vivo via transferring miR-23a-3p.144 Similarly, Zhang et al. unveiled that umbilical cord MSC-derived exosomal miR-146a-5p could attenuate microglia-mediated neuroinflammation after OGD in vitro, while improving behavioral deficits and microglia activation in vivo via the IRAK1/TRAF6 signaling pathway.145 Secondly, the anti-inflammatory effect of SC-exo therapy could be enhanced by modifying the exosomes. Yoon and co-workers established tumor susceptibility gene (TSG)101-overexpressing human NSCs, thereby increasing exosome secretion.146 The engineered exosomes not only attenuated LDH release and proinflammatory factors in vitro, but also reduced infarction volume, inhibited DNA-damage pathway, and upregulated neurotrophic factors in vivo. Furthermore, Tian’s team broke new ground by ingeniously attaching RGD peptide onto an NSC-derived exosome membrane, which targeted the lesion region of the ischemic brain after intravenous administration, thereby suppressing the inflammatory response after cerebral ischemia by inhibiting the MAPK pathway.147 Interestingly, Gao et al. used induced NSCs (iNSCs) reprogrammed from mouse fibroblasts for stroke treatment. They showed that iNSC-derived exosomes, bearing similar therapeutic effects with NSC-derived ones, could not only promote neurogenesis but also inhibit neuroinflammation.148
Finally, some groups have targeted other aspects during stroke recovery, such as neurochemical injury and oxidative stress. Zhu et al. loaded brain-derived neurotrophic factor (BDNF) into exosomes derived from NSCs to construct engineered exosomes.149 In a model of H2O2-induced oxidative stress, exosome therapy significantly enhanced NSC survival. In a rat MCAO model, exosome therapy not only inhibited microglial activation, but also boosted the differentiation of endogenous NSCs into neurons. Collectively, BDNF-based modification of NSC-derived exosomes has improved effects in the treatment of ischemic stroke. On the other hand, miR-210-modified EPC-derived exosomes could protect neurons from hypoxia and reoxygenation (H/R)-induced apoptosis, oxidative stress, and decreased viability, thereby supporting the treatment of ischemic stroke.150,151 The exosomal miR-17-5p from ACE2-enriched EPC-derived exosomes could ameliorate cerebral ischemic injury in aged mice.152 In an intriguing study conducted by Xu and co-workers, combination of NSC-exo and EPC-exo with miR-210 and miR-123 overexpression exerted better therapeutic effects on ischemic stroke by protecting H/R injured neurons through the BDNF-TrkB and Nox2/ROS pathways.153
In contrast to ischemic stroke, hemorrhagic stroke poses a deadlier threat and worse disability in most survivors.154 miR-137 overexpression was found to boost the neuroprotective effects of EPC-derived exosomes against apoptosis, ferroptosis, and mitochondrial dysfunction in oxyhemoglobin-treated SH-SY5Y cells, an in vitro hemorrhagic stroke model, partially through the COX2/PGE2 pathway.155
Traumatic brain injury
Approximately 70 million patients suffer from traumatic brain injury (TBI) globally each year, which poses serious physical, psychosocial and economic threats.156 TBI can be categorized as primary injuries (e.g., axonal death, neuroinflammation, neurochemical change, and metabolic dysfunction) and secondary injuries (e.g., ischemic and hypoxic damage, cerebral edema, raised intracranial pressure, hydrocephalus, and infection).157 Each patient with a TBI has a unique set of circumstances depending on variables such as the location and severity of the injury, making medical and surgical treatment quite challenging.158 Therefore, systemic therapy using SC-exo may become a ‘one-size-fits-all’ option for managing TBI.
A series of animal studies published initially focused on the functional recovery and macroscopic aspects of MSC-derived exosome therapy. In a rat TBI model, exosome-treated animals showed significant improvement in spatial learning and sensorimotor function.159 In addition, exosome treatment significantly increased the number of newborn endothelial cells in the lesion boundary zone, and newborn immature and mature neurons in the dentate gyrus. In another rat TBI model with similar findings, exosomes derived from MSCs cultured in a 3D system provided better outcomes than those in a conventional 2D condition.160 In a monkey model of TBI to the primary motor cortex, exosome-treated animals returned to pre-operative grasp patterns and latency to retrieve a food reward in the first 3–5 weeks of recovery.161 In an even more complicated and clinically realistic large animal model, in which both TBI and hemorrhagic shock were investigated, exosome therapy attenuated the severity of neurologic injury and enabled faster neurologic recovery.162
In comparison, studies completed in recent years shed new light on the molecular mechanisms underlying SC-exo therapy for TBI. Chen et al. reported that adipose MSC-derived exosomes could promote functional recovery, suppress neuroinflammation, reduce neuronal apoptosis, and increase neurogenesis. This was achieved through the uptake of exosomes specifically by microglia and suppression of their activation by inhibiting the NF-κB & MAPK pathways.163 Wen et al. showed that bone marrow MSC-derived exosomes could reduce cell apoptosis in cortical tissue of mouse models of TBI, inhibit neuroinflammation, and promote the transformation of microglia to the anti-inflammatory phenotype. This was realized by the action of miR-181b on the IL-10/STAT3 pathway.164 Abedi et al. proved that NSC-derived exosomes could improve neurobehavioral performance, inhibit astrocyte neuroinflammation, enhance neurogenesis, while maintaining NSC stemness.165 A valuable additional finding was that exosomes seemed to be superior to the parent NSCs in terms of sensorimotor functional recovery. Finally, a dose-response and therapeutic window demonstrated that MSC-derived exosomes could improve angiogenesis and neurogenesis, and sensorimotor and cognitive function, while reducing neuroinflammation and hippocampal neuronal cell loss.166 Although 100 µg and 1 day might be the optimal dose and therapeutic window respectively, exosomes exhibited a wide range of effective doses for treatment of TBI within a therapeutic window of at least 7 days post-injury.
TBI and SCI are two of the most severe CNS traumas, which are increasingly recognized as global health priorities. The emerging evidence presented in Sections 3.3 and 4.2 are mutually beneficial for these two closely related research subspecialties. Henceforth, future research on SC-exo therapy for TBI and SCI could be either mechanism-based (e.g., the role of brain-gut axis167 transcriptional factors168 inflammasome169 and the complement system170) or modification-based (loading exosomes with drugs, e.g., immunomodulators171 antioxidants172 circular RNAs173 and microRNAs174).
Alzheimer’s disease
Unlike TBI and SCI, which are traumatic in nature, Alzheimer’s disease (AD) and Parkinson’s disease (PD) are the most common neurodegenerative diseases (NDD). The hallmarks of NDD include, but are not limited to, pathological protein aggregation, synaptic and neuronal network dysfunction, aberrant proteostasis, cytoskeletal abnormalities, altered energy homeostasis, DNA and RNA defects, inflammation, and neuronal cell death.175 AD is the most common form of dementia globally and accounts for 25 million cases.176 Currently, only two classes of drugs are approved for symptomatic AD treatment, including cholinesterase inhibitors and NMDA antagonists. Although several therapeutics are actively undergoing clinical trials, none of them are near curative for AD.177 The challenges of brain-drug delivery, e.g., the blood-brain barrier (BBB) and pharmacokinetic drawbacks, are very likely to be solved by nanosized exosomes, which are additionally packaged with potent biomolecules. Most SC-exo therapy involves amyloid-β (Aβ), which is positioned at the center of AD pathophysiology.178
The initial work focused on the clearance of aggregation of the pathological protein, Aβ peptide. The intracerebral injection of MSC-derived exosomes by Elia and co-workers reduced Aβ plaque burden and dystrophic neurites in both the cortex and hippocampus in the early stages of a preclinical model of AD.179 In addition, using immunoblotting, the authors confirmed the presence of Neprilysin, a neutral endopeptidase capable of Aβ degradation, in the exosome’s lysates and its mRNA.
Some teams have focused on relieving synaptic dysfunction and oxidative stress. Wang et al. found that MSC-derived exosomes could rescue synaptic impairment and improve cognitive behavior in APP/PS1 mice, while alleviating exogenous Aβ-induced inducible nitric oxide synthase (iNOS) expression.180 Instead of using MSC-derived exosomes, Li et al. administered NSC-derived exosomes and enhanced mitochondrial function, sirtuin 1 activation, synaptic activity, and rescued cognitive deficits.181 Using alternative methods, Huber et al. noticed that heat shock-induced exosomes derived from NSCs exhibited greater neuroprotection against oxidative stress as well as Aβ-induced neurotoxicity.182
Some teams have centered their research around energy homeostasis. Chen et al. found that MSC-derived exosomes could improve brain glucose metabolism and cognitive function in AD transgenic mice using 18F-FDG PET/CT imaging and NOR testing, respectively.183
Some teams have focused on microglial neuroinflammation. In Zavatti’s cellular study, it was found that amniotic fluid MSC-derived exosomes could mitigate neuroinflammatory microglial phenotype and recover neurotoxicity from Aβ using LPS-stimulated BV2 microglia and SH-SY5Y neuroblastoma cells as models, respectively.184 Ding et al. showed that umbilical cord MSC-derived exosomes could alleviate neuroinflammation and reduce Aβ deposition by modulating microglial activation, thereby increasing spatial learning and memory function in AD mice.185
Some teams have focused on neuronal cell death and neurogenesis hoping to counteract AD progression. Reza-Zaldivar and co-workers gave MSC-derived exosomes to AD mice and the SC-exo therapy stimulated neurogenesis in the subventricular zone and alleviated Aβ-induced cognitive impairment.186 These effects are comparable to those shown in the MSCs.
Some teams have focused on the BBB, the dysfunction of which leads to increased permeability, microbleeds, impaired glucose transport, and degeneration of pericytes and endothelial cells.187 Liu et al. indicated that BBB breakdown in 5xFAD (familial Alzheimer’s disease) mice occurred at 4 months of age, and more importantly, treatment with NSCs-derived exosomes reversed AD-caused BBB deficiency.188
Finally, some groups have focused on improving the technicality of SC-exo therapy for AD. When exosomes are injected intravenously, they could be tracked in other organs instead of the targeted regions in the brain. Cui et al. conjugated MSC-derived exosomes with CNS-specific rabies viral glycoprotein (RVG) to target them to the brain of transgenic AD mice.189 The modified exosomes not only exhibited increased delivery to the cortex and hippocampus, but also significantly improved learning and memory capabilities with reduced Aβ deposition. On the other hand, Gao et al. obtained iNSCs through somatic cell reprogramming, which opened a new window for sourcing therapeutic exosomes. They demonstrated that iNSC-derived exosomes, bearing comparable therapeutic effects with NSC-derived ones, could mitigate various AD phenotypes, e.g., cognitive function, Aβ deposition, neuroinflammation, and neuroregeneration, in a preclinical mouse model.190
Parkinson’s disease
Parkinson’s disease is the second most common neurodegenerative disease among the elderly, affecting more than 6 million patients worldwide.191 PD is caused by the necrosis of dopaminergic neurons in the substantia nigra and the presence of protein inclusions named Lewy bodies. The molecular pathophysiology includes α-synuclein proteostasis, mitochondrial dysfunction, oxidative stress, calcium imbalance, and neuroinflammation.192
In a study using bone marrow MSC-derived exosomes, Huang et al. discovered that exosome-derived TNF-stimulated gene-6 (TSG-6) could attenuate 1-methyl-4-phenylpyridinium ion (MPP + , metabolite of a neurotoxin MPTP)-induced neurotoxicity. In this in vitro PD model using SH-SY5Y and SK-N-SH cells, the exosomal anti-PD progression effect was found to be mediated through the STAT3/miR-7/NEDD4 axis.193
In a study using NSC-derived exosomes, Lee et al. revealed that SC-exo therapy could help to prevent the neuropathology and progression of PD.194 Working in vitro on SH-SY5Y and BV2 cells, NSC-derived exosomes could reduce the intracellular reactive oxygen species (ROS) and associated apoptotic pathways. Working in vivo on 6-hydroxydopamine-induced PD mice, NSC-derived exosomes could downregulate pro-inflammatory factors and significantly reduce dopaminergic neuronal loss. The presence of NSC-specific microRNAs, such as miR-182-5p, miR-183-5p, miR-9 and let-7, was confirmed and found to be involved in cell differentiation, neurotrophic function, and immune modulation.
