Abstract
The incidence of metabolism-related diseases like obesity and type 2 diabetes mellitus has reached pandemic levels worldwide and increased gradually. Most of them are listed on the table of high-risk factors for malignancy, and metabolic disorders systematically or locally contribute to cancer progression and poor prognosis of patients. Importantly, adipose tissue is fundamental to the occurrence and development of these metabolic disorders. White adipose tissue stores excessive energy, while thermogenic fat including brown and beige adipose tissue dissipates energy to generate heat. In addition to thermogenesis, beige and brown adipocytes also function as dynamic secretory cells and a metabolic sink of nutrients, like glucose, fatty acids, and amino acids. Accordingly, strategies that activate and expand thermogenic adipose tissue offer therapeutic promise to combat overweight, diabetes, and other metabolic disorders through increasing energy expenditure and enhancing glucose tolerance. With a better understanding of its origins and biological functions and the advances in imaging techniques detecting thermogenesis, the roles of thermogenic adipose tissue in tumors have been revealed gradually. On the one hand, enhanced browning of subcutaneous fatty tissue results in weight loss and cancer-associated cachexia. On the other hand, locally activated thermogenic adipocytes in the tumor microenvironment accelerate cancer progression by offering fuel sources and is likely to develop resistance to chemotherapy. Here, we enumerate current knowledge about the significant advances made in the origin and physiological functions of thermogenic fat. In addition, we discuss the multiple roles of thermogenic adipocytes in different tumors. Ultimately, we summarize imaging technologies for identifying thermogenic adipose tissue and pharmacologic agents via modulating thermogenesis in preclinical experiments and clinical trials.
Similar content being viewed by others
Background
According to the global epidemiological data from the World Health Organization (WHO), cancer is the first or second leading cause of death in 112 of 185 countries, and it ranks third to fourth and fifth to ninth in an additional 23 and 48 countries, respectively.1 Moreover, cancer is also expected to rank as the single most significant obstacle to prolonging life expectancy in the 21st century globally.2 Making matters worse, the diagnosis and treatment of cancer were hampered by the worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which resulted in health care setting closures and then possibly lead to a temporary drop in cancer incidence followed by an uptick in advanced-stage disease and consequently increased mortality.3,4 For example, cancer screenings for breast and colorectal cancers dropped precipitously at the beginning of the SARS-CoV-2 pandemic, which may affect cancer-related morbidity and mortality.5 More sadly, oncologists state that more than 1.9 million new cancer cases and almost 600 thousand cancer deaths are predicted to occur in the United States in 2022 even without taking into account the shock of the SARS-CoV-2 pandemic.6 Overall, the burden of cancer incidence and mortality is rapidly growing worldwide.
Cancer cells undergo a reprogramming of metabolism to support biomass production and ATP generation and maintain a redox state in contexts where nutrients of the microenvironment are limiting.7,8 To supply pivotal biosynthetic pathways with precursors, the anabolism and catabolism of certain nutrients are upregulated in tumor cells. For example, the Warburg effect was firstly observed in the 1920s, and it occurred in many human tumors.9,10 Classical signaling pathways, multiple intracellular and extracellular proteins, plentiful transcription factors, and key metabolic enzymes participate in the regulation of cancer metabolism, and it constitutes a complex interactive network.11 Given that, the drug targeted metabolism suggests a potential strategy of precise treatment, and some metabolic drugs currently in clinical trials emphasize the potential effectiveness.12 In addition, targeting key regulators involved in metabolic reprogramming can improve response to chemotherapy, radiotherapy, and immunotherapy in various types of cancer.13,14
Adipose tissue is generally considered a metabolically active organ with key roles in the modulation of whole-body energy homeostasis, and impairments of its function are directly associated with a variety of metabolic diseases, including obesity, cardiovascular diseases, type 2 diabetes mellitus (T2DM), and cancer.15,16 Historically, two types of distinct mature adipocytes—white and brown fat cells were recognized to exist in humans. Because of the difference in morphology and function, white adipocytes are major energy storage sites, while brown adipocytes function as burning energy.17 Significantly, another type of adipocytes appears in white fat depots but these fat cells exhibit a substantial capacity for induction of thermogenesis. Given that these adipocytes and classical brown fat derive from different cellular lineages and thus, this specific type of adipocytes is called beige adipocytes, and the process of white-to-beige conversion is called the browning of white adipose tissue (WAT).18,19 Current evidence showed that altered thermogenic fat consisting of brown and beige adipocytes was related to metabolic diseases, such as overweight and T2DM, and targeting thermogenic mechanisms can offer a new therapeutic strategy.20,21
The mask of thermogenic fat abnormality in cancer is being gradually unveiled due to a better understanding made advances in the cellular and functional complexity of thermogenic adipose tissue. However, how thermogenic adipose tissue affects the biological behavior of cancer cells remains obscure. So in this review, we concentrate on clarifying the origin and physiological functions of thermogenic fat as well as its role in tumors and emphasizing the potential clinical application value of thermogenic adipose tissue.
The developmental origin and anatomical location of thermogenic adipocytes
Generally, the defining feature of active thermogenic adipocytes has been the expression of the mitochondrial protein uncoupling protein 1 (UCP1) and multilocular lipid droplet appearance. Therefore, both brown and beige adipocytes belong to thermogenic fat cells. Despite these similarities, beige and brown adipocytes are recognized as two distinct cell types because each of them has unique biological characteristics beyond thermogenesis, suggesting cellular heterogeneity of thermogenic fat22,23,24 (Fig. 1).
The biogenesis of different adipocytes and their characteristics. The three mature adipocytes including white adipocytes, beige adipocytes, and brown adipocytes can derive from progenitors via de novo differentiation. Importantly, white adipocytes can reinstall the thermogenic program by mitochondria biogenesis in response to cold and certain other stimuli. When external stimuli are withdrawn, mitochondria-enriched beige adipocytes transform into dormant adipocytes that resemble white adipocytes. In addition, the three types of adipocytes have distinct morphology and anatomical location. White adipocytes are mostly distributed in white adipose tissue, existing in various subcutaneous and intra-abdominal depots and contributing to the storage and release of energy. Stimulated by beigeing factors, beige adipocytes appear in white adipose tissue sporadically. Compared with beige adipocytes, brown adipocytes are an embryonic-origin cell type and cluster in designated depots like interscapular brown adipose tissue depots of mice and infants. However, both brown and beige fat cells are capable of thermogenesis because they have multilocular lipid droplets and densely packed mitochondria. Part of the HE images in this figure is generated from the Human Protein Atlas. This figure was created on BioRender.com with permission for publication
Origin of brown adipocytes
In mammals, the developmental origin of classical brown fat cells is evolutionally conserved. Concretely, brown adipocytes develop prenatally, and their fate is determined by mid-gestation. It implies that the thermogenic function of brown adipose tissue (BAT) is completely activated at birth, which is of supreme importance to newborn animals because neonates of many larger animals including humans are based on the non-shivering thermogenesis of BAT to maintain normal body temperature.25,26,27 Pulse-chase lineage tracing studies suggest that most brown fat cells originate from precursor cells in the embryonic mesoderm developmentally, and these precursors transiently express somite markers, such as paired-box protein 3 (Pax3), myogenic factor 5 (Myf5), and mesenchyme homeobox 1 (Meox1), engrailed 1 (En1).17,28 In rodents, the major BAT depots are distributed in the interscapular region covering axillary, interscapular, and cervical pads embedded in and around deep back muscles. Some of the BAT depots in humans are anatomically analogous to those in rodents. In addition to the three pads mentioned above, humans also possess BAT depots in four anatomic regions, such as abdominal, mediastinal, supraclavicular, and paraspinal. However, some of the BAT depots regress and are absent in adults, for example, the BAT depot in the interscapular region is most dominant in infants and gradually declines with growing.29,30,31
Origin of beige adipocytes
Although beige adipocytes share many morphological and biochemical characteristics with classical brown adipocytes, they have distinguishing phenotypic and functional features.32 Compared to brown adipocytes, beige adipocytes develop postnatally, and they are a recruitable cell type derived from non-dermomyotome cells in WAT depots.33 It is currently accepted that the recruitment of beige adipocytes occurs in two ways. Primarily, beige fat cells can arise via de novo differentiation from adipocyte progenitors. Then, the rouse of the thermogenic phenotype by dormant cells also contributed to the recruitment of beige adipocytes in WAT depots.15 It is commonly recognized that both direct de novo biogenesis and white-to-beige conversion engaging progenitor cells occur in vivo, with the contribution from each pathway influenced by experimental conditions. For example, the researchers used the model of Adipo-Chaser (inducible adiponectin–Cre lineage tracing) mice and suggested that the majority of beige adipocytes arose from de novo biogenesis when the mice are at thermoneutrality (30 °C) and subsequently exposed to cold (6 °C). By contrast, if the mice are transferred from ambient temperature (20–23 °C) to cold (6 °C), only almost half of the beige fat cells are derived via de novo adipogenesis, with the remaining beige adipocytes generating from the dormant adipocytes.23,34,35 Except for the influence of external cues, the mechanism of recruiting beige adipocytes may vary depending on the heterogeneity of progenitors. As an illustrative example, cold, as the most well-known thermogenic stimulus, can promote de novo beige adipocyte differentiation from α smooth muscle actin (αSMA)-positive stromal progenitor cells through triggering intracellular signaling, including cyclic adenosine monophosphate (cAMP) signaling via stimulation of β-adrenergic receptor (β-AR). By contrast, myogenic PDGFRα + progenitors can be activated by thermal stress in the absence of β-AR signaling and turn to beige fat.36,37,38 In addition to the existence of multiple types of progenitors, beige adipocytes are composed of several subpopulations, such as glycolytic beige fat using mostly glucose as a metabolic fuel, and lipolytic beige fat tending to consume free fatty acids (FAs).39,40 Intriguingly, there is an important phenomenon: the recruited beige fat cells can transform into dormant adipocytes with white fat characteristics morphologically and functionally when the external stimulus is removed, and this process is referred to as beige-to-white conversion.41,42 In terms of the distribution of beige fat throughout the body, beige adipocytes sporadically reside within WAT depots in the postnatal stage. It is universally accepted that beige adipocytes exist mainly in subcutaneous WAT, including anterior subcutaneous and inguinal WAT, and suprascapular fat depots.17,26,43 However, the developmental features of thermogenic fat cells in some anatomical sites remain obscure. For example, perivascular adipose tissue (PVAT) was widely perceived to be brown fat depots. Nevertheless, recent studies suggest that PVAT shows characteristics of beige adipose tissue in humans and BAT-like in mice. More precisely, PVAT is not always brown-like in mice or beige-like in humans, depending on the anatomic location and environmental or metabolic context.44,45
In sum, the reinstallation and loss of thermogenesis in beige fat are adapted to altered external conditions. The mechanism of recruiting beige adipocytes may vary depending on the nature of the stimulus and of heterogeneity beige adipocytes in various metabolic diseases including cancer, which will be infusive areas for future research.