Multiple sclerosis
Multiple sclerosis (MS) is the most common non-traumatic, neurodegenerative, and disabling CNS disease affecting young adults. The pathological hallmark of MS is the formation of demyelinating lesions in the brain and spinal cord, with an inflammatory and autoimmune involvement.195 Currently licensed disease-modifying therapies include interferon-based, immunomodulatory, immunosuppressive, and immune reconstitution drugs.196 A few preliminary studies have highlighted the potential of MSC-derived exosomes for MS treatment.
In an animal experiment using experimental autoimmune encephalomyelitis (EAE) rats, Li et al. showed that SC-exo therapy significantly decreased neural behavioral scores, reduced the infiltration of inflammatory cells into the CNS, and decreased demyelination.197 In addition, exosome treatment upregulated M2-related cytokines while downregulating M1-related ones by regulating the polarization of microglia.
In an animal study using two mouse models of demyelination (the EAE model and the cuprizone diet model), Zhang et al. found that SC-exo therapy could promote remyelination by acting both directly on oligodendrocyte (OL) progenitors and indirectly on microglia.198 MSC-derived exosomes could improve neurological outcomes, increase the numbers of newly generated and mature OLs, decrease Aβ precursor protein density, decrease neuroinflammation by shifting from M1 to M2 phenotype, and inhibit the TLR2/IRAK1/NF-κB pathway.
Other neurosurgical and related diseases
The surgical potential of MSC- and NSC-derived exosome therapy in four major types of neurosurgical or neurological diseases has been thoroughly discussed above. In addition to vascular disruption-, trauma-, neurodegeneration-, and autoimmune-related disorders, other diseases have been proven suitable targets for SC-exo therapy recently (Table 3). These include, but are not limited to: 1, dementia, such as vascular dementia.199 HIV-associated neurocognitive disorders200 and radiation-induced cognitive dysfunction201,202; 2, functional disorders, such as epilepsy203 and mechanical allodynia204; 3, congenital abnormalities, such as spina bifida aperta205; 4, neuropsychological conditions, such as depression206 and stress207; 5, brain aging208,209,210; 6, iatrogenic brain problems, such as deep hypothermic circulatory arrest.211
Plastic surgery and SC-Exo therapy
Wound healing occurs in all parts of the human body, with cutaneous wounds being the most common. The highest wound-related expenses were attributed to surgical wounds followed by diabetic ulcers.212 The overall but overlapping phases of wound healing include hemostasis, inflammation, angiogenesis, proliferation and remodeling, each of which is governed by distinct cell types and modulated by various signaling pathways.213 More than half of relevant work using SC-exo therapy to boost cutaneous wound healing is MSC-based (Table 4).
In the inflammatory stage, exosomes could inhibit the proliferation of peripheral blood mononuclear cells and promote the transformation of regulatory T cells in vitro, and reduce the number of lymphocytic infiltrations in the skin.214 In addition, exosomes could reduce IgE, eosinophil and mast cell count, and downregulate inflammatory cytokines.215 In the angiogenic stage, educated exosomes (e.g., atorvastatin and deferoxamine) could promote angiogenesis in diabetic wounds via the Akt/eNOS and PTEN/PI3K/Akt pathways.216,217,218 EPC-derived exosomes could accelerate cutaneous wound healing by promoting angiogenesis219 through the Erk1/2 pathway220 and p53 pathway.221 In the proliferative stage, stem cell-derived exosomes could promote the proliferation and migration of fibroblasts and keratinocytes. Some were achieved through the PI3K/Akt222 Akt/HIF-1α223 ERK1/2224 and Wnt/β-catenin225 pathways, while others through inhibition of LATS2226 PPARγ227 and AIF nucleus translocation228 In the final remodeling stage of wound healing, granulation tissue is replaced by permanent scar, during which abnormal wound healing might occur (e.g., keloids and hypertrophic scars). MSC-derived exosomes could suppress fibroblast-myofibroblast transition via the TGF-β/Smad2 pathway229,230 and increase collagen synthesis in early stage and reduce in late stage231 thereby reducing scar formation.
Furthermore, ESC-derived exosomes were found to exert similar therapeutic effect for wound healing to MSC-derived ones. Chen et al. used human ESC-derived exosomes to help healing of pressure ulcer.232 They noticed that exosomes could ameliorate endothelial senescence by activating Nrf2 and recover aging-related angiogenic dysfunction, thereby accelerating wound healing.232 In addition, Bae et al. revealed that the exosomal mmu-miR-291a-3p from ESCs could inhibit cellular senescence in human dermal fibroblasts through the TGF-β receptor 2 pathway, thereby accelerating the excisional skin wound healing process.233
In addition to wound healing, other plastic surgery-related diseases have been proven to be suitable targets for SC-exo therapy (Table 4). These include, but are not limited to: 1, skin grafting, such as skin flaps234; 2, tissue loss, such as craniofacial defect235; 3, autoimmune skin diseases, such as scleroderma236; 4, skin infections, such as leishmaniasis237; 5, hair transplantation, such as for alopecia238; 6, skin aging239
General surgery and SC-Exo therapy
As a major subspecialty of general surgery, hepatobiliary surgery has attracted tremendous attention to SC-exo therapy. Firstly, acute liver injury (ALI)/acute liver failure (ALF) is a rare but challenging syndrome manifested by hepatic dysfunction, coagulopathy, encephalopathy, and multiorgan failure. About 60% of cases with ALF require and undergo orthotopic liver transplantation or result in death.240 In one study, Lin’s team focused on the cell death aspect of ALI, and found that MSC-derived exosomes could protect against ferroptosis via stabilization of SLC7A11 in carbon tetrachloride-induced ALI.241 Alternatively, Shao’s team focused on the pre-isolation modification of the exosomes, and revealed that exosomes derived from umbilical cord MSCs could ameliorate IL-6-induced ALI through exosomal miR-455-3p.242 Secondly, in contrast to ALI, liver fibrosis occurs when the liver sustains a chronic injury, which may progress into cirrhosis, liver failure, hepatocellular carcinoma, and even death.243 Ma et al. discovered that MSC-originated exosomal circDIDO1 could suppress hepatic stellate cell activation by miR-141-3p/PTEN/Akt pathway in human liver fibrosis.244 In addition, Wang et al. found that exosomes derived from 3D human ESC spheroids could attenuate hepatic stellate cell activation and inhibit liver fibrosis through inactivation of the Smad pathway by exosomal miR-6766-3p.245 For those with end-stage liver fibrosis needing a liver transplant, liver ischemia reperfusion injury (IRI) is a serious complication for graft dysfunction and organ rejection.246 Yang et al. demonstrated that bone marrow MSC-derived exosomes could relieve hepatic IRI, reduce hepatocyte apoptosis, and decrease liver enzyme levels by enhancing autophagy.247 Du et al. showed that exosomes from iPSC-derived MSCs could protect liver against hepatic IRI via activating sphingosine kinase and the sphingosine-1-phosphate pathway.248 Thirdly, non-alcoholic fatty liver disease (NAFLD) is known to adversely affect stroke recovery. Using a type 2 diabetes mellitus mouse model, Venkat et al. demonstrated that HSC-derived exosomes could simultaneously reduce liver dysfunction and improve neurological and cognitive function.249 Lastly, acute pancreatitis is an unpredictable and potentially lethal disease, the prognosis of which mainly depends on whether it develops into multiple organ dysfunction syndrome.250 Chen et al. revealed that exosomes from iPSC-derived MSCs could improve myocardial injury caused by severe acute pancreatitis through the Akt/Nrf2/HO-1 pathway.251
Peripheral artery disease affects 200 million patients worldwide and, in its most severe stage, can cause critical limb ischemia, subjecting patients to increased risk of cardiovascular events, amputation and death.252 As a cell-free therapy, placenta MSC-derived exosome infusion could enhance angiogenesis in a murine auricle ischemic injury model using laser Doppler blood flow analysis.253 Mechanistically, MSC-derived exosomes not only promote tube-like structure formation in vitro, but also mobilize endothelial cells into subcutaneous Matrigel plug in vivo, mainly through exosomal pro-angiogenic microRNAs, such as miR-30b.254 In addition, HSC-derived exosomes could repair ischemic hindlimb in mice by improving limb perfusion, capillary density, motor function and their amputation.255 This was most likely caused by internalization of exosomal miR-126-3p by endothelial cells relative to smooth muscle cells and fibroblasts. Human iPSC-derived exosomes demonstrated similar neoangiogenic effect through the exosomal miR-199b-5p.256 On the other hand, endovascular re-canalization is increasingly being used to reestablish blood flow to ischemic areas and restore tissue loss or gangrene for patients with peripheral artery disease.257 Three independent teams all proved that EPC-derived exosomes could promote vascular repair and accelerate reendothelialization in rat models of balloon-induced vascular injury by enhancing endothelial cell function.258,259,260 In addition, Kong et al. demonstrated similar protective effect of EPC-derived exosomes against balloon injury by inhibiting neo-intimal hyperplasia.261 This was achieved through promotion of reendothelialization and suppression of restenosis rather than through the direct inhibition of proliferation and migration of smooth muscle cells.
McCulloh and his co-workers published an intriguing study on the SC-exo therapy for necrotizing enterocolitis (NEC) which has an overall mortality of over 30% for premature infants requiring surgery.262 The authors compared the therapeutic effect of exosomes derived from four different types of stem cells, i.e., amniotic fluid MSCs, bone marrow MSCs, amniotic fluid NSCs and neonatal enteric NSCs.263 When injected at a concentration of at least 4 × 108, all types of SC-exo were shown to reduce the incidence and severity of experimental NEC as effectively as their parental stem cells.
Sepsis is a deadly and potentially preventable complication in general surgery, in which microvascular dysfunction leads to multi-organ failure and mortality.264 Using a murine sepsis model by cecal ligation and puncture (CLP), Zhou and co-workers demonstrated that EPC-derived exosomes could improve sepsis outcome.265 This was manifested by reduced lung and renal vascular leakage, improved organ function, and increased survival through the exosomal miR-126-5p and miR-126-3p. Similarly, Liu et al. exhibited protective effect of EPC-derived exosomes on sepsis-induced organ damage and immune suppression by the exosomal miR-382-3p through the IκBα/NF-κB pathway.266
Cardiothoracic surgery and SC-Exo therapy
The world’s leading mortality is ischemic heart disease (IHD) which is primarily caused by obstructive coronary atherosclerosis. The rupture of an atherosclerotic plaque is the most common trigger of acute arterial thrombosis causing myocardial infarction (MI). Prolonged oxygen deprivation to the myocardium can lead to cardiomyocyte death. Although timely reperfusion is essential, myocardial IRI might occur, thus mitigating the beneficial effects of reperfusion. Despite modern coronary reperfusion, the mortality and morbidity associated with the development of heart failure as a consequence of acute MI remain substantial, highlighting the importance of next-generation cardioprotective therapies, such as SC-exo.267
The work conducted by Xing et al. focused on atherosclerosis. The adipose MSC-derived exosomal miR-342-5p was shown to protect endothelial cells against atherosclerosis by targeting PPP1R12B in a H2O2-challenged HUVEC model.268 The work conducted by Peng et al. and Gao et al. focused on MI. The exosomal miR-25-3p from MSCs could alleviate MI by targeting pro-apoptotic proteins and EZH2 in an OGD cardiomyocytes model and left anterior descending artery ligation animal model.269 Similarly, human iPSC-derived exosomes could improve recovery from MI without increasing the frequency of arrhythmogenic complications in a swine model.270 The work conducted by Wen et al. and Santoso et al. focused on the death of cardiomyocytes. MSC-derived exosomes could ameliorate cardiomyocyte apoptosis in hypoxic conditions through miR-144 by targeting the PTEN/Akt pathway271 whereas iPSC-derived exosomes could regulate autophagy in hypoxic cardiomyocytes.272 Both Katsur et al. and Chen et al. focused on myocardial IRI. The former team found that exosomes derived from non-cardiomyocyte-related cells, i.e., CTX0E03 NSCs, could reduce infarct size while delaying cardiomyocyte mitochondrial permeability transition pore opening through the JAK/STAT pathway.273 The latter team discovered that MSC-derived exosomal miR-143-3p could suppress myocardial IRI by regulating autophagy via the CHK2-Beclin2 pathway.274 Finally, Chen and co-workers proved that bone marrow MSC-derived exosomes could attenuate cardiac hypertrophy and fibrosis in pressure overload-induced remodeling, thereby providing a promising potential treatment for heart failure.275
In comparison to MSC-derived exosomes, exosomes derived from ESCs exhibited comparable therapeutic effect for cardiac conditions. In terms of protection of cardiomyocytes, Khan et al. discovered that the ESC-derived exosomal miR-294 could improve cardiomyocyte survival, promote neovascularization and inhibit fibrosis after MI, thereby augmenting post-MI cardiac function.276 In addition, Tavakoli Dargagni et al. demonstrated that ESC-derived exosomes could alleviate doxorubicin-induced cardiotoxicity by inhibiting TLR4-NLRP3-mediated pyroptotic cell death in cardiomyocytes.277 Similarly, Singla’s team showed that ESC-derived exosomes could improve cardiac remodeling by enhancing anti-inflammatory M2 macrophages and reducing inflammation-induced pyroptosis.278 In terms of management of heart failure, Pang et al. exhibited that ESC-derived exosomes could attenuate heart failure, improve cardiac function and promote myocardial angiogenesis through the FGF2 signaling in a transverse aortic constriction-induced heart failure model.279 Using a coronary artery occlusion-induced heart failure model, Kervadec et al. showed that exosomes secreted by ESC-derived cardiovascular progenitors could recover cardiac functions such as reduced left ventricular end-systolic and end-diastolic volumes.280 Finally, the same research group later demonstrated that exosomes derived from more readily available cell sources, e.g., iPSCs, were capable of cardioprotective effects similar to those offered by ESC-derived ones.281
Other important subtypes of SC-Exo, such as iPSC-exo, HSC-exo and EPC-exo, have also exhibited cardio-protective effects. For example, exosomes secreted by iPSCs could exert cytoprotective effects on maintaining intracellular Ca2+ homeostasis and promoting cardiomyocyte survival, thereby improving recovery from MI.282 HSC-exo could reduce the cardiac injury-related indices and the degree of cardiac fibrosis while elevating the ejection fraction in an animal model of heart failure.283 In addition, systemic infusion of HSC-derived exosomes could improve ischemic cardiomyopathy in a rat model of acute MI, with additional benefits in treating the side effects such as kidney damage.284 Modification of HSCs using sonic hedgehog (Shh), an angiogenic factor, could preserve cardiac function after acute MI by delivery of exosomal Shh to ischemic myocardium.285 Ke et al. proved that EPC-derived exosomes could enhance the proliferation and angiogenesis of cardiac fibroblasts by activating mesenchymal-endothelial transition and decreasing the expression of HMGB1286 and later revealed that the exosomal miR-218-5p and miR-363-3p from EPC-derived exosomes could ameliorate MI by targeting the p53/JMY pathway.287 In an interesting study by Yue et al., IL-10 deficiency-induced systemic inflammation was found to compromise the reparative properties of EPC-derived exosomes on myocardial repair by upregulating integrin-linked kinase (ILK) enrichment in exosomes, and ILK-mediated activation of NF-κB pathway in recipient cells.288
In terms of treatment of thoracic disorders, Liu et al. proved that human ESCs-derived exosomes could alleviate inflammation, prevent excessive collagen deposition and preserve alveolar architecture in the lungs of mice with bleomycin-induced pulmonary fibrosis.289 This was achieved by the exosomal mi-17-5p targeting thrombospondin-2. Similarly, Zhou et al. demonstrated that the exosomal miR-302a-3p from iPSC-derived exosomes could suppress M2 macrophages via TET1, thereby mitigating pulmonary fibrosis.290 Liu et al. showed that EPC-derived exosomes could inhibit pulmonary artery smooth muscle cells proliferation and their resistance to apoptosis by regulating the Mitofusin-2 and Ras-Raf-ERK1/2 pathways, thereby acting as a potential therapeutic candidate for the treatment of pulmonary arterial hypertension.291 Two independent teams both revealed that human EPC-derived exosomes could improve outcomes of the LPS-induced acute lung injury partially through the delivery of miR-126 into the injured alveolus.292,293 Zhang et al. found that EPC-derived exosomes could improve the bioactivity of pulmonary microvascular endothelial cells and protect them from hyperoxic injury in the developing lung vasculature, thereby contributing to the treatment of bronchopulmonary dysplasia.294 Moreover, Montay-Gruel et al. demonstrated that human ESC-derived exosomes could improve the adverse late normal tissue complications associated with exposure of the lungs to ionizing radiation, such as those encountered during postoperative treatment of lung cancer.295
Urology and SC-Exo therapy
Chronic kidney disease (CKD) is a syndrome characterized by persistent changes in kidney structure, function, or both, affecting 10−14% of the global population.296 The most common pathological feature and final manifestation of CKD is some form of renal fibrosis. Kidney fibrosis occurs when wound healing is deregulated, which leads to excessive accumulation of ECM proteins, such as collagen and fibronectin. In their study, Liu et al. discovered that bone marrow MSC-derived exosomes could alleviate vascular calcification, a detrimental indicator of morbidity and mortality in CKD.297 This was achieved through exosomal miR-381-3p by targeting NFAT5, which was further verified in severe arterial calcification in dialysis patients. In alternative studies, several groups demonstrated the capacity of bone marrow MSC-derived exosomes in treating renal fibrosis, each with a distinct mechanistic interpretation. In a cellular study, Yin et al. found that exosomes could prevent TGF-β1-induced epithelial-mesenchymal transition of renal tubular epithelial cells by transporting Nedd4L, which activates autophagy. In a 5/6 subtotal nephrotomy rat model, Liu et al. revealed that exosomes could improve renal function and reduce fibrotic size by regulating the Smurf2/Smad7 axis.298 In a unilateral ureteral occlusion-induced interstitial fibrosis mouse model, Lu et al. demonstrated that exosomes could improve renal fibrosis by reducing the polarization of M1 and M2 macrophages by activating EP2 receptors.299
Acute kidney injury (AKI) and CKD are closely connected, with each a risk factor for developing the other. Renal IRI is a leading cause of AKI and acute kidney failure.300 Lim et al. proved that exosomes from iPSC-derived MSCs could correct serum creatinine level, tubular necrosis, apoptosis, inflammatory cytokine production, and oxidative stress in AKI mice by activating the ERK1/2 signaling pathway.301 In a similar work by Zhang et al., the exosomal miR-21-5p from EPCs were found to alleviate sepsis-induced AKI by inhibiting RUNX1 expression in CLP rats.302
Otorhinolaryngology and head & neck surgery and SC-Exo therapy
Hearing loss is the most common sensory deficit worldwide, affecting nearly 20% of the global population.303 The causes of sensorineural hearing loss (SNHL) can be very diverse, such as presbycusis, ototoxic medication-induced, noise-induced, and idiopathic sudden SNHL. Tsai’s team demonstrated that umbilical cord MSC-derived exosomes could rescue the loss of outer hair cells and repair cochlear damage in cisplatin-induced hearing loss.304 The underlying mechanism for the cochlea-protective effects is mediated by the miRNAs (e.g., miR-125a-5p and miR-125b-5p) and remodeling factors (e.g., fibronectin and galectin-3).
Cochlear IRI is one of the main reasons for idiopathic sudden and noise-induced SNHL, which can lead to irreversible damage of sensory hair cells and bipolar cochlear spiral ganglion.305 Its pathophysiology includes oxidative stress, excess cell death and dysregulated inflammation. Hao et al. discovered that exosomes derived from miR-21-overexpressing NSCs could prevent hearing loss from IRI by inhibiting the inflammatory process in the mouse cochlea.306 This was evidenced by a reduced auditory brainstem response threshold, upregulated IL-10 and downregulated TNF-α and IL-1β.
Hypothyroidism is a very common disease which could result from thyroidectomy or radioactive ablation to treat hyperthyroidism or thyroid cancer.307 Stem cell therapy and stem cell-derived exosome therapy have emerged as a promising management for hypothyroidism through thyroid regeneration. Using an in vitro culture system of thyroid lobes, Degosserie et al. suggested that EPC-derived exosomes could facilitate thyrocyte organization into thyroid follicles and lumen expansion (i.e., folliculogenesis), which was promoted by laminin-α1.308
Temporomandibular joint (TMJ) disorders are the second most common musculoskeletal condition affecting 31% of adults and 11% of children.309 Like other synovial joints in the body, TMJ is also prone to OA, sharing common pathophysiological processes (Section 3.2). Zhang and co-workers proved that MSC-derived exosomes could alleviate TMJ OA in an immunocompetent rabbit model by attenuating inflammation and restoring matrix homeostasis.310 The exosome-mediated joint repair was attributed to adenosine activation of Akt, ERK and AMPK signaling, as well as enhanced s-GAG synthesis.
Ophthalmology and SC-Exo therapy
Acquired optic neuropathy is a major cause of blindness in adults, and has various etiologies, such as vascular, inflammatory, traumatic, toxic, compressive, and nutritional etiologies. Retinal ganglion cell (RGC) loss is the hallmark of optic neuropathies, via multiple cell death pathways.311 Mead and Tomarev found that bone marrow MSC-derived exosomes could promote survival of RGCs, and regeneration of their axons, in a rat optic nerve crush model.312 The exosomal neuroprotective and neuritogenic effects were accomplished through exosomal miRNAs, demonstrating a cell-free potential for traumatic and degenerative ocular diseases.
Retinal degenerative diseases (e.g., age-related macular degeneration and retinitis pigmentosa) are the leading cause of bilateral irreversible vision loss worldwide.313 It is characterized by progressive degeneration of photoreceptors, RGCs or retinal pigment epithelium (RPE) cells. Bian’s team discovered that exosomes derived from NSCs could preserve photoreceptors, visual function and prevent thinning of the outer nuclear layer in an RCS retinal degeneration rat model.314 This was achieved by marked inactivation of microglial inflammation via exosomal targeting of TNF-α, IL-1β and COX-2. In two consecutive studies, Gao’s team showed that ESC-derived exosomes could alleviate retinal degeneration by enhancing the proliferation and retrodifferentiation of retinal Müller cells as replacement retinal neuronal precursors. On one hand, this was accomplished by regulating the expression of Oct4 in Müller cells through exosomal HSP90.315 On the other hand, activation of the Wnt signaling pathway by delivering BDNF protein to Müller cells also played an important role.316 Furthermore, using a rat model of inherited retinal degeneration, Park et al. demonstrated that both subretinal and intravitreal injection of human HSC-derived exosomes could provide functional rescue of a degenerating retina.317
Failed healing of corneal defect often leads to corneal blindness which has been reported as second only to cataract in the leading causes of blindness.318 The most severe and recalcitrant cases would need corneal transplantation. Wang et al. compared exosomes derived from iPSCs and MSCs as therapeutic providers for the treatment of corneal epithelial defects.319 It was found that both types of exosomes could promote proliferation, cell cycle progression and migration while inhibiting apoptosis in vitro, and accelerate corneal epithelium defect healing in vivo. More importantly, the iPSC-derived exosomes had a stronger therapeutic effect than the MSC-derived exosomes.
Corneal transplantation is one of the most successful forms of solid organ transplantation. However, graft rejection can occur in up to 90% of high-risk recipients.320 Both innate and adaptive immunity are the predominant reason for graft failure. Immunosuppressive drugs have shown only partial effectiveness. Jia et al. showed that MSC-derived exosomes could cross biological barriers and prolong graft survival time in a rat model of corneal allograft rejection.321 This was likely caused by inhibition of the infiltration of CD4+ and CD25+ T cells and the reduction of IFN-γ and CXCL11 via the Th1 signaling pathway.