The multifaced roles of thermogenic fat
The most important biological role of brown and beige fat cells is to participate in the process of non-shivering thermogenesis that involves uncoupling protein 1 (UCP1, a characterized thermogenic factor highly expressed in beige and brown adipocytes)-dependent and UCP1-independent mechanisms.46 Beyond the capability of producing heat, thermogenic fat is also a metabolic pool for glucose, lipid, and branched-chain amino acids (BCAAs).47 In addition, the accumulation of brown or beige adipose tissue is coupled with anti-inflammation, anti-fibrosis, and angiogenesis. Moreover, thermogenic fat considered a secretory organism can secrete various molecules to mediate communication with diverse organs and tissues via autocrine, paracrine, and endocrine.48,49 Numerous preclinical studies and comprehensive reviews on the biofunction of thermogenic adipose tissue in physiology have been reported and will be discussed briefly here. Instead, we focus on the influence caused by breaking brown and beige fat homeostasis on metabolic diseases (Fig. 2).
The multiple roles of thermogenic adipocytes in metabolic homeostasis. Thermogenic fat is generally considered a metabolic sink for glucose, lipid, and BCAAs. In addition, brown or beige adipocytes have an impact on neighbor cells by secreting batokines. Moreover, thermogenic adipose tissue is also recognized as an endocrine organ, regulating the gene expression or functions in distant organs, such as the heart, liver, muscle, and brain. BCAA branched-chain amino acids, LNAA the large neutral amino acid transporter, FA fatty acid FATP fatty acid transport protein, NE norepinephrine, β3-AR β3-adrenergic receptor, PKA protein kinase A, HSL hormone-sensitive lipase, LD lipid droplet, TCA tricarboxylic acid, UCP1 uncoupling protein 1, PRDM16 PR domain containing 16, GLUT1/4 glucose transporter 1/4, FGF21 fibroblast growth factor-21, NRG4 neuregulin 4, PLTP phospholipid transfer protein, VEGF vascular endothelial growth factor, Metrnl meteorin-like hormone, NT-3 neurotrophin 3. This figure was created on BioRender.com with permission for publication
Non-shivering thermogenesis
The mechanisms of thermogenesis consist of classical UCP1-dependent and novel UCP1-independent pathways.50 As the name implies, the activation of the former pathway is mediated by UCP1 and relies on the transcription co-regulatory protein PR domain containing 16 (PRDM16).51 The futile metabolic cycling mechanisms consist of Ca2+ cycling, creatine-dependent substrate cycling, and triacylglycerol (TAG) futile cycle are the basis of UCP1-independent thermogenesis, which has no other effect but the consumption of ATP and dissipation of energy.52,53,54,55
Intimately closed to the malfunction of thermogenesis, obesity is often described as a disorder of energy intake and expenditure.56 Up to now, abundant evidence has proved the fact that impaired heat production can give rise to obesity. For example, inhibition of the key transcriptional factor PRDM16 or reduction of UCP1 which is both involved in the UCP1-dependent pathway can drive obesity.57,58 Similarly, in the process of UCP1-independent thermogenesis, suppression of any one of the three futile metabolic cycling mechanisms can cause obesity. For instance, genetic depletion of creatine metabolism in adipocytes impairs diet-induced thermogenesis that limits weight gain in response to caloric excess and then develops obesity.59 Importantly, obesity is a high-risk factor for metabolic diseases, including angiocardiopathy, T2DM, and malignancy.60,61,62 Therefore, induction of the browning process to enhance the capability of thermogenesis in the whole body holds a promising therapeutic potential to combat obesity and its complications.48,63,64
Roles in glucose metabolism
As an important substrate for fueling thermogenesis, glucose can be actively transported into thermogenic adipocytes which is a common characteristic. Typically, glucose uptake in beige and brown adipocytes is stimulated by insulin. In this process, thermogenic adipocytes serve as a cellular ‘rheostat’ that senses the glucose status and contributes significantly to whole-body energy homeostasis.65,66 Given that brown and beige adipocytes can take up glucose positively, 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography (FDG-PET/CT) was used as an effective method to characterize and quantify thermogenic depots volume in humans, and the prevalence of thermogenic adipose tissue assessed by 18F-FDG-PET/CT was highly correlated with a lower incidence of T2DM.60,67,68,69
Insulin stimulation of glucose uptake via glucose transporters in fat tissues is essential for regulating systemic blood glucose levels. Concretely, glucose transporter type 4 (GLUT4) known as the predominant insulin-responsive glucose transporter redistributes within adipocytes in response to insulin, and this net redistribution of GLUT4 from intracellular storage to the plasma membrane is generically called “GLUT4 translocation”.70,71,72 For example, defective GLUT4 translocation or inhibition of GLUT4 in adipocytes induces systemic glucose homeostasis dysregulation and leads to a risk of developing T2DM.73,74 Importantly, thermogenic fat cells can take in more glucose via the GLUT1 than that white fat cells solely rely on GLUT4.17,75 So, given the significant role of thermogenic adipocytes performed in regulating glucose, the impaired capability of thermogenic fat tissue may give rise to metabolic disorders in vivo. Indeed, evidence from an early study demonstrated that genetic deletion of brown adipocytes leads to the development of obesity.76 Besides, researchers found that brown fat transplantation significantly reduces fat mass and enhanced glucose metabolism and insulin sensitivity in mice models.43,77 Similarly, it has been proved that elevated UCP1 levels in human white adipocytes improve glucose uptake by 40%.78 Moreover, a recent study found that PRDM16, the key transcriptional regulator of beige adipocytes de novo synthesis, in SAT was significantly lower in T2DM and prediabetes as compared to the normal glucose tolerance group.79 Consistently, PRDM16 transgenic mice showed enhanced glucose tolerance, high energy expenditure, and limited weight gain in response to high-fat feed.80 Therefore, a wealth of evidence mentioned above suggests that targeting brown or beige fat cells may have therapeutic utility in disorders of glucose homeostasis. For example, treatment with the β3-adrenergic receptor (β3-AR) agonist, such as mirabegron which was approved for treating overactive bladder, can improve glucose and insulin homeostasis.81,82,83
Roles in lipid metabolism
Although thermogenic fat is characterized by a high rate of glucose uptake, FAs are generally recognized as the primary fuel for mitochondrial uncoupling respiration. In general, upon activation by long-chain FAs, UCP1 increases the conductance of the inner mitochondrial membrane (IMM) to make thermogenic adipocytes mitochondria generate heat.30,84,85 As for the source of FAs, it is believed that it is mainly released from WAT rather than de novo lipolysis in BAT which was initially thought to be the primary fuel source.86,87 The BAT takes up FAs via plasma membrane FA transport proteins such as CD36 or FAs transport protein 1 (FATP1), and impairment of FATP1 inhibits FA uptake into BAT and suppresses thermogenesis. By contrast, promoting the translocation of the FA transporters FATP1 and CD36 to the cell membrane can increase FAs uptake into thermogenic adipocytes.88,89 Therefore, 18F-fluoro-thiaheptadecanoic acid (18F-THA)-PET/CT is applied to assess the activity of brown or beige fat tissues.90
Given the fact that the FAs constitute a preferred energetic substrate for most thermogenic adipocytes, increasing evidence has shown that thermogenic fat exerts effects on systemic lipid metabolism. As illustrative examples, BAT activity can control vascular lipoprotein homeostasis by boosting triglyceride-rich lipoproteins (TRL) turnover and channeling lipids into BAT, and this process crucially relies on local lipoprotein lipase (LPL) activity and CD36 in mice models by short-term cold exposure.91 In line with this, BAT after both acute cold and a highly selective β3-AR agonist CL316,243 treatment accelerates intravascular LPL-mediated lipolysis of TRL, and TRL-derived FAs are the most prominent energy source for energy replenishment in BAT.92 More recently, researchers found that endothelial cells of BAT internalize entire TRL particles and follow the endosomal pathway for lysosomal acid lipase (LAL)-dependent hydrolysis.93 Further, impaired thermogenesis of mice showed a remarkable increase in plasma triglyceride levels in response to cold exposure.94 In addition to decreasing pro-atherogenic remnant lipoproteins in hyperlipidemic mice, both cold-induced and pharmacological thermogenic activation confers atheroprotective properties via increasing the levels of high-density lipoprotein (HDL) cholesterol and promoting reverse cholesterol transport.95 Besides, a more recent clinical trial study comes to a similar conclusion that human BAT metabolic activity can be improved after mirabegron treatment chronically and consequently contribute to elevations of the beneficial lipoprotein biomarkers in plasma like HDL and apolipoprotein A1 (ApoA1), as well as total bile acids.96 According to current evidence, activation of brown or beige adipose tissue seems to be beneficial to human health through regulating lipid metabolism. Of note, current research also emphasizes the importance of UCP1-independent thermogenesis based on ATP-dependent futile cycling, and how it impacts the whole-body lipid homeostasis dynamically remains unclear.