Obstetrics and gynecology and SC-Exo therapy
Primary ovarian insufficiency (POI), or premature ovarian failure (POF), is defined as loss of ovarian function before the age of 40. Non-genetic causes of POI include autoimmune disorders, metabolic conditions, infections, and iatrogenic procedures (e.g., chemotherapy, radiotherapy, and surgery). Women with POI suffer from various complications, such as osteoporosis, infertility, cardiovascular disorders and depression.322 Although promptly initiating hormone replacement therapy is critical to control these symptoms and complications, it fails to restore ovarian function. Currently-tested experimental therapies include mitochondrial activation, in vitro activation, stem cell therapy, and exosome therapy.323
The work conducted by Li et al. showed that umbilical cord MSC-derived exosomes could improve ovarian function in a cyclophosphamide (CTX)-induced POI mouse model.324 The SC-exo therapy not only restored ovarian function-related hormone levels and the number of ovarian follicles, but also improved the reproductive ability of POI mice. In addition, the exosomes promoted the proliferation of ovarian granulosa cells (GCs) by regulating the Hippo pathway, and the effect was neutralized by a YAP inhibitor. Similar results were obtained using exosomes from iPSC-derived MSCs.325 The work performed by Ding et al. illustrated that umbilical cord MSC-derived exosomes could restore ovarian phenotype and function in a POI mouse model, promote proliferation of CTX-damaged human GCs and oocytes, and alleviate ROS accumulation by delivering exosomal miR-17-5p and targeting its downstream mRNA SIRT7.326 It was further elucidated that miR-17-5p down-regulated PARP1, γH2AX, and XRCC6 expression by inhibiting SIRT7. Lastly, the work completed by Yang et al. revealed that bone marrow MSC-derived exosomes could recover the estrus cycle, increase the number of basal and sinus follicles, increase estradiol E2 and anti-Mullerian hormone levels, and reduce follicle stimulating hormone and luteinizing hormone levels in a chemotherapy-induced POF rat model.327 Mechanistically, this was achieved by exosomal miR-114-5p that targets PTEN.
From preclinical studies to clinical trials of exosome therapy
Many preclinical studies, as discussed in Sections 3 to 11, have confirmed the advantages of MSC-derived and NSC-derived exosomes to treat many diseases spanning the subspecialties of surgical practice. Without restricting the scope to stem cell-derived exosomes only, many clinical trials have demonstrated the role of exosomes to be twofold: biological markers and therapeutic agents. A search on ClinicalTrials.gov using ‘exosome therapy’, ‘exosome treatment’, and ‘exosome’ as keywords generated 188 records. However, only 60 (32%) of these directly relate to interventional studies using exosomes as therapeutic agents (Table 5). The rest, especially oncology-related trials, mostly used exosomes as biomarkers, such as key players during disease pathogenesis (e.g., NCT04288141, NCT04154332), diagnostic markers and guidance before treatment (e.g., NCT04629079, NCT03791073, NCT05451342, NCT03432806), monitoring indices and predictive tools for treatment efficiency (e.g., NCT05427227, NCT04499794, NCT04852653, NCT05370105, NCT03800121, NCT05370105, NCT05328089), and prognostic indicators after treatment (e.g., NCT06026735, NCT05705583, NCT05411445, NCT04167722, NCT05575622). The clinical applications of exosomes as biomarkers have been extensively explored in other reviews328,329,330,331,332 and are therefore beyond discussion in this review. In terms of the clinical characteristics of the 60 clinical trials on exosome therapy (Table 5), there are several highlights worth mentioning.
Firstly, the spectrum of diseases covered is very broad, as both surgical and medical conditions are included. These include many surgical disorders discussed in Sections 3 to 11, such as orthopedic diseases (osteoarthritis of the knee in NCT05060107, bone loss in NCT04998058, and intervertebral disc degeneration in NCT04849429), neurosurgical diseases (ischemic stroke in NCT03384433 and Alzheimer’s disease in NCT04388982), plastic surgical diseases (cutaneous wound healing in NCT02565264 and NCT05475418), general surgical diseases (liver cirrhosis in NCT05871463), cardiothoracic diseases (myocardial infarction in NCT05669144), and ophthalmology diseases (retinitis pigmentosa in NCT05413148). In other words, some preclinical studies have not yet developed into clinical trials. These include, but are not limited to, exosome therapy for fracture333 spinal cord injury334 traumatic brain injury335 acute liver injury336 and hearing loss304,306 which might serve as future directions for exosome therapy-related clinical trials.
Secondly, an increasing number of clinical trials focused on two medical conditions, i.e., COVID-19 (16 trials, 27%) and cancer (5 trials, 8%). MSC-derived exosomes can manage viral infection and lung damage in COVID-19 through both reparative actions and regenerative effects.337 The former manifests as blockage of viral entry and replication, and suppression of the cytokine storm, whereas the latter as prevention of inflammation, and fluid-clearance and restoration of lung permeability. In addition, exosomes can be engineered into a drug (e.g., CD24, a potent immune regulator) delivery platform338 and even a vaccine339 to combat COVID-19. In contrast to the MSC-derived exosomes for COVID-19 management, exosomes used for cancer treatment mainly rely on non-stem cells and cargo engineering. This is partially because MSC-derived exosomes demonstrate controversial effects on tumorigenesis and metastasis.340,341 Although the exact interaction between MSC-derived exosomes and tumor cells remains open to debate, scientists have overcome several obstacles by modifying exosomal cargos to deliver antioncogenic nucleic acids and anticancer medications, and exosomal membranes for specific tumor targeting.342,343,344,345,346
Finally, among the 40 clinical trials using stem cell-derived exosomes for disease treatment, 38 (95%) used MSC and 2 (5%) used iPSC as the cellular source for exosomes. However, this differs significantly from the preclinical studies discussed in Sections 3 to 11. The lack of use of NSC-derived exosomes in clinical trials might be partially because of the supply constraints of their parental cells.347 Currently, NSCs can be obtained from three sources348 (Fig. 1a): 1, isolation from primary CNS tissues (e.g., adult and fetal brain); 2, differentiation from pluripotent stem cells (e.g., iPSCs and ESCs); 3, reprogramming of somatic cells (e.g., fibroblasts and blood cells) to iNSCs.349 Recent studies have developed fibroblast-derived iNSCs, opening a new window for obtaining exosomes from NSC-like cells. These iNSC-exo could not only promote cell survival and proliferation no less than NSC-exo in vitro350,351 but also enhance recovery after ischemic stroke148 and mitigate AD-like phenotypes in preclinical models.190 Therefore, iNSCs might be an excellent cellular source to produce clinical-grade exosomes in clinical trials.
In summary, the progression from preclinical studies to clinical trials of exosome therapy has been expeditious. However, issues like insufficient clinical indications for exosome treatment and limited sources for parental stem cells remain to be addressed. In addition, once the limitations (Table 1) in upscaling of manufacturing, compliance with good manufacturing practice, and regulatory framework are overcome329 stem cell-derived exosome therapy will soon be incorporated into clinical practice and serve at the patient’s bedside.
Conclusion and future perspectives
Exosomes have been pursued recently as a cell-free alternative to stem cell-based therapy. ESC-, iPSC-, HSC-, MSC-, NSC- and EPC-derived exosomes are of particular interest, partially due to the pluripotency or multipotency of their parental cells. After going through production and purification with or without modification, stem cell-derived exosomes have demonstrated tremendous potential in treating numerous diseases encountered during surgical practice. These are exemplified by disorders in orthopedic surgery (e.g., fracture, osteoarthritis, and spinal cord injury); neurosurgery (e.g., ischemic stroke, traumatic brain injury, and Alzheimer’s disease); plastic surgery (e.g., wound healing); general surgery (e.g., acute liver injury); cardiothoracic surgery (e.g., myocardial infarction); urology (e.g., chronic kidney disease); head and neck surgery (e.g., sensorineural hearing loss); ophthalmology (e.g., acquired optic neuropathies), and gynecology (e.g., primary ovarian insufficiency). Mechanistically, the diverse therapeutic effects of stem cell-derived exosomes are achieved through disease-specific cellular and tissue responses (e.g., tissue regeneration, anti-inflammation, anti-cell death, immunomodulation, and anti-oxidative stress) and tissue-specific molecular signaling pathways (e.g., Wnt/β-catenin, PTEN/PI3K/Akt/HIF-1α, MAPK, and JAK/STAT pathways). Collectively, stem cell-derived exosome therapy has been proven to be a potent and versatile surrogate to stem cell therapy in the surgical arena.
Future emphasis of clinical applications of stem cell-derived exosomes should be placed on various nodes of this therapeutic pipeline. Firstly, targeting the pretherapeutic large-scale production of exosomes, a high-throughput cellular source as well as a reproducible and scalable production and isolation protocol are required. Compared to the static system growing monolayer cells, dynamic system in the form of bioreactor, e.g., hollow-fiber bioreactor and stirred tank bioreactor, can increase the efficiency by producing copious cells and exosomes in a short period of time. However, the phenotype of parental cells and derived exosomes might change due to physical and shear stress encountered in a reactor. Thus, the working parameters of the bioreactor must be optimized to facilitate large-scale production of stem cell-derived exosomes. Secondly, targeting the therapeutic modality of exosomes, delivery methods other than systemic administration need to be explored. When delivered through the venous system, exosomes are rapidly cleared from blood circulation and accumulate in the liver, spleen and lungs, which can be overcome by local delivery. Various biomaterials have been recently used to protect, assist and augment locally delivered exosomes to maximize their therapeutic effects. These biomaterials could be designed according to their sources (e.g., natural, synthetic, and hybrid polymers), format (e.g., scaffold, patch, spray, and microneedle), and responsiveness (e.g., temperature, pH, and protein), thereby allowing disease-specific customization. Lastly, targeting the therapeutic indications of exosome therapy, more diseases than the ones discussed in this review should be included into future preclinical studies and clinical trials. For example, airway inflammatory conditions (e.g., allergic rhinitis and asthma) could be suitable candidates for exosome therapy, considering the immunomodulatory effect of MSC-derived exosomes. Disorders that are best treated using surgical implants (e.g., cochlear implant, intraocular lenses, and contraceptive intrauterine devices) could be managed in the form of implant-based local release of exosomes. In addition to primary diseases, secondary conditions including surgical operation- and general anesthesia-related complications (e.g., cognitive impairment, wound paresthesia, and malignant hyperthermia) might become therapeutic targets of exosome therapy. Collectively, efforts to upscale exosome production in conjunction with multimodal exosome delivery will accelerate the clinical applications of stem cell-derived exosomes in a rapidly expanding disease spectrum.
References
Kimbrel, E. A. & Lanza, R. Next-generation stem cells - ushering in a new era of cell-based therapies. Nat. Rev. Drug. Discov. 19, 463–479 (2020).
Puri, M. C. & Nagy, A. Concise review: embryonic stem cells versus induced pluripotent stem cells: the game is on. Stem Cells. 30, 10–14 (2012).
Ng, A. P. & Alexander, W. S. Haematopoietic stem cells: past, present and future. Cell Death Discov. 3, 17002 (2017).
Naji, A. et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol. Life Sci. 76, 3323–3348 (2019).
Tang, Y., Yu, P. & Cheng, L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 8, e3108 (2017).
Chambers, S. E. J. et al. Current concepts on endothelial stem cells definition, location, and markers. Stem Cells Transl. Med. 10, S54–S61 (2021).
Hoang, D. M. et al. Stem cell-based therapy for human diseases. Signal Transduct. Target Ther. 7, 272 (2022).
Zakrzewski, W., Dobrzynski, M., Szymonowicz, M. & Rybak, Z. Stem cells: past, present, and future. Stem Cell Res. Ther. 10, 68 (2019).
Zhang, K. & Cheng, K. Stem cell-derived exosome versus stem cell therapy. Nat. Rev. Bioeng. 1, 608–609 (2023).
Zhang, Y. et al. Exosome: A Review Of Its Classification, Isolation Techniques, Storage, Diagnostic And Targeted Therapy Applications. Int. J. Nanomed. 15, 6917–6934 (2020).
Vizoso, F. J. et al. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 18, 1852–1875 (2017).
Ren, K. Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology. 107, 271–284 (2019).
Hastuti, S. et al. hUMSC vs. hUMSC-Exosome: which one is better for epilepsy? Pharmaceuticals. 15, 1247–1261 (2022).
Carr, N. J. The pathology of healing and repair. Surgery 40, 13–19 (2022).
Peshkova, M. et al. Targeting inflammation and regeneration: scaffolds, extracellular vesicles, and nanotechnologies as cell-free dual-target therapeutic strategies. Int. J. Mol. Sci. 23, 13796–13813 (2022).
Wang, H., Huber, C. C. & Li, X. P. Mesenchymal and neural stem cell-derived exosomes in treating alzheimeras disease. Bioengineering 10, 253–266 (2023).
Li, X. et al. Neural stem/progenitor cell-derived extracellular vesicles: a novel therapy for neurological diseases and beyond. MedComm 4, e214 (2023).
Norouzi-Barough, L., Shirian, S., Gorji, A. & Sadeghi, M. Therapeutic potential of mesenchymal stem cell-derived exosomes as a cell-free therapy approach for the treatment of skin, bone, and cartilage defects. Connect Tissue Res. 63, 83–96 (2022).
Doyle, L. M. & Wang, M. Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 8, 727–750 (2019).
Pegtel, D. M. & Gould, S. J. Exosomes. Annu. Rev. Biochem. 88, 487–514 (2019).
Gurung, S., Perocheau, D., Touramanidou, L. & Baruteau, J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 19, 47 (2021).
Zhang, Y., Liu, Y., Liu, H. & Tang, W. H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 9, 19 (2019).
Gholami Farashah, M. S. et al. Bone marrow mesenchymal stem cell’s exosomes as key nanoparticles in osteogenesis and bone regeneration: specific capacity based on cell type. Mol. Biol. Rep. 49, 12203–12218 (2022).
Qing, L., Chen, H., Tang, J. & Jia, X. Exosomes and their MicroRNA cargo: new players in peripheral nerve regeneration. Neurorehabilit. Neural Repair. 32, 765–776 (2018).
Kwok, Z. H., Wang, C. & Jin, Y. Extracellular vesicle transportation and uptake by recipient cells: a critical process to regulate human diseases. Processes 9, 273–294 (2021).
Kimiz-Gebologlu, I. & Oncel, S. S. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control Release 347, 533–543 (2022).
Hamzah, R. N., Alghazali, K. M., Biris, A. S. & Griffin, R. J. Exosome traceability and cell source dependence on composition and cell-cell cross talk. Int. J. Mol. Sci. 22, 5346–5362 (2021).
Li, M. et al. Exosomes from different cells: characteristics, modifications, and therapeutic applications. Eur. J. Med. Chem. 207, 112784 (2020).
Shan, X. et al. The biogenesis, biological functions, and applications of macrophage-derived exosomes. Front. Mol. Biosci. 8, 715461 (2021).
Wang, Y. et al. Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 11, 924 (2020).
Liu, J., Wu, F. & Zhou, H. Macrophage-derived exosomes in cancers: biogenesis, functions and therapeutic applications. Immunol. Lett. 227, 102–108 (2020).
Elashiry, M., Elsayed, R. & Cutler, C. W. Exogenous and endogenous dendritic cell-derived exosomes: lessons learned for immunotherapy and disease pathogenesis. Cells. 11, 115–136 (2021).
Pitt, J. M. et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Investig. 126, 1224–1232 (2016).
Kok, V. C. & Yu, C. C. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int. J. Nanomed. 15, 8019–8036 (2020).
Naseri, M. et al. Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology 9, 1779991 (2020).
Kluszczynska, K. et al. Methods for the determination of the purity of exosomes. Curr. Pharm. Des. 25, 4464–4485 (2019).
Zhou, B. et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 5, 144 (2020).
Boukouris, S. & Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin. Appl. 9, 358–367 (2015).
Gurunathan, S. et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 8, 307–342 (2019).
Bei, H. P. et al. Bone-a-Petite: engineering exosomes towards Bone, osteochondral, and cartilage repair. Small. 17, e2101741 (2021).
Yang, X. X., Sun, C., Wang, L. & Guo, X. L. New insight into isolation, identification techniques and medical applications of exosomes. J. Control Release 308, 119–129 (2019).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science. 367, 640–654 (2020).
Li, X. et al. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 3, 011503 (2019).
Khayambashi, P. et al. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int. J. Mol. Sci. 22, 684–698 (2021).
Hussen, B. M. et al. Strategies to overcome the main challenges of the use of exosomes as drug carrier for cancer therapy. Cancer Cell Int. 22, 323 (2022).
Shao, J., Zaro, J. & Shen, Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int. J. Nanomed. 15, 9355–9371 (2020).
Tian, Y. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35, 2383–2390 (2014).
Kim, M. S. et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12, 655–664 (2016).
Ohno, S. et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21, 185–191 (2013).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Zhu, Q. et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv. Sci. 6, 1801899 (2019).
Nakase, I. & Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 5, 10112 (2015).
Wu, Y., Deng, W. & Klinke, D. J. 2nd Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst. 140, 6631–6642 (2015).
Maas, S. L. et al. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J. Control Release. 200, 87–96 (2015).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles. 7, 1535750 (2018).
Witwer, K. W. et al. Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J. Extracell Vesicles. 10, e12182 (2021).
Maroto, R. et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell Vesicles. 6, 1359478 (2017).
Yamashita, T., Takahashi, Y. & Takakura, Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 41, 835–842 (2018).
Bosch, S. et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 6, 36162 (2016).
Charoenviriyakul, C., Takahashi, Y., Nishikawa, M. & Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 553, 1–7 (2018).
Kusuma, G. D. et al. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front. Pharmacol. 9, 1199 (2018).
Bahney, C. S. et al. Cellular biology of fracture healing. J. Orthop. Res. 37, 35–50 (2019).
Einhorn, T. A. & Gerstenfeld, L. C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 11, 45–54 (2015).
Yang, Z. et al. Exosomes: a friend or foe for osteoporotic fracture? Front. Endocrinol. 12, 679914 (2021).
Furuta, T. et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 5, 1620–1630 (2016).
Zhang, L. et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 11, 38 (2020).
Jia, Y. et al. Exosomes secreted by young mesenchymal stem cells promote new bone formation during distraction osteogenesis in older rats. Calcif. Tissue Int. 106, 509–517 (2020).
Jia, Y. et al. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res. Ther. 10, 12 (2019).
O’Brien, K. et al. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 21, 585–606 (2020).
Yu, H., Zhang, J., Liu, X. & Li, Y. microRNA-136-5p from bone marrow mesenchymal stem cell-derived exosomes facilitates fracture healing by targeting LRP4 to activate the Wnt/beta-catenin pathway. Bone Joint Res. 10, 744–758 (2021).
Jiang, Y., Zhang, J., Li, Z. & Jia, G. Bone marrow mesenchymal stem cell-derived exosomal miR-25 regulates the ubiquitination and degradation of Runx2 by SMURF1 to promote fracture healing in mice. Front. Med. 7, 577578 (2020).
Wang, Y. et al. Obesity regulates miR-467/HoxA10 axis on osteogenic differentiation and fracture healing by BMSC-derived exosome LncRNA H19. J. Cell Mol. Med. 25, 1712–1724 (2021).
Behera, J., Kumar, A., Voor, M. J. & Tyagi, N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 11, 7715–7734 (2021).
Liang, B. et al. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 10, 335 (2019).
Lu, G. D., Cheng, P., Liu, T. & Wang, Z. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Front. Cell Dev. Biol. 8, 608521 (2020).
Liu, W. et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 103, 196–212 (2020).
Katz, J. N., Arant, K. R. & Loeser, R. F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 325, 568–578 (2021).
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Primers. 2, 16072 (2016).
Wu, J. et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 206, 87–100 (2019).
Liu, Y. et al. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 17, 2411–2422 (2018).
Kong, R. et al. Synovial mesenchymal stem cell-derived exosomal miR-320c enhances chondrogenesis by targeting ADAM19. Future Med. Chem. 14, 81–96 (2022).
Mao, G. et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 9, 247 (2018).
Tao, S. C. et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7, 180–195 (2017).
Wang, R., Xu, B. & Xu, H. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17, 2756–2765 (2018).
Zhu, Y. et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 8, 64 (2017).
Jiang, K., Jiang, T., Chen, Y. & Mao, X. Mesenchymal stem cell-derived exosomes modulate chondrocyte glutamine metabolism to alleviate osteoarthritis progression. Mediat. Inflamm. 2021, 2979124 (2021).
Huang, Y. et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 promotes osteoblast proliferation and differentiation in osteoarthritis by reducing Elf3. J. Cell Mol. Med. 25, 7734–7745 (2021).
Zhang, S. et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 156, 16–27 (2018).
Tian, X. et al. Gingival mesenchymal stem cell-derived exosomes are immunosuppressive in preventing collagen-induced arthritis. J. Cell Mol. Med. 26, 693–708 (2022).
Cho, Y. et al. Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp. Mol. Med. 53, 1689–1696 (2021).
Ding, W. et al. Spinal cord injury: the global incidence, prevalence, and disability from the global burden of disease Study 2019. Spine 47, 1532–1540 (2022).
Ahuja, C. S. et al. Traumatic spinal cord injury. Nat. Rev. Dis. Primers. 3, 17018 (2017).
Ahuja, C. S. & Fehlings, M. Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl. Med. 5, 914–924 (2016).
Ma, K. et al. Insulin-like growth factor-1 enhances neuroprotective effects of neural stem cell exosomes after spinal cord injury via an miR-219a-2-3p/YY1 mechanism. Aging 11, 12278–12294 (2019).
Zhang, L. & Han, P. Neural stem cell-derived exosomes suppress neuronal cell apoptosis by activating autophagy via miR-374-5p/STK-4 axis in spinal cord injury. J. Musculoskelet. Neuronal Interact. 22, 411–421 (2022).
Shao, C. et al. Mesenchymal stem cell derived exosomes suppress neuronal cell ferroptosis Via lncGm36569/miR-5627-5p/FSP1 axis in acute spinal cord injury. Stem Cell Rev. Rep. 18, 1127–1142 (2022).
Nakazaki, M. et al. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-beta upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J. Extracell Vesicles. 10, e12137 (2021).
Liu, W. et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflamm. 17, 47 (2020).
Huang, J. H. et al. Extracellular vesicles derived from epidural fat-mesenchymal stem cells attenuate NLRP3 inflammasome activation and improve functional recovery after spinal cord injury. Neurochem. Res. 45, 760–771 (2020).
Yuan, F. et al. Endothelial progenitor cell-derived exosomes promote anti-inflammatory macrophages via SOCS3/JAK2/STAT3 axis and improve the outcome of spinal cord injury. J. Neuroinflamm. 20, 156 (2023).
Zhong, D. et al. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp. Biol. Med. 245, 54–65 (2020).
Chen, J. et al. Exosomes derived from nerve stem cells loaded with FTY720 promote the recovery after spinal cord injury in rats by PTEN/AKT signal pathway. J. Immunol. Res. 2021, 8100298 (2021).
Zhu, S. et al. Versatile subtypes of pericytes and their roles in spinal cord injury repair, bone development and repair. Bone Res. 10, 30 (2022).
Zhou, Y. et al. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen. Res. 17, 194–202, (2022).
Lai, X. et al. miR-146a-5p-modified hUCMSC-derived exosomes facilitate spinal cord function recovery by targeting neurotoxic astrocytes. Stem Cell Res. Ther. 13, 487 (2022).
Li, S. et al. Exosomes derived from NGF-overexpressing bone marrow mesenchymal stem cell sheet promote spinal cord injury repair in a mouse model. Neurochem. Int. 157, 105339 (2022).
Zhou, W. et al. Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res. Ther. 12, 174 (2021).
Perez, N. E. et al. Neurogenic bladder physiology, pathogenesis, and management after spinal cord injury. J. Pers. Med. 12, 968–982 (2022).
Vila Pouca, M. C. P., Parente, M. P. L., Jorge, R. M. N. & Ashton-Miller, J. A. Injuries in muscle-tendon-bone units: a systematic review considering the role of passive tissue fatigue. Orthop. J. Sports Med. 9, 23259671211020731 (2021).
Thomopoulos, S., Parks, W. C., Rifkin, D. B. & Derwin, K. A. Mechanisms of tendon injury and repair. J. Orthop. Res. 33, 832–839 (2015).
Nakamura, Y. et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 589, 1257–1265 (2015).
Chen, S. H. et al. Extracellular vesicles of adipose-derived stem cells promote the healing of traumatized achilles tendons. Int. J. Mol. Sci. 22, 12373–12388 (2021).
Brindisino, F. et al. Rotator cuff repair vs. nonoperative treatment: a systematic review with meta-analysis. J. Shoulder Elbow Surg. 30, 2648–2659 (2021).
Wang, C. et al. Exosomes isolated from adipose-derived stem cells: a new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am. J. Sports Med. 47, 3247–3255 (2019).