Roles in amino acid metabolism
Apart from glucose and FAs, BCAAs including valine, leucine, and isoleucine have also been demonstrated to support thermogenesis in mice and humans, and active BCAAs oxidation is required for optimal thermogenesis in turn.97 As an illustrative example, BAT positively uptake and catabolize BCAAs to produce heat in febrile mice and rats.98 Additionally, BAT actively promotes BCAAs clearance in circulation via utilizing BCAAs in the mitochondria for thermogenesis in mice and humans in response to cold exposure. Moreover, defective BCAAs catabolism in beige and brown adipose tissue causes impaired thermogenesis.97 In line with this, knocking out genes associated with heat production in mice displays an inability to thermoregulate and aberrant BCAAs and FAs metabolism.99 Sadly, several previous studies have suggested that high BCAAs levels in plasm and impaired metabolism of BCAAs are associated with the occurrence of T2DM,100,101,102 partially related to activating the mammalian target of rapamycin complex 1 (mTORC1) and protein kinase Cε (PKCε).103 Remarkably, BCAAs possibly have a significant impact on adipocyte differentiation more than a simple energetic fuel in thermogenic adipocytes.104 Compared to proliferating cells, differentiated adipocyte cells displayed enhanced BCAAs catabolism, and inhibition of BCAAs catabolism compromised adipogenesis.105 More precisely, Sirtuin 4 (SIRT4) enhances BCAAs catabolism by activating methylcrotonyl-coenzyme A carboxylase (MCCC), then increased BCAAs catabolism stimulates peroxisome proliferator-activated receptor-gamma (PPARγ) that is a pivotal regulator of adipogenesis. Further, decreased SIRT4 expression is found in the adipose tissue of diabetic mouse models.106 To sum up, although an interactive network was initially established among thermogenic fat, BCAAs, and metabolic diseases, the underlying mechanisms remain obscure. So to comprehensively understand the roles they play calls for further experiments and clinical trials.
Secretory functions act locally and distantly
Beyond heat generation, solid evidence has suggested that some of the physiological effects of beige and brown adipocytes are mediated by releasing small molecules, defined as batokines. Some of the adipokines released by brown or beige adipocytes have been identified by the transcriptomic, proteomic, and metabolomic analysis, including proteins, lipids, and metabolites. Furthermore, the broad number of articles suggest that these secretory batokines mainly act in an autocrine, paracrine, or endocrine manner to regulate neighboring cells and distant organs.107,108,109,110,111
Generally, batokines can act on cells within the adipose tissue and promote adipogenesis, angiogenesis, neurite outgrowth, and immune cell interactions. In particular, the thermogenic activity of beige and brown fat cells can be enhanced by bone morphogenetic protein-8b (BMP8b), fibroblast growth factor-21 (FGF21), Follistatin-like 1 (FSTL1), and the cytokine interleukin-6 (IL-6), or inhibited by the soluble form of the LDL receptor 11 (sLR11), which are secreted by themselves with autocrine actions.112,113,114,115,116 Additionally, other small molecules secreted by brown or beige adipocytes regulate locally other cell types.117 For example, neurotrophin 3 (NT-3) and vascular endothelial growth factor (VEGF) secreted by thermogenic adipocytes can promote sympathetic innervation and target endothelial cells to induce vascularization of brown and beige adipose tissue, respectively.118,119,120 Besides, thermogenic adipocytes also have an impact on immune cells through releasing meteorin-like hormone (Metrnl) which promotes eosinophil activation to produce IL-4, leading to the recruitment of alternatively activated M2 macrophages and the increased expression of anti-inflammatory gene programs.121,122,123
Beyond these local effects, batokines can impact distant tissues in an endocrine fashion, such as the liver, heart, skeletal muscle, and central nervous system (CNS).110 Recently, plenty of studies have shown that thermogenic adipocytes can target CNS and subsequently regulate systemic energy balance and food intake. Furthermore, batokines such as FGF21 might influence sympathetic nervous system activity and circadian behavior.124,125 As an interactive network, CNS also has an influence on the browning of adipose tissue and facilitating heat generation. As an illustrative example, researchers newly have demonstrated that a population of GABAergic neurons in the dorsolateral portion of the dorsal raphe nucleus (DRN) is capable of regulating thermogenesis through both direct and indirect pathways.126,127 Given current evidence, the browning of fat is beneficial to cardiometabolic. Preclinical experiments have suggested that some of the factors released by thermogenic fat cells such as FGF21 increase cardiac substrate oxidation and protect the heart from hypertensive cardiac remodeling.128 Consistent with this result, a large retrospective study published lately demonstrated that the presence of thermogenic adipocytes is correlated to a low prevalence of cardiovascular diseases, including hypertension, coronary artery disease, congestive heart failure, and cerebrovascular disease.60 Current evidence also suggests the crosstalk between thermogenic adipocytes and hepatocytes through batokines. More precisely, activated thermogenic adipocytes secret neuregulin 4 (NRG4), which then acts on hepatocytes to decrease de novo lipogenesis and protect the liver from damage.129 Alcohol consumption or direct alcohol administration can stimulate BAT to secrete some adipokines, such as adiponectin, which suppresses hepatocyte injury and death.130 Furthermore, a recent study showed that BAT is a major source of exosomal miRNAs in humans and mice, and several microRNAs packaged into extracellular vesicles such as microRNA (miR)-99b may inhibit hepatic FGF21 generation.131 Besides, researchers identified a previously ignored batokine phospholipid transfer protein (PLTP) by using proteomics and transcriptomics in human thermogenic adipose tissue, which regulates metabolism in the liver.132 In addition, there is some documentation of crosstalk between thermogenic adipose tissues and skeletal muscle in mammals. For instance, the FA derivative 12,13-dihydroxy-(9Z)-octadecenoic acid (12,13-diHOME) is increased within beige and brown fat in response to exercise or cold exposure to enhance thermogenesis, leading to the enhanced FAs uptake and oxidation of myocytes in an endocrine manner.88,133 Moreover, loss of interferon regulatory factor 4 (IRF4) induces myogenic gene expression like the secreted factor myostatin in BAT, a classical muscle mass negative regulator that results in impaired mitochondrial function and diminished exercise capacity.134
Collectively, these publications have identified plenty of candidate batokines from mammals, and some of them remain mysterious. Further research is warranted into this aspect to comprehensively understand the secretome of beige or brown adipocytes, and describe the action mode of each of these molecules in every single metabolic disease, including malignancy which is seem to be forgotten by researchers studying thermogenic fat.
The regulation of thermogenic fat in metabolic diseases
Currently, it is widely accepted that the activity of thermogenic adipose tissue declines during the development of metabolic disorders.29,135 Therefore, thermogenic fat is emerging as an attractive and promising target for therapeutic intervention in metabolic diseases like obesity and T2DM, because of its capacity to utilize glucose and lipids for thermogenesis and release molecules locally or distantly that contribute to a hypermetabolism state of the whole body.
Obesity
Obesity is defined by the WHO as excessive fat accumulation that might impair health and is diagnosed at a body mass index (BMI) ≥30 kg/m2.136 The prevalence of obesity has already reached pandemic levels worldwide, thereby substantially increasing the risk of other metabolic diseases such as T2DM, fatty liver disease, hypertension, and several cancers.137 These non-communicable diseases(NCDs) lead to a lower standard of living and a decline in life expectancy.138 In terms of pathogenesis, the fundamental cause of obesity is a long-term energy imbalance between too many calories intaked and too few calories expended. Examples, overeating (like emotional eating, peer pressure, snacking), low energy expenditure (like aging, neuroendocrine factors, sarcopenia, and medications), or physical inactivity (like chronic fatigue, low fitness level, emotional barriers, and joint pain) can influence the chronic positive energy balance, thus subsequently causing obesity.139,140 Besides, obesity might be considered a heritable trait. The heritability of BMI has been estimated as 30–40% based on a recent study.141 Furthermore, some investigations found that loss of function in genetic levels for leptin, leptin receptor, melanocortin 4 receptor, and others might cause severe obesity in humans.142,143 Therefore, obesity most likely results from a concerted interplay of genetic and environmental factors.
During the development of obesity, adipose tissue can expand by de novo synthesis and enlargement of existing adipocytes, whereas the mass of activated thermogenic fat is decreased. Compared with white fat, brown and beige adipose tissue is beneficial for combating obesity by enhanced lipolysis of triglycerides and the oxidation of FAs.144,145 Previous research discovered that expression of thermogenic genes reduced and adipocytes shifted from multilocular to unilocular appearance in mice fed a high-fat diet compared to mice fed a normal diet.146 Consistently, investigators found that lean men had more activated BAT than obese men by using 18F-FDG-PET/CT.29 The reason for the reduced thermogenesis in obesity is that B lymphocytes and macrophages infiltrate thermogenic fat, and subsequently suppress UCP1 expression via releasing tumor necrosis factor-α (TNFα).147,148,149 In turn, impaired thermogenesis of adipose tissue can drive obesity. Give an illustrative example, selectively genetic depletion of glycine amidinotransferase (GATM) in fat (Adipo-Gatm KO) or the cell surface creatine transporter (CRT) in fat (AdCrTKO) substantially reduces adipocyte creatine, inhibits thermogenesis and energy expenditure, then driving obesity as a consequence.59,150 Consistently, adipocyte-selective inactivation of creatine kinase B (CKB, is indispensable for futile creatine cycling-based thermogenesis) in mice disturbs glucose homeostasis and diminishes thermogenic capacity, subsequently increasing predisposition to obesity.55 Therefore, stimulating the thermogenesis of adipose tissue is an effective approach with which to curb obesity.151 As a classical external clue, cold can upregulate thermogenic gene expression of human fat via inducing the secretory of irisin and FGF21.152 Besides, the cold-induced 12,13-diHOME increases FAs uptake into brown adipocytes by promoting the translocation of the FA transporters FATP1 and CD36 to the cell membrane, which resulted in decreased levels of serum triglycerides.88 In addition to cold, exercise also can induce WAT lipolysis and BAT thermogenesis in vivo by increasing the plasma level of the tricarboxylic acid cycle intermediate α-ketoglutaric acid (AKG) which is also negatively correlated with BMI. Mechanically, the AKG stimulates the secretion of adrenaline through 2-oxoglutarate receptor 1 (OXGR1) expressed in adrenal glands and causes muscle hypertrophy and fat loss consequently.153 Moreover, refeeding-induced mesencephalic astrocyte-derived neurotrophic factor (Manf) curbs diet-induced obesity by directly promoting the browning of adipocytes via the p38 MAPK pathway.154 Of note, a variety of phytochemicals have been shown in the literature to counteract weight gain via adipose thermogenesis.155 Currently, investigators identified phytochemical hyperforin (HPF) as an agent to combat obesity via using the Connectivity Map (CMAP) approach. More specifically, they found that HPF directly targeted dihydrolipoamide S-acetyltransferase (DLAT) and thereby enhanced the capacity of heat generation by activating AMPK and PGC1A.156 However, stimulating the thermogenesis of the whole body has limited applications because of the potential risk of cardiovascular diseases and other complications caused by hypermetabolism.157,158 Thus, safer approaches to achieving beige fat activation are required. Polyethylene glycol (PEG)-crosslinked polydopamine nanoparticle (PDA) is a safe and injectable photothermal hydrogel that converts near-infrared (NIR) light input into accurately controlled temperature output.159,160 Recently, researchers achieved local hyperthermia of fat in vivo by using PEG-PDA hydrogel and treated obesity without adverse effects as a consequence. Mechanically, local hyperthermia activates heat shock factor 1 (HSF1), and enhanced HSF1 regulates Hnrnpa2b1 transcription, consequently increasing the mRNA stability of key metabolic genes.161 In conclusion, the manipulation of thermogenic activity in adipose tissue is regarded as a potential strategy in the treatment of obesity.
T2DM
T2DM is arguably one of the largest epidemics ever seen globally, leading to the ninth major cause of death. The number of people with T2DM has doubled in the last several decades and is projected to rise further to almost 700 million by 2045.162,163 Worryingly, the incidence and prevalence of T2DM in younger adults (aged <40 years) have risen sharply since the 2000s.164 Young-onset T2DM presents a more aggressive disease phenotype because it has a more severe and rapid deterioration of β-cell function and is more likely to develop complications.165 The reasons for the escalating epidemic of T2DM are multiple, including population aging, economic development, a sugar-rich diet, and a sedentary existence.166 For example, the incidence of T2DM increases proportionally with BMI.167 Besides, large prospective studies have shown that the rise in body weight over time dramatically fuels the prevalence of T2DM.168 Consistently, BMI exceeding the upper limit (25 kg/m2) was associated strongly and positively with mortality attributed to diabetes.136 In conclusion, substantial evidence directly points out that adiposity has an impact on the development of T2DM.169
Accumulation of excess WAT is detrimental to metabolic health, while the activation of thermogenic fat has a beneficial influence on diabetes.170 Indeed, plenty of research has demonstrated that glucose uptake is increased in activated thermogenic fat.67,135 Moreover, cold-induced activation of thermal adipose tissue improves overall glucose disposal and insulin sensitivity.171,172,173 Give an illustrative example, overweight men with T2DM who received a short-term cold acclimation appeared to enhanced thermogenic activity and improved whole-body insulin sensitivity.172 In general, thermogenic adipose tissue intakes glucose from circulation, contributing to glucose clearance and the decreased demand for insulin secretion by β-cells.174 Mechanically, boosting glucose uptake into brown or beige adipose tissue consists of two different mechanisms: insulin-dependent and insulin-independent. Insulin is a major regulator of glucose uptake in most tissues, including BAT, WAT, and skeletal muscle glucose. However, heat production-related glucose uptake into thermogenic fat has been suggested to be independent of insulin signaling, primarily dependent on GLUT1 transporter in an adrenergic-promoted manner.175,176,177 In addition to decreasing glucose directly, peripheral lipid clearance also indirectly benefits β-cells and aids in restoring peripheral insulin sensitivity.178 Collectively, beige and brown fat confers beneficial effects on glucose metabolism and insulin sensitivity, which may be used as an underlying therapeutic target for the treatment of T2DM.
Cardiovascular diseases
Non-communicable diseases (NCDs) are the leading cause of death and ill health, which account for seven of ten deaths around the world.179 Of the NCDs, cardiovascular disease (CVD) is now the leading cause of mortality and morbidity worldwide.180 In China, CVD causes 40% of deaths in the Chinese population.181 Over 95% of all CVD deaths are attributable to six conditions: ischemic heart disease (IHD), stroke, heart failure, cardiomyopathy, rheumatic heart disease (RHD), and atrial fibrillation (AF).182,183 Of note, the incidence of CVD has increased among young adults (defined in general as individuals aged 18–45 years) in the past 2 decades, largely because of a high prevalence of risk factors for CVD, such as obesity, physical inactivity, and poor diet.184 Indeed, obesity contributes directly to the incidence of cardiovascular risk factors like dyslipidemia, T2DM, and hypertension. Further, obesity also leads to CVD mortality independently of other cardiovascular risk factors.185 Based on epidemical data, the prevalence of obesity was responsible for 4 million deaths in 2015, with two-thirds of this number attributed to CVD.186 Specifically, CVD caused by obesity is closely related to excess adipose accumulation and metabolic abnormalities. As an active endocrine and paracrine organ, excessive adipose tissue releases multiple hormonses and cytokines, such as leptin, adiponectin, IL-6, and TNF-α, which result in diabetes, cardiovascular inflammation, increased blood pressure level, fibrinolysis, and atherosclerosis.187,188,189 Accordingly, physical activity can attenuate the adverse effects of obesity on CVD events.190
Generally, thermogenic fat potentially exerts beneficial metabolic and cardiovascular effects through stimulating energy expenditure, attenuating cardiac remodeling, and suppressing the inflammatory response.191,192,193 Firstly, the activation of thermal fat plays a protective role in the vascular system. In animal studies, increased BAT activation stimulated by beta-adrenaline can diminish the progress of hypercholesterolemia and protect from atherosclerosis development in mice with hyperlipidemia.39 Consistently, researchers used 18F-FDG-PET/CT to evaluate the relationship between 18F-FDG uptake in supraclavicular BAT to arterial inflammation and subsequent CVD events in humans, and the results suggested that increased supraclavicular BAT activity is inversely associated with arterial inflammation.194 In addition, PGC1A can not only assist carbon monoxide to complete vasodilation but also regulate vascular senescence negatively.195,196 Remarkably, most vessels in the body are surrounded by PVAT which shares thermogenic adipose-like properties like high mitochondrial density and increased expression of UCP1.197 In a previous study, investigators found that activation of PVAT during cold acclimation improved endothelial dysfunction and attenuated the development of atherosclerosis.198 In line with it, knockdown of BMP4 (which transforms white adipocyte to beige adipocyte) in PVAT inhibited the expression of thermogenic genes and aggravated endothelial inflammation in a co-culture system. Accordingly, overexpression of BMP4 in adipose tissues enhanced the thermogenic activity of PVAT and protects against atherosclerosis in mice.199 Moreover, the PVAT also secretes multiple vasorelaxant factors such as adiponectin,200 gasotransmitters hydrogen sulfide,201 nitric oxide,202 and palmitic methyl ester,203 and these vasorelaxant factors can alleviate blood pressure in the microcirculatory system. Of note, epicardial adipose tissue (EAT) is part of the VAT that surrounds the heart, excessive accumulation of whom is considered a risk factor for the incidence of coronary artery diseases.204,205,206 Currently, multiple reports of UCP1, PGC1A, and PRDM16 expression have established their presence in human EAT, which functionally corresponded with downregulation in the production of reactive oxygen species and immune responses. Furthermore, thermogenic genes expressions in EAT were recongnized as protective factors against coronary artery disease and heart failure with reduced left ventricular ejection fraction.207,208,209 Secondly, brown or beige adipose tissue are capable of improving cardiac remodeling. By contrast, mice with genetic ablation of UCP1 is susceptible to hypertension, cardiomyopathy, and fibrosis.210 In line with it, compared with the control group, UCP1 knock-out mice displayed fibrosis, augmented myocardial injury, and decreased survival rates when isoproterenol was administered. Interestingly, the cardiac parameters and survival of UCP1 knock-out mice were improved after receiving a BAT transplant from the controls.211 The molecular mechanism underlying the role of BAT in pathological cardiac remodeling is mediated by FGF21. Specifically, activation of the adenosine 2 A (A2A) receptor in thermogenic adipocytes can attenuate hypertensive cardiac remolding by inducing themselves to release FGF21.128,212 Incidentally, factors like natriuretic peptides produced by the heart could regulate the thermogenic activity of adipocytes via the cyclic guanosine monophosphate (cGMP)–protein kinase G (PKG)–p38 signaling pathway.213 Finally, thermogenic fat can cause a decline in the incidence of CVD and associated adverse events by reducing glucose and lipid levels.214 According to the characteristics of thermogenic fat, it can be speculated that stimulating the browning of adipose tissue in a specific region can be protective for cardiovascular system.
The crosstalk between thermogenic adipose tissue and cancer
There is growing evidence to indicate that the dysregulation of adipose tissue is closely linked with metabolic diseases in rodents and humans, such as T2DM, obesity, fatty liver, and pancreatitis. Besides, some of them have been considered high-risk factors for multiple tumors. Recently, there is a systematic understanding that WAT is capable of promoting tumor growth, metastasis, and chemoresistance.16,215,216,217 In short, WAT generally accelerates tumor progression through the following three pathways. Firstly, white adipocytes fuel tumor growth by providing nutrients like FAs and glutamine.218,219 Secondly, inflammatory adipokines secreted by WAT, such as IL-6, leptin, and adiponectin, can influence STAT3, AKT, JNK, and MEK/ERK signal pathways in cancer cells directly, consequently upregulating the expression of proliferation and invasion-related genes.220,221,222 Finally, adipocytes are also involved in building a beneficial microenvironment for cancer cells. For example, adipocytes can decrease the natural killer cell toxicity, activate the inflammatory phenotype of macrophages, and promote fibrosis and vascularization in tumors.223,224,225,226 But compared to the non-thermal adipocytes, the pathophysiological mechanisms of brown or beige adipocytes that regulate malignant biological properties remain elusive in multiple types of cancer (Fig. 3).