Wang, C. et al. Adipose stem cell-derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am. J. Sports Med. 48, 1456–1464 (2020).
Huang, Y. et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res. Ther. 11, 496 (2020).
Compston, J. E., McClung, M. R. & Leslie, W. D. Osteoporosis. Lancet 393, 364–376 (2019).
Ren, L. et al. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem. Biophys. Res. Commun. 508, 138–144 (2019).
Yang, B. C. et al. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem. Biophys. Res. Commun. 524, 883–889 (2020).
Yahao, G. & Xinjia, W. The role and mechanism of exosomes from umbilical cord mesenchymal stem cells in inducing osteogenesis and preventing osteoporosis. Cell Transplant. 30, 9636897211057465 (2021).
Gordon, T. Peripheral nerve regeneration and muscle reinnervation. Int. J. Mol. Sci. 21, 8652–8675 (2020).
Bucan, V. et al. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol. Neurobiol. 56, 1812–1824 (2019).
Chen, J. et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J. Cell Physiol. 234, 23097–23110 (2019).
Li, C. et al. Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: involvement of TSG-6/NF-kappaB/NLRP3 signaling pathway. Exp. Neurol. 356, 114139 (2022).
Fine, N. et al. Intervertebral disc degeneration and osteoarthritis: a common molecular disease spectrum. Nat. Rev. Rheumatol. 19, 136–152 (2023).
Cheng, X. et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell Mol. Med. 22, 261–276 (2018).
Yu, Y. et al. Human embryonic stem-cell-derived exosomes repress NLRP3 inflammasome to alleviate pyroptosis in nucleus pulposus cells by transmitting miR-302c. Int. J. Mol. Sci. 24, 7664–7678 (2023).
Hu, Y. et al. Exosomes derived from bone mesenchymal stem cells alleviate compression-induced nucleus pulposus cell apoptosis by inhibiting oxidative stress. Oxid. Med. Cell Longev. 2021, 2310025 (2021).
Petek, D., Hannouche, D. & Suva, D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open Rev. 4, 85–97 (2019).
Liu, X. et al. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 13, 232–244 (2017).
Zuo, R. et al. Exosomes derived from human CD34(+) stem cells transfected with miR-26a prevent glucocorticoid-induced osteonecrosis of the femoral head by promoting angiogenesis and osteogenesis. Stem Cell Res. Ther. 10, 321 (2019).
Campbell, B. C. V. & Khatri, P. Stroke. Lancet. 396, 129–142 (2020).
Campbell, B. C. V. et al. Ischaemic stroke. Nat. Rev. Dis. Primers. 5, 70 (2019).
Fisher, M. & Savitz, S. I. Pharmacological brain cytoprotection in acute ischaemic stroke — renewed hope in the reperfusion era. Nat. Rev. Neurol. 18, 193–202 (2022).
Luo, H. et al. miR-150-3p enhances neuroprotective effects of neural stem cell exosomes after hypoxic-ischemic brain injury by targeting CASP2. Neurosci. Lett. 779, 136635 (2022).
Pan, J., Wu, T., Chen, B. & Wu, H. Exosomes derived from endothelial progenitor cells ameliorate glyoxylate deprivation (OGD)-induced neuronal apoptosis by delivering miR-221-3p. Histol. Histopathol. 38, 423–430 (2023).
Huang, R., Cheng, T. & Lai, X. Mechanism of ischemic brain injury repair by endothelial progenitor cell-derived exosomes. Mol. Med. Rep. 26, 269–278 (2022).
Zhang, G. et al. Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. J. Adv. Res. 24, 435–445 (2020).
Kang, X. et al. Exosomes derived from hypoxic bone marrow mesenchymal stem cells rescue OGD-induced injury in neural cells by suppressing NLRP3 inflammasome-mediated pyroptosis. Exp. Cell Res. 405, 112635 (2021).
Li, W. Y. et al. Exosomes derived from human induced pluripotent stem cell-derived neural progenitor cells protect neuronal function under ischemic conditions. Neural Regen. Res. 16, 2064–2070, (2021).
Sun, X. et al. Stem cell-derived exosomes protect astrocyte cultures from in vitro ischemia and decrease injury as post-stroke intravenous therapy. Front. Cell Neurosci. 13, 394 (2019).
Wei, R. et al. Zeb2/Axin2-Enriched BMSC-Derived exosomes promote post-stroke functional recovery by enhancing neurogenesis and neural plasticity. J. Mol. Neurosci. 72, 69–81 (2022).
Wang, J. et al. Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci. Ther. 26, 1255–1265 (2020).
Dong, C. et al. Mesenchymal stem cell-derived exosomes improved cerebral infarction via transferring miR-23a-3p to activate microglia. Neuromol. Med. 24, 290–298 (2022).
Zhang, Z. et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 13, 3060–3079 (2021).
Yoon, E. J. et al. The neuroprotective effects of exosomes derived from TSG101-overexpressing human neural stem cells in a stroke model. Int. J. Mol. Sci. 23, 9532–9546 (2022).
Tian, T. et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics 11, 6507–6521 (2021).
Gao, G. et al. Induced neural stem/progenitor cell-derived extracellular vesicles promote recovery post-stroke. Clin. Transl. Med. 12, e936 (2022).
Zhu, Z. H. et al. Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regen. Res. 18, 404–409, (2023).
Yerrapragada, S. M. et al. The protective effects of miR-210 modified endothelial progenitor cells released exosomes in hypoxia/reoxygenation injured neurons. Exp. Neurol. 358, 114211 (2022).
Ma, X. et al. Loading MiR-210 in endothelial progenitor cells derived exosomes boosts their beneficial effects on hypoxia/reoxygeneation-injured human endothelial cells via protecting mitochondrial function. Cell Physiol. Biochem. 46, 664–675 (2018).
Pan, Q. et al. MiR-17-5p mediates the effects of ACE2-Enriched endothelial progenitor cell-derived exosomes on ameliorating cerebral ischemic injury in aged mice. Mol. Neurobiol. 60, 3534–3552 (2023).
Xu, X. et al. Combination of EPC-EXs and NPC-EXs with miR-126 and miR-210 overexpression produces better therapeutic effects on ischemic stroke by protecting neurons through the Nox2/ROS and BDNF/TrkB pathways. Exp. Neurol. 359, 114235 (2023).
Morotti, A. & Goldstein, J. N. Diagnosis and management of acute intracerebral hemorrhage. Emerg. Med. Clin. N. Am. 34, 883–899 (2016).
Li, Y. et al. miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res. Ther. 11, 330 (2020).
Wiles, M. D. Management of traumatic brain injury: a narrative review of current evidence. Anaesthesia. 77, 102–112 (2022).
McKee, A. C. & Daneshvar, D. H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 127, 45–66 (2015).
Galgano, M. et al. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 26, 1118–1130 (2017).
Zhang, Y. et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 122, 856–867 (2015).
Zhang, Y. et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 111, 69–81 (2017).
Moore, T. L. et al. Mesenchymal derived exosomes enhance recovery of motor function in a monkey model of cortical injury. Restor. Neurol. Neurosci. 37, 347–362 (2019).
Williams, A. M. et al. Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J. Neurotrauma. 36, 54–60 (2019).
Chen, Y. et al. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 12, 18274–18296 (2020).
Wen, L. et al. Exosomes derived from bone marrow mesenchymal stem cells inhibit neuroinflammation after traumatic brain injury. Neural Regen. Res. 17, 2717–2724, (2022).
Abedi, M., Hajinejad, M., Atabi, F. & Sahab-Negah, S. Exosome derived from human neural stem cells improves motor activity and neurogenesis in a traumatic brain injury model. Biomed. Res. Int. 2022, 6409346 (2022).
Zhang, Y. et al. Mesenchymal stem cell-derived exosomes improve functional recovery in rats after traumatic brain injury: a dose-response and therapeutic window study. Neurorehabil. Neural Repair. 34, 616–626 (2020).
Wallace, D. J. et al. Spinal cord injury and the human microbiome: beyond the brain-gut axis. Neurosurg. Focus. 46, E11 (2019).
Kumar, S. et al. Transcriptional factors and protein biomarkers as target therapeutics in traumatic spinal cord and brain injury. Curr. Neuropharmacol. 18, 1092–1105 (2020).
Mortezaee, K., Khanlarkhani, N., Beyer, C. & Zendedel, A. Inflammasome: its role in traumatic brain and spinal cord injury. J. Cell Physiol. 233, 5160–5169 (2018).
Roselli, F., Karasu, E., Volpe, C. & Huber-Lang, M. Medusa’s head: the complement system in traumatic brain and spinal cord injury. J. Neurotrauma. 35, 226–240 (2018).
Putatunda, R., Bethea, J. R. & Hu, W. H. Potential immunotherapies for traumatic brain and spinal cord injury. Chin. J. Traumatol. 21, 125–136 (2018).
Bains, M. & Hall, E. D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta. 1822, 675–684 (2012).
Yuan, J. et al. Role of circular ribonucleic acids in the treatment of traumatic brain and spinal cord injury. Mol. Neurobiol. 57, 4296–4304 (2020).
Sun, P. et al. MicroRNA-based therapeutics in central nervous system injuries. J. Cereb. Blood Flow Metab. 38, 1125–1148 (2018).
Wilson, D. M. 3rd et al. Hallmarks of neurodegenerative diseases. Cell. 186, 693–714 (2023).
Erkkinen, M. G., Kim, M. O. & Geschwind, M. D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect. Biol. 10, 3118–3163 (2018).
Breijyeh, Z. & Karaman, R. Comprehensive review on alzheimeras disease: causes and treatment. Molecules. 25, 5789–5816 (2020).
Hampel, H. et al. The amyloid-beta pathway in Alzheimer’s disease. Mol. Psychiatry. 26, 5481–5503 (2021).
Elia, C. A. et al. Intracerebral injection of extracellular vesicles from mesenchymal stem cells exerts reduced abeta plaque burden in early stages of a preclinical model of Alzheimer’s disease. Cells. 8, 1059–1078 (2019).
Wang, S. S., Jia, J. & Wang, Z. Mesenchymal stem cell-derived extracellular vesicles suppresses inos expression and ameliorates neural impairment in Alzheimer’s disease mice. J. Alzheimers Dis. 61, 1005–1013 (2018).
Li, B. et al. Impact of neural stem cell-derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. J. Neurochem. 154, 502–518 (2020).
Huber, C. C. et al. Heat shock-induced extracellular vesicles derived from neural stem cells confer marked neuroprotection against oxidative stress and amyloid-beta-caused neurotoxicity. Mol. Neurobiol. 59, 7404–7412 (2022).
Chen, Y. A. et al. Mesenchymal stem cell-derived exosomes ameliorate Alzheimer’s disease pathology and improve cognitive deficits. Biomedicines. 9, 594–612 (2021).
Zavatti, M. et al. Exosomes derived from human amniotic fluid mesenchymal stem cells preserve microglia and neuron cells from Abeta. Int. J. Mol. Sci. 23, 4967–4980 (2022).
Ding, M. et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem. Res. 43, 2165–2177 (2018).
Reza-Zaldivar, E. E. et al. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 14, 1626–1634, (2019).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Liu, Y., Huber, C. C. & Wang, H. Disrupted blood-brain barrier in 5xFAD mouse model of Alzheimer’s disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem. Biophys. Res. Commun. 525, 192–196 (2020).
Cui, G. H. et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing. 16, 10 (2019).
Gao, G. et al. Neural stem cell-derived extracellular vesicles mitigate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Signal Transduct. Target Ther. 8, 228 (2023).
Marino, B. L. B. et al. Parkinson’s disease: a review from pathophysiology to treatment. Mini Rev, Med, Chem. 20, 754–767 (2020).
Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Primers. 3, 17013 (2017).
Huang, D., Zhang, M. & Tan, Z. Bone marrow stem cell-exo-derived TSG-6 Attenuates 1-Methyl-4-Phenylpyridinium+-Induced Neurotoxicity via the STAT3/miR-7/NEDD4/LRRK2 Axis. J. Neuropathol. Exp. Neurol. 81, 621–634 (2022).
Lee, E. J. et al. Human neural stem cell-derived extracellular vesicles protect against Parkinson’s disease pathologies. J. Nanobiotechnol. 20, 198 (2022).