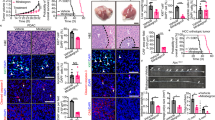
The role of thermogenic adipose tissue in malignancy. Polypeptides, metabolites, or other certain mediators derived from cancerous cells initial the conversion of white-to-beige locally and distantly. On the one hand, activated beige adipocytes in SAT lead to lipolysis and energy expenditure, subsequently contributing to cancer-associated cachexia. On the other hand, the adjacent thermogenic fat can directly promote tumor growth and metastasis by secreting specific molecules, such as lactate. Accordingly, pharmacologically inhibiting the browning of white adipose tissue slows cancer progression and improves the outcomes for patients. ZAG zinc-α2-glycoprotein, PTHRP parathyroid-hormone-related protein, LIF leukemia inhibitory factor, GDF15 growth differentiation factor 15, ADM adrenomedullin, IL-6 the cytokine interleukin-6, β3-AR β3-adrenergic receptor, TKI tyrosine kinase inhibitor, Arctii Fructus the extract of Arctium lappa. This figure was created on BioRender.com with permission for publication
Cancer cachexia
Cancer-associated cachexia is a multifactorial syndrome characterized by weakness, loss of fat, and muscle wasting, which is driven by a variable combination of reduced food intake and metabolic changes, such as elevated energy expenditure, excess catabolism, and systemic inflammation. Moreover, cancer cachexia has commonly been considered the main inducement of complications in patients with malignancy, leading to reduced quality of life and poor outcomes.227,228,229,230 Over the years, plenty of evidence has shown that thermogenic fat contributes to cancer cachexia owing to its key role in heat production and energy balance.231,232 Brown or beige fat is an active tissue associated with hypermetabolism because of the high presence of UCP1, which leads to thermogenesis and energetic inefficiency. Thus, a switch from white to thermogenic fat contributes to cancer cachexia by increasing systemic energy expenditure. Notably, the WAT browning generally takes place during the initial steps of cancer cachexia, preceding the loss of muscle.233,234,235 During cachexia progression, the process of WAT browning can be triggered by pro-inflammatory mediators including IL-6 and the lipid-mobilizing factor zinc-α2-glycoprotein (ZAG),219,236,237 or by tumor-derived compounds like parathyroid-hormone-related protein (PTHRP),238,239,240 miR‐146‐5p,241 leukemia inhibitory factor (LIF),242 growth differentiation factor 15 (GDF15),243 or by sympathetic secretion, such as catecholamine.244 According to the fact that WAT browning usually occurs in the early stage of cancer cachexia, the biomarkers involved in browning are capable of becoming novel biochemical indicators for prognosis or potential targets for treatment.
The adipose tissue microenvironment in cancer development and progression
The adipose tissue microenvironment (ATME) consists of multiple cell types, such as adipocytes, stromal cells, immune cells, vascular endothelial cells, and fibroblasts.245 Importantly, there’s plenty of evidence that ATME is associated with a variety of metabolic diseases, including malignancy.246,247 Based on currently available evidence, the ATME promotes tumor initiation and progression in multiple ways. First, adipocytes can secrete nutrients and adipokines into the microenvironment, contributing to cancer cell proliferation and invasion. Give an illustrative example, leptin released into ATME by white adipocytes can blind to the leptin receptor which is highly abundant in many tumors, then synergize with plenty of different oncogenes, cytokines, and growth factors by impacting the JAK-2/STAT, PI3K/AKT-1, and MAPK/ ERK1/2 signaling pathways.248,249 Second, it has been proved that adipose tissue accelerates cancer development and enhances tumor resistance to chemotherapy or immunotherapy through impinge on immune cells in the microenvironment. For instance, adipocytes release cellular contents into the microenvironment through pyroptosis or necrosis in response to external clues like hypoxia or low pO2 and mechanical stress, then triggering the accumulation of phagocytic macrophages consequently.250,251 Of note, their accumulation is frequently correlated to poor outcomes and therapeutic resistance in cancer patients.252,253,254 In addition, adipocytes are capable of recruiting other inflammatory cells by secreting chemokines into ATME, such as monocyte chemoattractant protein 1 (MCP1) and TNF.255 Next, infiltrating inflammatory cells like mast cells produce multiple proteases such as cathepsin S, leading to cancer progression and chemotherapy resistance. Besides, the elastase of neutrophils causes insulin resistance and elevated levels of free insulin by cleaving insulin receptor substrate 1 (IRS1), contributing to enhanced phosphoinositide 3-kinase (PI3K) signaling within tumor cells.256,257,258 Third, adipocytes can modulate endothelial cells and promote tumor vascularization to accelerate cancer progression. On the one hand, adipocytes in ATME strongly support tumor growth and enhanced angiogenesis by releasing particular molecules directly in hepatocellular carcinoma. On the other hand, adipocytes recruit and activate macrophages through a CCL2/IL-1β/CXCL12 signaling pathway, and activated macrophages in turn promote stromal vascularization and angiogenesis.259,260 Notably, the survival time of patients with breast cancer who are treated with anti-vascular endothelial growth factor (VEGF) is not extended. Potential mechanisms may include the upregulation of IL-6 and/or FGF-2.261 In addition, vascular inflammation caused by adipokines like leptin leads to elevated permeability, which is indispensable for cancer metastasis.262,263 Finally, adipocytes can affect the biological behavior of carcinoma by modifying the extracellular matrix (ECM) structure which is characterized by a high degree of fibrosis in ATME.264 Typically, the researchers observed an abundant fibrotic response in tumor areas that were enriched in adipocytes or located adjacent to adipose tissue. Moreover, the interaction between pancreatic stellate cells and adipocytes promotes matrix remodeling and impairs vascular perfusion, leading to tumor growth and ineffective chemotherapy.265 Given that, the multiple roles of white fat microenvironment have been described by many investigations during tumor initiation and progression. Nevertheless, how the thermogenic adipocytes in ATME affect tumor development and metastasis is mysterious. To make it clear, we may need to identify subpopulations of adipocytes in tumor-associated ATME by single-nucleus sequencing (sNuc-seq) or single-cell RNA sequencing (scRNA-seq) technique.
The roles of thermogenic adipocytes differ in metabolism-related cancers
The interaction (except cachexia) between thermogenic adipose tissue and cancer cells differs in tumor types. Nowadays, our understanding of it is limited but promising, which is expected to provide a novel strategy for diagnosis, therapy, or prognosis.
Liver cancer
Hepatic carcinoma is the fourth leading cause of cancer-related mortality worldwide, which generally arises in a background of hepatitis and cirrhosis. Primary liver cancer commonly consists of hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (iCCA), and other rare tumors like hepatoblastoma and fibrolamellar carcinoma. On the basis of recent epidemic data, HCC alone accounts for 80–90% of primary liver cancers, and iCCA accounts for 10–15%.266,267,268 It is generally recognized that chronic inflammation is the most important contributor to the incidence and development of primary liver cancer. These chronic inflammation originate from hepatitis B or C virus (HBV or HCV) infections, metabolic disorders (including excess body weight, diabetes, impaired glucose tolerance, metabolic syndrome, alcoholic steatohepatitis (ASH), and nonalcoholic fatty liver disease (NAFLD), smoking, chronic toxin exposure, and parasite infection, and the majority of them are potentially modifiable risk factors.269,270 Notably, although vaccination programs and antiviral therapies have led to a decrease in HCC incidence, NAFLD has become the fastest-growing cause of HCC owing to the increasing prevalence of obesity.271 NAFLD is diagnosed using either imaging or a liver biopsy assessment with the presence of steatosis in more than 5% of hepatocytes in individuals, who consume little or no alcohol (<30 g per day for men and <20 g per day for women)and without metabolic risk factors (for example, obesity and type 2 diabetes) or other chronic liver diseases(for example, Wilson’s disease, congenital or acquired lipodystrophy).272,273 In general, NAFLD encompasses a disease continuum from nonalcoholic fatty liver (NAFL, the non-progressive subtype of NAFLD) to nonalcoholic steatohepatitis (NASH, the progressive subtype of NAFLD), which is characterized by necroinflammation and faster fibrosis progression than NAFL. Recently, there are some great reviews summarizing the pathogenetic mechanisms of the transition from NAFL to NASH and even HCC.274,275,276 Thus, we put more emphasis on the key roles of thermogenic adipocytes in the dynamic transformation process from NAFLD to HCC. According to recent investigations, thermogenic fat is induced to increase the energy expenditure of the whole body, and then alleviate obesity and hepatic steatosis simultaneously.277,278,279 Give some examples, exercise training is the most effective strategy to prevent obesity and NAFLD, partially because β-aminoisobutyric acid secreted from myocytes promotes a switch from WAT to thermogenic phenotype and enhances fatty acid β-oxidation in hepatocytes both through the PPARα signaling pathway, and perhaps IL-6-induced upregulated thermogenesis also play a part in alleviating NAFLD.280,281 In addition to aerobics, substantial strong evidence proved that some natural plant extracts could attenuate NAFLD by enhancing adipocytes browning and energy metabolism, such as the platycodon grandiflorus root (a Korean medicinal food, increases the expression of thermogenic genes like SIRT1, PPARα, PGC1A, and UCP1, which accompanied changes in fatty acid oxidation and energy expenditure),282 pomegranate seed oil (increases the levels of thermogenic genes and hepatic HO-1, along with decreased inflammatory adipokines and hepatic fibrosis),283 the Diospyros kaki fruit and Citrus unshiu mixture (activates fatty acid β-oxidation and thermogenesis, and inhibits lipogenesis and cholesterol synthesis via suppression of sterol regulatory element-binding protein 1 (SREBP-1) and SREBP-2 and its target genes),284 palmitoleic acid (increase lipid metabolism in adipocytes),285 cyanidin-3-O-β-glucoside (the most abundant monomer of anthocyanins, reduces adipokines secretion and lipid accumulation in HepG2 cells),286 the ARPS (polysaccharides from Anoectochilus roxburghii (Wall.) Lindl, promotes fat thermogenesis via the AMPK/SIRT1/PGC1A signaling pathway).287 Consistent with the above findings, activating thermogenesis in the protein level like the voltage-dependent anion channel 1 (VDAC1)-based peptide R-Tf-D-LP4 (a mitochondrial protein with multiple functions, like regulating cellular metabolism and energy expenditure) or disrupting the thermogenic suppressor gene nuclear receptor-interacting protein 1 (NRIP1, suppress glucose transport, fatty acid oxidation, mitochondrial respiration, UCP1 expression) by CRISPR enhances lipid metabolism in the liver, offering a promising therapeutic approach for liver steatosis.288,289 Conversely, the impairment of thermogenesis leads to metabolic disorders and liver steatosis.290,291,292 Of note, UCP1 in brown or beige adipocytes can be leveraged to antagonize inflammation of NAFLD. Mechanically, increased succinate in liver tissue drives inflammation through blinding to succinate receptor 1 (SUCNR1) in liver-resident stellate cell and macrophage populations and activated thermogenic adipocytes in mice protect against SUCNR1-dependent inflammatory infiltration in the liver.293 In conclusion, thermogenic adipocytes prevent the progression of NAFLD and thereby reduce the occurrence of HCC through increasing energy expenditure and suppressing liver inflammation. By contrast, the crosstalk between thermal adipocytes and hepatic cancer cells is barely revealed. Just one previous research found that the thermogenesis signaling pathway was upregulated in HCC patients without fibrosis by functional enrichment analysis, which might predict survival in HCC patients.294 Thus, comprehensive investigations on the interaction between thermal adipocytes and liver cancer cells are urgent and meaningful.
Renal cell carcinoma
Renal cell carcinoma (RCC) denotes cancer that originated from the renal epithelium and accounts for more than 90% of cancers in the kidney. Epidemiologically, represents the sixth most frequently diagnosed cancer in men and the tenth in women, respectively, and its incidence rates have been increasing. Based on histological and cytogenetic signatures, RCC is divided into several subtypes. Approximately 80% of RCC individuals are diagnosed with clear cell RCC (ccRCC), and up to a third of cases will present with or develop metastases.295,296,297 Of note, ccRCC can invade into surrounding perinephric adipose tissue (PAT), which distributes between the renal capsule and renal fascia, and this process is associated with some adverse perioperative outcomes and poor prognosis.298,299,300 Given that cancers are capable of reprogramming noncancerous neighboring cells to fuel tumor growth via providing additional nutrients, the potential protumorigenic relationship between ccRCC cells and PAT has been gradually studied in recent years. For example, recent research has shown that melatonin promotes tumor slimming and suppress tumor progression by activating transcriptional coactivator PGC1A and lipid browning programs.301 In agreement with this finding, PRDM16 is epigenetically silenced in RCC, and restoration of PRDM16 represses tumor growth. Specifically, researchers used RNA-Seq analysis to find that PRDM16 disrupts the transcriptome of cancer cells like semaphorin 5B (SEMA5B), which is a hypoxia-inducible factor (HIF) target gene highly expressed in RCC that promotes in vivo tumor growth.302 However, a new study has drawn the opposite conclusion that ccRCC promotes adipocyte browning to enhance tumor growth. More precisely, ccRCC cells activate PAT browning through the secretion of PTHRP, then the thermogenic adipocytes increase lactate secretion and promote ccRCC cell proliferation. Notably, tyrosine kinase inhibitors (TKI) often used to treat ccRCC, such as sunitinib, have been shown to activate adipocytes browning, and the combination therapy of TKI plus browning inhibitor present a more-complete suppression of ccRCC.303 The mentioned above result seems to be controversial for now, but it will be explained clearly after systematically analyzing transcriptomics and metabolomics of cancer cell-reprogrammed adipocytes and comprehensively clarifying how browning of adipose tissue impacts other members in the tumor microenvironment (TME), such as immune cells.
Pancreatic cancer
Pancreatic cancer (PC) is one of the most serious diseases which has a poor outcome and its five-year survival rate remains less than 10% in the U.S. and 7.2% in China, which is often due to a lack of early detection and effective treatment. Besides, extensive research has shown that PC also ranks 4th and 6th the cancer-associated deaths in the U.S. and China, respectively. What’s more, PC has an increasing incidence of 13 per 100,000 people per year, and it is projected to become the second leading cause of cancer-related deaths in the U.S. by 2030.2,6,304,305 Multiple evidence has shown that obesity and T2DM are modifiable risk factors associated with the development of PC, which implies that the metabolic abnormity of the whole-body fat contributes to tumor progression.265,306,307 Previously, researchers found a phenotypic switch from WAT to brown fat in Kras-pancreatic cancer mice, which was associated with high-expressed UCP1 caused by chronic inflammation and IL-6.234 Consistent with this finding, a recent study described 3 phases of metabolic and soft-tissue changes before PC diagnosis, and the reduction of subcutaneous adipose tissue (SAT) was observed during phases 2 and 3 (start 18 months before PC identification). In addition, SAT wasting was likely related to the browning of adipocytes, because overexpression of UCP1 in SAT exposed to PC exosomes was tested in mice and patients with PC.308 Mechanically, the browning of SAT may be induced by exosomal adrenomedullin shed from cancer cells via activating the p38 and the extracellular signal-regulated kinase (ERK)1/2 and the mitogen-activated protein kinases (MAPKs) signaling axis.309 Of note, it is commonly believed that thermogenic fat can accelerate the intake of glucose and improve insulin resistance in obese and T2DM. However, hyperglycemia is detected in quite a few PC patients before or after the diagnosis of PC, although the browning of SAT is considered a phenomenon of decreasing blood glucose.310,311,312 Revealing the underlying mechanism of this seemly controversial phenomenon promisingly provides a novel view for new-onset diabetes in patients with PC. In addition to SAT, pancreatic fat accumulation is linked to chronic pancreatitis, pancreatic neoplasms, disturbed glucose metabolism, and impaired insulin secretion. Given that some cancers have been observed to promote browning of adjacent WAT, whether the browning of intrapancreatic adipose tissue occurs and the crosstalk between brown pancreatic fat and tumor required further research.215,313,314
Breast cancer
Breast cancer (BC) is the most common cancer diagnosed in many countries including the US (excluding skin cancers) and China, which is the second leading cause of cancer death among women after lung cancer and remains the primary tumor-associated cause of disease burden for women. In addition, it is important to notice that BC is also the most commonly diagnosed cancer type in young adults aged 30 to 39 years.315,316,317,318 In line with PC, overweight and obesity are highly related to the prevalence of BC in postmenopausal females. Moreover, the breast adipose tissue is impacted by surrounding cancer cells, and vice-versa modifies the TME in favor of cancer via browning of WAT.319 For example, a recent study demonstrated that markers for BAT and beige adipocytes were highly expressed in BC xenografts, implicating that thermal characteristics could play a vital role in BC progression.320 In agreement with this finding, newly published articles have suggested that BC mammospheres can secrete adrenomedullin to induce the browning of adjacent adipocytes and lipolysis. Furthermore, activated thermogenic adipocytes in the breast can modulate the behavior of mammary epithelial cells and promote tumor progression in both tumor and non-tumor mice.321,322 In addition, a large cohort clinical trial showed that chemotherapy harmed the activity of BAT, which maybe explain why weight gain during chemotherapy.323 In conclusion, comprehensively understanding the interaction between BC cells and adjacent adipose tissue will path a novel way to combat breast tumor progression.
Gastric cancer
Tumors derived from the stomach are a global health problem, with more than 1 million people newly diagnosed with gastric cancer (GC) worldwide each year. Although chemotherapy, radiotherapy, surgery, and immunotherapy all have proven efficacy in GC, it does represent the third most common cancer-related death worldwide and is responsible for >700,000 deaths annually.324,325,326 There are various factors contributing to tumorigenesis of the stomach, including Helicobacter pylori infection, lifestyle factors, and genetic risk factors.327 Recently, substantial preclinical experiments and clinical trials have demonstrated that adipose tissue in a particular position (such as SAT) is associated with the prognosis of GC patients.328,329,330,331,332 However, the function of WAT browning in GC was reported by merely several articles. For instance, researchers found that exosomes released from GC cells could deliver circular RNAs-133 into preadipocytes, promoting the differentiation of preadipocytes into mature adipocytes with thermogenic phenotype via stimulating PRDM16 and suppressing miR-133.333 Similarly, a new study has suggested that exosomal miR-155 from GC suppresses adipogenesis and enhances brown adipocytes differentiation in adipose mesenchymal stem cells via CCAAT/enhancer-binding protein (C/EBP) β, which causes cancer-associated cachexia.334 In general, more research is needed for understanding the impact of browning on other GC phenotypes.
Colorectal cancer
Colorectal cancer (CRC) is the main contributor to global cancer mortality, accounting for roughly 1.9 million new cases and 0.9 million deaths per year worldwide. Optimistically, the incidence and mortality are gradually stable and even slightly declined in developed countries owing to nationwide screening programs and increased uptake of colonoscopy in general. Nevertheless, new cases of early-onset CRC (generally defined as CRC diagnosed before the age of 50 years) have recently been increasing globally, which exhibits different clinical manifestations, pathological characteristics, and molecular features compared to later-onset CRC patients. As with most cancers, body fat and obesity are modifiable risk factors increasing CRC incidence.335,336,337,338 Although the biofunction of thermogenic adipocytes is less well understood, it has engaged researchers’ interest recently. Give an example, they find that intestinal disease tolerance is preferentially established in thermoneutral mice, protecting them from injury-induced colitis and inflammation-induced colon cancer. Besides, the underlying mechanism is mediated by an unexpected crosstalk between thermogenic adipocytes and intestinal epithelial cells.339 In addition, more direct evidence has shown that the expression of UCP1 is significantly associated with better overall survival of CRC in a cohort study.340 Furthermore, a signature consisting of six biomarkers, including UCP1, signal transducer and activator of transcription 1 (STAT1), p-cofilin, LIM domain kinase 2 (LIMK2), the Forkhead transcription factor family member 3 (FOXP3), and inducible co-stimulator (ICOS), was identified as the best combination in terms of prognostic power.341,342,343 However, it is temporally unclear how thermogenic signaling improves the overall survival of CRC patients, so further study is necessary.
The clue of thermogenic adipose tissue is indirect and scattered in the rest of the cancers, thus which will not be discussed here. Collectively, cancer cachexia is the most common and mechanically clear impact caused by WAT browning. In contrast, the local inter-communication between thermogenic adipocytes and other cells in TME-like immune cells is unrevealed. Therefore, future research on this topic should be encouraged.
Clinical applications
Findings over the past two decades have comprehensively described the multiple functions of thermogenic adipocytes in mice and humans. Significantly, their abnormal activity is closely related to cancer and other metabolic diseases. Accordingly, investigators have been actively searching for effective tools and pharmacologic agents to identify and manipulate activated thermogenic fat, respectively, holding promise for combating malignancy and metabolic disorders clinically.
Identification of thermogenic fat
Different from WAT, thermogenic fat has distinctive features of ontogeny, bioenergetics, and physiological functions. These characteristics provide an opportunity to differentiate thermogenic fat from WAT by using imaging tools.344 Here, several common imaging methods for the assessment of thermogenic fat will be discussed in detail. Additionally, some novel molecular imaging modalities will be enumerated in Table 1.