Filippi, M. et al. Multiple sclerosis. Nat. Rev. Dis. Primers. 4, 43 (2018).
Dobson, R. & Giovannoni, G. Multiple sclerosis - a review. Eur. J. Neurol. 26, 27–40 (2019).
Li, Z. et al. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharmacol. 67, 268–280 (2019).
Zhang, J. et al. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp. Neurol. 347, 113895 (2022).
Qi, D. et al. HNSC exosome-derived MIAT improves cognitive disorders in rats with vascular dementia via the miR-34b-5p/CALB1 axis. Am. J. Transl. Res. 13, 10075–10093 (2021).
Branscome, H. et al. Retroviral infection of human neurospheres and use of stem Cell EVs to repair cellular damage. Sci. Rep. 12, 2019 (2022).
Leavitt, R. J., Acharya, M. M., Baulch, J. E. & Limoli, C. L. Extracellular vesicle-derived miR-124 resolves radiation-induced brain injury. Cancer Res. 80, 4266–4277 (2020).
Smith, S. M. et al. Functional equivalence of stem cell and stem cell-derived extracellular vesicle transplantation to repair the irradiated brain. Stem Cells Transl. Med. 9, 93–105 (2020).
Long, Q. et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA. 114, E3536–E3545 (2017).
Li, S. et al. Bone marrow mesenchymal stem cell-derived exosomes shuttling miR-150-5p alleviates mechanical allodynia in rats by targeting NOTCH2 in microglia. Mol. Med. 28, 133 (2022).
Ma, L. et al. Neural stem cell-derived exosomal netrin1 contributes to neuron differentiation of mesenchymal stem cells in therapy of spinal bifida aperta. Stem Cells Transl. Med. 11, 539–551 (2022).
Guo, H. et al. Bone marrow mesenchymal stem cells-derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA-26a expression. Int. Immunopharmacol. 82, 106285 (2020).
Niu, Y., Wang, X., Li, M. & Niu, B. Exosomes from human umbilical cord Mesenchymal stem cells attenuates stress-induced hippocampal dysfunctions. Metab. Brain Dis. 35, 1329–1340 (2020).
Natale, F. et al. Neural stem cell-derived extracellular vesicles counteract insulin resistance-induced senescence of neurogenic niche. Stem Cells 40, 318–331 (2022).
Spinelli, M. et al. Neural stem cell-derived exosomes revert HFD-dependent memory impairment via CREB-BDNF Signalling. Int. J. Mol. Sci. 21, 8994–9008 (2020).
Zhang, Y. et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548, 52–57 (2017).
Kong, L. Y. et al. Mesenchymal stem cell-derived exosomes rescue oxygen-glucose deprivation-induced injury in endothelial cells. Curr. Neurovasc. Res. 17, 155–163 (2020).
Almadani, Y. H., Vorstenbosch, J., Davison, P. G. & Murphy, A. M. Wound healing: a comprehensive review. Semin. Plast Surg. 35, 141–144 (2021).
Rodrigues, M., Kosaric, N., Bonham, C. A., Gurtner, G. C. & Wound Healing: a cellular perspective. Physiol. Rev. 99, 665–706 (2019).
Wang, M., Zhao, Y. & Zhang, Q. Human mesenchymal stem cell-derived exosomes accelerate wound healing of mice eczema. J. Dermatolog. Treat. 33, 1401–1405 (2022).
Cho, B. S., Kim, J. O., Ha, D. H. & Yi, Y. W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 9, 187 (2018).
Qiu, X. et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 53, e12830 (2020).
Yu, M. et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 11, 350 (2020).
Ding, J. et al. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed. Res. Int. 2019, 9742765 (2019).
Li, X., Jiang, C. & Zhao, J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J. Diabetes Complications. 30, 986–992 (2016).
Zhang, J. et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int. J. Biol. Sci. 12, 1472–1487 (2016).
Xu, J. et al. miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab. Syndr. Obes. 13, 1259–1270 (2020).
Zhang, W. et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 370, 333–342 (2018).
Zhang, Y. et al. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1alpha axis. J. Mol. Histol. 51, 375–383 (2020).
Kim, S., Lee, S. K., Kim, H. & Kim, T. M. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int. J. Mol. Sci. 19, 3119–3134 (2018).
He, L. et al. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/beta-catenin pathway. Biosci. Rep. 40, 549–561 (2020).
Gao, S. et al. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res. Ther. 11, 56 (2020).
Li, P. et al. Endothelial progenitor cell derived exosomes mediated miR-182-5p delivery accelerate diabetic wound healing via down-regulating PPARG. Int. J. Med. Sci. 20, 468–481 (2023).
Zhao, G. et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res. Ther. 11, 174 (2020).
Fang, S. et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth Factor-β/SMAD2 pathway during wound healing. Stem Cells Transl. Med. 5, 1425–1439 (2016).
Hu, J., Chen, Y., Huang, Y. & Su, Y. Human umbilical cord mesenchymal stem cell-derived exosomes suppress dermal fibroblasts-myofibroblats transition via inhibiting the TGF-beta1/Smad 2/3 signaling pathway. Exp. Mol. Pathol. 115, 104468 (2020).
Hu, L. et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 6, 32993 (2016).
Chen, B. et al. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res. Ther. 10, 142 (2019).
Bae, Y. U. et al. Embryonic stem cell-derived mmu-miR-291a-3p inhibits cellular senescence in human dermal fibroblasts through the TGF-beta Receptor 2 pathway. J. Gerontol. A 74, 1359–1367 (2019).
Bai, Y. et al. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 500, 310–317 (2018).
Liu, Y. et al. Exosomes derived from stem cells from apical papilla promote craniofacial soft tissue regeneration by enhancing Cdc42-mediated vascularization. Stem Cell Res. Ther. 12, 76 (2021).
Li, M. et al. Mesenchymal stem cell-derived exosomes ameliorate dermal fibrosis in a Murine model of Bleomycin-Induced Scleroderma. Stem Cells Dev. 30, 981–990 (2021).
Koken, G. Y., Abamor, E. S., Allahverdiyev, A. & Karaoz, E. Wharton Jelly derived mesenchymal stem Cell’s exosomes demonstrate significant antileishmanial and wound healing effects in combination with Aloe-Emodin: an in vitro study. J. Pharm. Sci. 111, 3232–3242 (2022).
Cao, L. et al. Neural progenitor cell-derived nanovesicles promote hair follicle growth via miR-100. J. Nanobiotechnol. 19, 20 (2021).
Oh, M. et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 19, 1715–1732 (2018).
Stravitz, R. T. & Kramer, D. J. Management of acute liver failure. Nat. Rev. Gastroenterol. Hepatol. 6, 542–553 (2009).
Lin, F. et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 13, 271 (2022).
Shao, M. et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res. Ther. 11, 37 (2020).
Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18, 151–166 (2021).
Ma, L. et al. Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv. 29, 440–453 (2022).
Wang, N. et al. 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFbetaRII-SMADS pathway. J .Nanobiotechnol. 19, 437 (2021).
Hirao, H., Nakamura, K. & Kupiec-Weglinski, J. W. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat. Rev. Gastroenterol. Hepatol. 19, 239–256 (2022).
Yang, B. et al. Bone marrow mesenchymal stem cell-derived hepatocyte-like cell exosomes reduce hepatic ischemia/reperfusion injury by enhancing autophagy. Stem Cells Dev. 29, 372–379 (2020).
Du, Y. et al. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) protect liver against hepatic ischemia/ reperfusion injury via activating sphingosine kinase and Sphingosine-1-Phosphate signaling pathway. Cell. Physiol. Biochem. 43, 611–625 (2017).
Venkat, P. et al. Therapeutic effects of CD133 + Exosomes on liver function after stroke in type 2 diabetic mice. Front. Neurosci. 17, 1061485 (2023).
Boxhoorn, L. et al. Acute pancreatitis. Lancet 396, 726–734 (2020).
Chen, M. et al. Exosomes from human induced pluripotent stem cells derived mesenchymal stem cells improved myocardial injury caused by severe acute pancreatitis through activating Akt/Nrf2/HO-1 axis. Cell Cycle. 21, 1578–1589 (2022).
Uccioli, L. et al. Critical limb ischemia: current challenges and future prospects. Vasc. Health Risk Manag. 14, 63–74 (2018).
Komaki, M. et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res. Ther. 8, 219 (2017).
Gong, M. et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8, 45200–45212 (2017).
Mathiyalagan, P. et al. Angiogenic Mechanisms of Human CD34(+) Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ. Res. 120, 1466–1476 (2017).
Ye, M. et al. Exosomes derived from human induced pluripotent stem cells-endothelia cells promotes postnatal angiogenesis in mice bearing ischemic limbs. Int. J. Biol. Sci. 15, 158–168 (2019).
Lazar, A. & Morrissey, N. Recent advances in endovascular treatment of peripheral arterial disease. F1000Res. 9, 122–126 (2020).
Li, X. et al. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy. 18, 253–262 (2016).
Hu, H. et al. Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21-5p to modulate Thrombospondin-1 expression. Clin. Sci. 133, 1629–1644 (2019).
Hu, H., Jiang, C., Li, R. & Zhao, J. Comparison of endothelial cell- and endothelial progenitor cell-derived exosomes in promoting vascular endothelial cell repair. Int. J. Clin. Exp. Pathol. 12, 2793–2800 (2019).
Kong, J. et al. Exosomes of endothelial progenitor cells inhibit neointima formation after carotid artery injury. J. Surg. Res. 232, 398–407 (2018).
Thyoka, M. et al. Advanced necrotizing enterocolitis part 1: mortality. Eur. J. Pediatr. Surg. 22, 8–12 (2012).
McCulloh, C. J. et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J. Pediatr. Surg. 53, 1215–1220 (2018).
Moore, L. J. et al. Sepsis in general surgery: a deadly complication. Am. J. Surg. 198, 868–874 (2009).
Zhou, Y. et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol. Ther. 26, 1375–1384 (2018).
Liu, Y. et al. Protective effect of endothelial progenitor cell-derived exosomal microRNA-382-3p on sepsis-induced organ damage and immune suppression in mice. Am. J. Transl. Res. 14, 6856–6873 (2022).
Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 17, 773–789 (2020).
Xing, X. et al. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging 12, 3880–3898 (2020).
Peng, Y. et al. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 11, 317 (2020).
Gao, L. et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci. Transl. Med. 12, 317–331 (2020).
Wen, Z. et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 11, 36 (2020).
Santoso, M. R. et al. Exosomes from induced pluripotent stem cell-derived cardiomyocytes promote autophagy for myocardial repair. J. Am. Heart Assoc. 9, e014345 (2020).
Katsur, M. et al. Exosomes from neuronal stem cells may protect the heart from ischaemia/reperfusion injury via JAK1/2 and gp130. J. Cell. Mol. Med. 25, 4455–4465 (2021).
Chen, G. et al. Mesenchymal stem cell-derived exosomal miR-143-3p suppresses myocardial ischemia-reperfusion injury by regulating autophagy. Life Sci. 280, 119742 (2021).
Chen, F. et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate cardiac hypertrophy and fibrosis in pressure overload induced remodeling. In Vitro Cell. Dev. Biol. Anim. 56, 567–576 (2020).
Khan, M. et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 117, 52–64 (2015).
Tavakoli Dargani, Z. & Singla, D. K. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am. J. Physiol. Heart Circ. Physiol. 317, H460–H471 (2019).
Singla, D. K., Johnson, T. A. & Tavakoli Dargani, Z. Exosome treatment enhances anti-inflammatory M2 macrophages and reduces inflammation-induced pyroptosis in doxorubicin-induced cardiomyopathy. Cells. 8, 1224–1244 (2019).
Pang, Y. et al. Embryonic stem cell-derived exosomes attenuate transverse aortic constriction induced heart failure by increasing angiogenesis. Front. Cardiovasc. Med. 8, 638771 (2021).
Kervadec, A. et al. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J. Heart Lung Transplant. 35, 795–807 (2016).
El Harane, N. et al. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur. Heart J. 39, 1835–1847 (2018).
Li, H. et al. Exosomes secreted by endothelial cells derived from human induced pluripotent stem cells improve recovery from myocardial infarction in mice. Stem Cell. Res. Ther. 14, 278 (2023).