PET
PET is the most frequently used imaging method for the assessment of thermogenic adipose tissue, and 18F- FDG-PET/CT is currently the most frequently used method for visualizing activated thermogenic fat in mammals and humans. 18F-FDG-PET/CT scans are commonly conducted for cancer diagnosis, staging, and surveillance. Clinicians can identify suspected malignancies and metastases by measuring the uptake of radiolabelled glucose.345 Since thermogenic adipocytes are good at intaking glucose in the same way, reports firstly described increased uptake of the glucose analog in supraclavicular fat two decades ago, suggesting the presence of metabolically active BAT in adult humans.346 After that, plenty of research sprung up and proved the prevalence of active thermogenic adipose tissue in humans. Moreover, investigators developed Brown Adipose Reporting Criteria in Imaging Studies (BARCIST 1.0) criteria to produce the diagnosis based on scoring thermogenic fat.67,68,135,347,348 In recent years, 18F-FDG-PET/CT is used to assess the distribution and activity of adipose tissue, rather than diagnosing and staging cancers.349 A series of clinical trials have demonstrated that BAT activity on 18F-FDG-PET/CT is prevalent and it is negatively associated with lymph nodes and distant metastasis.350,351 In contrast, a new clinical study consisting of multiple cancers suggested that elevated thermogenic fat volume is linked with lower cancer-associated death.352 Furthermore, another study suggested that patients with active cancer have more BAT compared to patients with successfully treated cancer.353 In conclusion, 18F-FDG-PET/CT of thermogenic fat could serve as a novel measurement for the outcome of cancer patients, but more large clinical trials are needed.
MRI
Although 18F-FDG-PET/CT remains the gold standard for the detection and quantification of thermogenic fat in humans, other novel imaging techniques have sprung up like magnetic resonance imaging (MRI). Compared with PET, MRI has better spatial resolution at a lower cost and is much safer as they do not involve the injection of radioactive tracers.354,355 There are already some comprehensive and updated reviews of MRI mechanisms and applications for thermogenic adipose tissue, so we mainly focus on its role in tumors.356,357,358 In KRASG12D P53R172H Pdx‐Cre+/+ (KPC) mouse, tumor progression induced loss of fat thermogenesis, which was detected by MRI.359 Consistently, the water-fat separated MRI could identify and quantify the BAT in C57BL/6 mice that were inoculated orthotopically with Pan02 tumor cells.360 Furthermore, dynamic wasting of BAT can be captured by a dedicated high-Fifield (7 Tesla) Bruker 7T ClinScan MRI combined with an infrared camera in the ovarian tumor mouse model.361 Unfortunately, the information about MRI identifying thermogenic fat in clinical patients with malignancy is little.
Imaging of thermogenic fat using other technologies
Apart from PET and MRI, plenty of other modalities have been developed to identify thermogenic adipose tissue, such as single-photon emission computerized tomography (SPECT), near-infrared fluorescence imaging (NIRFI), contrast-enhanced ultrasound (CEUS), near-infrared spectroscopy (NIRS), infrared thermography (IRT).344,355 These advanced imaging techniques have their advantages and disadvantages, respectively, and further study is necessary for validating their practical applications.
Therapeutic strategies of thermogenic fat
Given the role of thermal fat in nutrient metabolism and energy expenditure as well as its impact on other tissues, activating thermogenic adipose tissue provides a promising therapeutic strategy for curbing obesity, T2DM, and other metabolic diseases. Although the overwhelming benefits have been demonstrated in some metabolic diseases, the adverse side effects of browning should not be ignored, like cachexia and cardiovascular events in hypermetabolic conditions. In turn, blocking WAT browning might be exploited for clinical benefit in hypermetabolic patients. Collectively, we have to figure out how we can preserve the therapeutic effects by manipulating thermogenesis, while also eliminating many of the unexpected side effects.362,363,364 Here, we summarize current clinical trials of targeting thermogenic adipose tissue pharmacologically for metabolic diseases in Table 2. In addition, we also cite some evidence to show the therapeutic potential of brown and beige fat in humans with malignancy.
Targeting activation of thermogenic adipose tissue
Activating the browning of adipose tissue is emerging as an interesting and promising method to curb metabolic disorders like obesity and T2DM because of its unique capacity to upregulate non-shivering thermogenesis.365 The most physiological external clues stimulating the thermogenesis of adipose tissue include cold exposure, diet, and exercise.366 Both the short-term cold exposure (10 °C, 4 h) and chronic cold exposure (6 °C, 10 days) induce WAT browning and UCP1-mediated thermogenesis-dependent glucose utilization.367,368 Further, intermittent cold exposure increases BAT thermogenesis and improves insulin sensitivity in humans consequently.369 In addition, exercise enhances glucose homeostasis and protects against metabolic diseases such as obesity and diabetes.370 Recently, investigators have demonstrated that exercise induces the phenotypic conversion of white adipocytes to thermogenic adipocytes through a range of mechanisms.371 During exercise, increased plasma epinephrine stimulates lipolysis through acting cAMP, PKA, and phosphorylates adipose triglyceride lipase (ATGL). Moreover, it has been demonstrated that exercise can stimulate the browning of WAT and increase energy consumption by promoting the expression of hypothalamic brain-derived neurotrophic factor (BDNF).372 Of note, various food ingredients have been considered potent stimuli inducing a phenotypic switch from white to beige adipocytes and activating thermogenesis. For instance, capsaicin and its analog capsinoids mimic the effects of cold exposure to decrease body fatness through the activation and recruitment of BAT through activating transient receptor potential (TRP) channels.373 Interestingly, intermittent fasting stimulates the thermogenic activity of BAT, which contributes to preventing obesity via altering the composition of the microbiota in mice.374 Besides, a variety of phytochemicals, including phenylpropanoids (e.g., artepillin C, resveratrol), flavonoids (e.g., catechin, quercetin), terpenoids (e.g., ginsenoside, fucoxanthin), alkaloids (e.g., capsaicin, caffeine), glycosides (e.g., oleuropein), and phenolic acid (for example, gallic acid) have been shown in the literature to counteract weight gain via adipose thermogenesis.155,375,376 Compared with the above approaches for activating thermal fat function, pharmacological products have a more specific effect with minimized adverse consequences (Table 2). Remarkably, recent preclinical investigations have shown that successful BAT transplantation models display improvements in glucose metabolism and insulin sensitivity, as well as reductions in body mass and decreased adiposity in recipients. These beneficial effects are mediated by several different mechanisms, including endocrine effects via the release of batokines.377 With the view of clinical application, progenitors or stem cell therapy is recognized as a more feasible strategy than tissue transplantation. Give an illustrative example, human adipose-derived stem cells were differentiated into brown adipocytes with rosiglitazone and then injected into mice every other week over 10 weeks, then the models injected with the thermogenic cells showed a loss of body weight and enhanced glucose tolerance.378
Although driving thermogenesis in preclinical and clinical studies of metabolic diseases like obesity showed exciting results, its beneficial effects on cancer patients have not been reported yet. Regarding previous investigations, the abnormal accumulation of WAT promotes tumorigenesis, progression, invasion, and metastasis.379 Thus, locally activating WAT browning and lipolysis, such as in the fatty liver and pancreas, might suppress oncogenesis prophylactically or inhibit tumor growth by an unknown signaling way. All of these hypotheses require strong evidence.
Targeting inhibition of thermogenic adipose tissue
On present evidence, hypermetabolic conditions including burns and cancer in which browning is detrimental to patient outcome.363 Now, activating thermogenic fat accelerates cancer-associated cachexia and promotes tumor growth, which implies that blocking the browning of adipose tissue seems to be an advantageous treatment for individuals with malignancy. In the skin tumor mice model, investigators found that WAT browning was an early event in the pathophysiology of cancer cachexia, and treatment with the selective β3-AR antagonist or nonsteroidal anti-inflammatory drug sulindac could ameliorate cachexia owing to the reduction of browning in subcutaneous WAT.234 In agreement with that, using the neutral sphingomyelinase inhibitor GW4869 or Arctii Fructus (the extract of Arctium lappa) improves cancer-induced cachexia and decreased mortality by inhibiting browning.380,381 Of note, Metformin, the first-line drug treatment for hyperglycemia and insulin resistance, has been considered a novel anticancer agent unexpectedly. The primary reason is that Metformin can inhibit the electron transport chain (ETC) and ATP synthesis, and it also can regulate AMP-activated protein kinase (AMPK) and mTORC1 in multiple ways.382,383 Furthermore, recent research demonstrated that treatment with Metformin could improve the tumor-induced wasting state and minimize cachexia in tumor-bearing rats, probably it prevented the pathological browning of WAT.384,385 So from the point of translational medicine, classical anticancer treatment combined with inhibiting fat browning might provide a promising option for patients.
Conclusions and perspective
Thermogenic adipocytes including brown and beige adipocytes have garnered considerable attention recently, mainly because both of them have similar impacts on thermoregulation and nutrient utilization. Although brown and beige adipocytes come from distinct origins during embryonic development and locate in different positions anatomically, they have multilocular lipid droplets, abundant mitochondria, and highly expressed UCP1. Of note, beige fat cells are reproducible in adults. More precisely, the emergence of beige adipocytes is an inducible process stimulated by external clues such as cold, exercise, and fasting. Therefore, manipulating the thermogenic program provide a promising therapeutic strategy for combating obesity, T2DM, cancer, and other diseases. Although multiple therapeutic strategies activating thermogenesis have been shown to work well in animal models, the majority of them do not apply to humans. Probably the heterogeneity in thermogenic adipose tissue between the two species make the translational application from mice to humans harder. Moreover, it should not be ignored that targeting thermogenic fat can give rise to adverse effects in vivo, such as cachexia, heart diseases, and other hypermetabolic disorders. Collectively, investigators and clinicians must consider it prudent how we can preserve the beneficial metabolic effects of browning and meanwhile eliminate many of the unexpected side effects.