Li, H. et al. Isolation of swine bone marrow Lin-/CD45-/CD133 + cells and cardio-protective effects of its exosomes. Stem Cell. Rev. Rep. 19, 213–229 (2023).
Angulski, A. B. B. et al. Systemic infusion of expanded CD133(+) cells and expanded CD133(+) cell-derived EVs for the treatment of ischemic cardiomyopathy in a rat model of AMI. Stem Cells Int. 2019, 4802578 (2019).
Mackie, A. R. et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ. Res. 111, 312–321 (2012).
Ke, X. et al. Human endothelial progenitor cell-derived exosomes increase proliferation and angiogenesis in cardiac fibroblasts by promoting the mesenchymal-endothelial transition and reducing high mobility Group Box 1 protein B1 expression. DNA Cell Biol. 36, 1018–1028 (2017).
Ke, X. et al. Exosomal miR-218-5p/miR-363-3p from endothelial progenitor cells ameliorate myocardial infarction by targeting the p53/JMY signaling pathway. Oxid. Med. Cell. Longev. 2021, 5529430 (2021).
Yue, Y. et al. Interleukin-10 deficiency alters endothelial progenitor cell-derived exosome reparative effect on myocardial repair via integrin-linked kinase enrichment. Circ. Res. 126, 315–329 (2020).
Liu, Q. et al. Exosomal miR-17-5p from human embryonic stem cells prevents pulmonary fibrosis by targeting thrombospondin-2. Stem Cell. Res. Ther. 14, 234 (2023).
Zhou, Y. et al. Exosomes derived from induced pluripotent stem cells suppresses M2-type macrophages during pulmonary fibrosis via miR-302a-3p/TET1 axis. Int. Immunopharmacol. 99, 108075 (2021).
Liu, P. et al. Endothelial progenitor cell-derived exosomes inhibit pulmonary artery smooth muscle cell in vitro proliferation and resistance to apoptosis by modulating the Mitofusin-2 and Ras-Raf-ERK1/2 signaling pathway. Eur. J. Pharmacol. 949, 175725 (2023).
Zhou, Y. et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit. Care. 23, 44 (2019).
Wu, X. et al. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp. Cell Res. 370, 13–23 (2018).
Zhang, X. et al. Exosomes secreted by endothelial progenitor cells improve the bioactivity of pulmonary microvascular endothelial cells exposed to hyperoxia in vitro. Ann. Transl. Med. 7, 254 (2019).
Montay-Gruel, P. et al. Extracellular vesicles for the treatment of radiation-induced normal tissue toxicity in the lung. Front. Oncol. 10, 602763 (2020).
Huang, R., Fu, P. & Ma, L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal. Transduct. Target Ther. 8, 129 (2023).
Liu, Y. et al. Bone marrow mesenchymal stem cell-derived exosomal microRNA-381-3p alleviates vascular calcification in chronic kidney disease by targeting NFAT5. Cell Death Dis. 13, 278 (2022).
Liu, Y. et al. Bone marrow mesenchymal stem cell-derived exosomes improve renal fibrosis via regulating Smurf 2/Smad 7. Front. Biosci. 27, 17 (2022).
Lu, Y. et al. Bone marrow mesenchymal stem cell-derived exosomes improve renal fibrosis by reducing the polarisation of M1 and M2 macrophages through the activation of EP2 receptors. IET Nanobiotechnol. 16, 14–24 (2022).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Primers. 7, 52 (2021).
Lim, S. W. et al. Alleviation of renal ischemia/reperfusion injury by exosomes from induced pluripotent stem cell-derived mesenchymal stem cells. Korean J. Intern. Med. 37, 411–424 (2022).
Zhang, Y. et al. Endothelial progenitor cells-derived exosomal microRNA-21-5p alleviates sepsis-induced acute kidney injury by inhibiting RUNX1 expression. Cell Death Dis. 12, 335 (2021).
Sheffield, A. M. & Smith, R. J. H. The epidemiology of deafness. Cold Spring Harb Perspect. Med. 9, 3258–3273 (2019).
Tsai, S. C. et al. Umbilical cord mesenchymal stromal cell-derived exosomes rescue the loss of outer hair cells and repair cochlear damage in cisplatin-injected mice. Int. J. Mol. Sci. 22, 6664–6687 (2021).
Tabuchi, K. et al. Ischemia-reperfusion injury of the cochlea: pharmacological strategies for cochlear protection and implications of glutamate and reactive oxygen species. Curr Neuropharmacol. 8, 128–134 (2010).
Hao, F. et al. Exosomes derived from microRNA-21 overexpressing neural progenitor cells prevent hearing loss from ischemia-reperfusion injury in mice via inhibiting the inflammatory process in the Cochlea. ACS Chem. Neurosci. 13, 2464–2472 (2022).
Chotigavanich, C. et al. Hypothyroidism after Hemithyroidectomy: the incidence and risk factors. J. Med. Assoc. Thai. 99, 77–83 (2016).
Degosserie, J. et al. Extracellular vesicles from endothelial progenitor cells promote thyroid follicle formation. J. Extracell. Vesicles. 7, 1487250 (2018).
Valesan, L. F. et al. Prevalence of temporomandibular joint disorders: a systematic review and meta-analysis. Clin. Oral. Investig. 25, 441–453 (2021).
Zhang, S. et al. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 200, 35–47 (2019).
Yang, Y. & Sun, X. Retinal ganglion cell death in glaucoma: advances and caveats. Curr. Eye Res. 48, 1–10 (2023).
Mead, B. & Tomarev, S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells. Transl. Med. 6, 1273–1285 (2017).
Van Gelder, R. N. et al. Regenerative and restorative medicine for eye disease. Nat. Med. 28, 1149–1156 (2022).
Bian, B. et al. Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. J. Extracell. Vesicles. 9, 1748931 (2020).
Ke, Y. et al. Human embryonic stem cell-derived extracellular vesicles alleviate retinal degeneration by upregulating Oct4 to promote retinal Muller cell retrodifferentiation via HSP90. Stem Cell. Res. Ther. 12, 21 (2021).
Gao, Y. et al. Embryonic stem cells-derived exosomes enhance retrodifferentiation of retinal Muller cells by delivering BDNF protein to activate Wnt pathway. Immunobiology 227, 152211 (2022).
Park, U. C. et al. Subretinal versus intravitreal administration of human CD34+ bone marrow-derived stem cells in a rat model of inherited retinal degeneration. Ann. Transl. Med. 9, 1275 (2021).
Ong, E. S. & Jeng, B. H. Current and future therapies for persistent corneal epithelial defects and neurotrophic keratopathy. Curr. Opin. Ophthalmol. 32, 262–267 (2021).
Wang, S. et al. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging 12, 19546–19562 (2020).
Zhu, J. et al. Role of immune cell diversity and heterogeneity in corneal graft survival: a systematic review and meta-analysis. J. Clin. Med. 10, 4667–4686 (2021).
Jia, Z. et al. Mesenchymal stem cell derived exosomes-based immunological signature in a rat model of corneal allograft rejection therapy.Front. Biosci. 86, (2022).
Huang, Q. Y. et al. Therapeutic options for premature ovarian insufficiency: an updated review. Reprod. Biol. Endocrinol. 20, 28 (2022).
Na, J. & Kim, G. J. Recent trends in stem cell therapy for premature ovarian insufficiency and its therapeutic potential: a review. J. Ovarian Res. 13, 74 (2020).
Li, Z. et al. Human umbilical cord mesenchymal stem cell-derived exosomes improve ovarian function and proliferation of premature ovarian insufficiency by regulating the hippo signaling pathway. Front. Endocrinol. 12, 711902 (2021).
Zhang, L. et al. Human pluripotent stem cell-mesenchymal stem cell-derived exosomes promote ovarian granulosa cell proliferation and attenuate cell apoptosis induced by cyclophosphamide in a POI-like Mouse Model. Molecules. 28, 2112–2129 (2023).
Ding, C. et al. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells. 38, 1137–1148 (2020).
Yang, M. et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Investig. 100, 342–352 (2020).
Chen, Y. S., Lin, E. Y., Chiou, T. W. & Harn, H. J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi. 32, 113–120 (2020).
Perocheau, D. et al. Clinical applications for exosomes: Are we there yet? Br. J. Pharmacol. 178, 2375–2392 (2021).
Rezaie, J., Feghhi, M. & Etemadi, T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun. Signal. 20, 145 (2022).
Wang, X. et al. Recent progress in exosome research: isolation, characterization and clinical applications. Cancer Gene Ther. 30, 1051–1065 (2023).
Santos, P. & Almeida, F. Exosome-based vaccines: history, current state, and clinical trials. Front. Immunol. 12, 711565 (2021).
Hao, Z. C. Stem cell-derived exosomes: a promising strategy for fracture healing. Cell Prolif. 50, 359–368 (2017).
Yang, Z. L. et al. The role of exosomes and exosomal noncoding RNAs from different cell sources in spinal cord injury. Front. Cell. Neurosci. 16, 882306 (2022).
Hade, M. D., Suire, C. N. & Suo, Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 10, 1959–2006 (2021).
Lou, G., Chen, Z., Zheng, M. & Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 49, e346 (2017).
Krishnan, A., Muthusamy, S., Fernandez, F. B. & Kasoju, N. Mesenchymal stem cell-derived extracellular vesicles in the management of COVID19-associated lung injury: a review on publications, clinical trials and patent landscape. Tissue Eng. Regen. Med. 19, 659–673 (2022).
Tsioulos, G. et al. Insights into CD24 and exosome physiology and potential role in view of recent advances in COVID-19 therapeutics: a narrative review. Life. 12, 1472–1487 (2022).
Yoo, K. H. Possibility of exosome‑based coronavirus disease 2019 vaccine (Review). Mol. Med. Rep. 25, 3625–3633 (2022).
Yassine, S. & Alaaeddine, N. Mesenchymal stem cell exosomes and cancer: controversies and prospects. Adv. Biol. 6, e2101050 (2022).
Vakhshiteh, F., Atyabi, F. & Ostad, S. N. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int. J. Nanomed. 14, 2847–2859 (2019).
Lin, Z. et al. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol. Cancer. 21, 179 (2022).
Xu, Z., Zeng, S., Gong, Z. & Yan, Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol. Cancer. 19, 160 (2020).
Nam, G. H. et al. Emerging prospects of exosomes for cancer treatment: from conventional therapy to immunotherapy. Adv. Mater. 32, e2002440 (2020).
Kim, H. Recent advances in exosome-based drug delivery for cancer therapy. Cancers. 13, 4435–4457 (2021).
Dai, J. et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal. Transduct. Target Ther. 5, 145 (2020).
Nie, L. et al. Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke. Cell Death Discov. 9, 215 (2023).
Fernandez-Munoz, B., Garcia-Delgado, A. B., Arribas-Arribas, B. & Sanchez-Pernaute, R. Human neural stem cells for cell-based medicinal products. Cells. 10, 2377–2402 (2021).
Shahbazi, E., Mirakhori, F., Ezzatizadeh, V. & Baharvand, H. Reprogramming of somatic cells to induced neural stem cells. Methods 133, 21–28 (2018).
Ma, Y. et al. Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal-regulated kinase pathways. Neurobiol. Dis. 124, 322–334 (2019).
Ma, Y. et al. Induced neural progenitor cell-derived extracellular vesicles promote neural progenitor cell survival via extracellular signal-regulated kinase pathway. CNS Neurosci. Ther. 27, 1605–1609 (2021).
Acknowledgements
This work is sponsored by the Fundamental Research Funds for the Central Universities. It was also supported by the National Natural Science Foundation of China (No.82271192).
Author information
Authors and Affiliations
Contributions
F.T.: conceptualization, formal analysis, investigation, resources, writing, visualization, funding acquisition; X.L.: software, methodology; Z.W.: data curation; J.L.: validation; K.S.: resources; J.Z.: project administration, supervision. All authors have read, discussed and approved the final version of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, F., Li, X., Wang, Z. et al. Clinical applications of stem cell-derived exosomes. Sig Transduct Target Ther 9, 17 (2024). https://doi.org/10.1038/s41392-023-01704-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01704-0
This article is cited by
-
Generalizable anchor aptamer strategy for loading nucleic acid therapeutics on exosomes
EMBO Molecular Medicine (2024)