In contrast to obesity or diabetes, the influence of thermogenic adipocytes is not one-sided anymore but double-edged during the initiation and progression of the tumor. Firstly, activated thermogenic adipocytes can prevent obesity, T2DM, and other metabolism-related diseases like NAFLD through increasing energy expenditure, enhancing glucose tolerance, or alleviating inflammation, and the majority of these metabolic diseases are generally considered high-risk factors for multiple tumors.366,386 However, the browning of adipocytes seems to support progression after tumor formation. Specifically, enhanced thermogenesis in SAT speeds up fat mass loss and cancer cachexia, thereby contributing to the poor outcome of cancer patients.387,388 In addition, the local activation of thermogenic adipocytes adjacent to the tumor can promote the proliferation and invasion of cancer cells by providing fuel sources like lactate. Ultimately, malignant cells are resistant to chemotherapy partly because chemotherapeutic drugs stimulate reinstallation of the thermogenic phenotype in WAT.303 Accordingly, the affection for manipulating thermogenic adipocytes is seemly quite different from preventing the onset of cancer to slowing tumor progression. Although targeting thermogenic adipocytes is not a reliable treatment for cancer patients based on current research, it has already presented its therapeutic potential in other metabolic conditions.152,389,390,391,392 Thus, the therapeutic strategy of regulating thermogenesis is an alternative option for metabolism-related cancer patients, if continue to conduct more research. Referring to the current status of investigating thermogenic fat, there are several aspects worth studying further in oncology.393,394,395 First, for example, it would be interesting to identify subpopulations of adipocytes or other cells in ATME in the different tumor contexts, to determine the origin of adipocytes, as well as their fate and phenotype (whether these cells form non-thermal or thermal fat, and the type of component they secrete). Besides, from the perspective of translational medicine, a novel detective technology is required for precisely distinguishing thermogenic fat from tumors and continuously evaluating the capacity of thermogenesis. Moreover, it is also necessary to explore how to achieve safe and effective beige fat activation in tumors locally, because inducing thermogenesis through traditional ways like cold stimuli or beta-adrenergic signaling possibly results in potential cardiovascular hazards.161
In conclusion, recent investigations provide new insights into the biology and pathology of thermogenic fat and preliminarily reveal its connection to metabolic diseases and malignancy, thereby suggesting that modulating the activity of thermogenic adipocytes holds promise for combating obesity, T2DM, cancer, and other metabolic diseases.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Stefan, N., Birkenfeld, A. L. & Schulze, M. B. Global pandemics interconnected—obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 17, 135–149 (2021).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
Fillon, M. Routine cancer screening rates rebound after deep drop from pandemic fear. CA Cancer J. Clin. 71, 366–368 (2021).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Sivanand, S. & Vander Heiden, M. G. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell 37, 147–156 (2020).
Cluntun, A. A., Lukey, M. J., Cerione, R. A. & Locasale, J. W. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3, 169–180 (2017).
Hay, N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635–649 (2016).
Sun, T., Liu, Z. & Yang, Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol. Cancer 19, 146 (2020).
Schworer, S., Vardhana, S. A. & Thompson, C. B. Cancer metabolism drives a stromal regenerative response. Cell Metab. 29, 576–591 (2019).
Counihan, J. L., Grossman, E. A. & Nomura, D. K. Cancer metabolism: current understanding and therapies. Chem. Rev. 118, 6893–6923 (2018).
Qin, C. et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol. Cancer 19, 50 (2020).
Leone, R. D. & Powell, J. D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 20, 516–531 (2020).
Shamsi, F., Wang, C. H. & Tseng, Y. H. The evolving view of thermogenic adipocytes—ontogeny, niche and function. Nat. Rev. Endocrinol. 17, 726–744 (2021).
Quail, D. F. & Dannenberg, A. J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15, 139–154 (2019).
Cohen, P. & Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 22, 393–409 (2021).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Kahn, C. R., Wang, G. & Lee, K. Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 129, 3990–4000 (2019).
Betz, M. J. & Enerback, S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat. Rev. Endocrinol. 14, 77–87 (2018).
Chouchani, E. T., Kazak, L. & Spiegelman, B. M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37 (2019).
Peres Valgas da Silva, C., Hernandez-Saavedra, D., White, J. D. & Stanford, K. I. Cold and exercise: therapeutic tools to activate brown adipose tissue and combat obesity. Biology 8, 9 (2019).
Shao, M. et al. Cellular origins of beige fat cells revisited. Diabetes 68, 1874–1885 (2019).
Henningsen, J. B. & Scheele, C. Brown adipose tissue: a metabolic regulator in a hypothalamic cross talk? Annu. Rev. Physiol. 83, 279–301 (2021).
Zhang, Z. et al. Non-shivering thermogenesis signalling regulation and potential therapeutic applications of brown adipose tissue. Int. J. Biol. Sci. 17, 2853–2870 (2021).
Wang, W. & Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702 (2016).
Li, L., Li, B., Li, M. & Speakman, J. R. Switching on the furnace: regulation of heat production in brown adipose tissue. Mol. Asp. Med 68, 60–73 (2019).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Leitner, B. P. et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl Acad. Sci. USA 114, 8649–8654 (2017).
Shinde, A. B., Song, A. & Wang, Q. A. Brown adipose tissue heterogeneity, energy metabolism, and beyond. Front. Endocrinol. 12, 651763 (2021).
McNeill, B. T., Suchacki, K. J. & Stimson, R. H. Mechanisms in endocrinology: human brown adipose tissue as a therapeutic target: warming up or cooling down? Eur. J. Endocrinol. 184, R243–R259 (2021).
Rui, L. Brown and beige adipose tissues in health and disease. Compr. Physiol. 7, 1281–1306 (2017).
Inagaki, T., Sakai, J. & Kajimura, S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 17, 480–495 (2016).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 (2013).
Barbatelli, G. et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–1253 (2010).
Gnad, T. et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 (2014).
Oguri, Y. et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell 182, 563–577 e520 (2020).
Chen, Y. et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565, 180–185 (2018).
Berbee, J. F. et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 6, 6356 (2015).
Jun, H. et al. Adrenergic-independent signaling via CHRNA2 regulates beige fat activation. Dev. Cell 54, 106–116 e105 (2020).
Cinti, S. Pink adipocytes. Trends Endocrinol. Metab. 29, 651–666 (2018).
Altshuler-Keylin, S. et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 24, 402–419 (2016).
Sidossis, L. & Kajimura, S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Investig. 125, 478–486 (2015).
Angueira, A. R. et al. Defining the lineage of thermogenic perivascular adipose tissue. Nat. Metab. 3, 469–484 (2021).
Chang, L., Garcia-Barrio, M. T. & Chen, Y. E. Perivascular adipose tissue regulates vascular function by targeting vascular smooth muscle cells. Arterioscler Thromb. Vasc. Biol. 40, 1094–1109 (2020).
Ikeda, K. & Yamada, T. UCP1 dependent and independent thermogenesis in brown and beige adipocytes. Front. Endocrinol. 11, 498 (2020).
Verkerke, A. R. P. & Kajimura, S. Oil does more than light the lamp: the multifaceted role of lipids in thermogenic fat. Developmental Cell 56, 1408–1416 (2021).
Brandao, B. B., Poojari, A. & Rabiee, A. Thermogenic fat: development, physiological function, and therapeutic potential. Int. J. Mol. Sci. 22, 5906 (2021).
Villarroya, J. et al. New insights into the secretory functions of brown adipose tissue. J. Endocrinol. 243, R19–R27 (2019).
Chang, S. H., Song, N. J., Choi, J. H., Yun, U. J. & Park, K. W. Mechanisms underlying UCP1 dependent and independent adipocyte thermogenesis. Obes. Rev. 20, 241–251 (2019).
Park, H. et al. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J. Clin. Investig. 129, 694–711 (2019).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465 (2017).
Wang, Z., Yu, X. & Chen, Y. Recruitment of thermogenic fat: trigger of fat burning. Front. Endocrinol. 12, 696505 (2021).
Rahbani, J. F. et al. Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature 590, 480–485 (2021).
Hall, K. D. & Guo, J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology 152, 1718–1727.e1713 (2017).
Damal Villivalam, S. et al. TET1 is a beige adipocyte-selective epigenetic suppressor of thermogenesis. Nat. Commun. 11, 4313 (2020).
Tian, Q. et al. Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice. Aging Cell 19, e13059 (2020).
Kazak, L. et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 26, 660–671 e663 (2017).
Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 (2021).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J. Clin. 67, 378–397 (2017).
The Lancet Diabetes, E. The obesity-cancer link: of increasing concern. Lancet Diabetes Endocrinol. 8, 175 (2020).
Bastias-Perez, M. et al. Impact of adaptive thermogenesis in mice on the treatment of obesity. Cells 9, 316 (2020).
Chen, K. Y. et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J. Biol. Chem. 295, 1926–1942 (2020).
Leto, D. & Saltiel, A. R. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 13, 383–396 (2012).
Tokarz, V. L., MacDonald, P. E. & Klip, A. The cell biology of systemic insulin function. J. Cell Biol. 217, 2273–2289 (2018).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Jaldin-Fincati, J. R., Pavarotti, M., Frendo-Cumbo, S., Bilan, P. J. & Klip, A. Update on GLUT4 vesicle traffic: a cornerstone of insulin action. Trends Endocrinol. Metab. 28, 597–611 (2017).
Bryant, N. J. & Gould, G. W. Insulin stimulated GLUT4 translocation—size is not everything! Curr. Opin. Cell Biol. 65, 28–34 (2020).
Brumfield, A. et al. Insulin-promoted mobilization of GLUT4 from a perinuclear storage site requires RAB10. Mol. Biol. Cell 32, 57–73 (2021).
Picatoste, B. et al. Defective insulin-stimulated GLUT4 translocation in brown adipocytes induces systemic glucose homeostasis dysregulation independent of thermogenesis in female mice. Mol. Metab. 53, 101305 (2021).
Wang, S. et al. Genetic evidence for an inhibitory role of tomosyn in insulin-stimulated GLUT4 exocytosis. Traffic 21, 636–646 (2020).
Beg, M., Zhang, W., McCourt, A. C. & Enerback, S. ATGL activity regulates GLUT1-mediated glucose uptake and lactate production via TXNIP stability in adipocytes. J. Biol. Chem. 296, 100332 (2021).
Lowell, B. B. et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 (1993).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 123, 215–223 (2013).
Tews, D. et al. Elevated UCP1 levels are sufficient to improve glucose uptake in human white adipocytes. Redox Biol. 26, 101286 (2019).
Mishra, B. K., Madhu, S. V., Aslam, M., Agarwal, V. & Banerjee, B. D. Adipose tissue expression of UCP1 and PRDM16 genes and their association with postprandial triglyceride metabolism and glucose intolerance. Diabetes Res. Clin. Pr. 182, 109115 (2021).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 121, 96–105 (2011).
Flier, J. S. Might beta3-adrenergic receptor agonists be useful in disorders of glucose homeostasis? J. Clin. Investig. 130, 2180–2182 (2020).
Cypess, A. M. et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
Keinan, O. et al. Glycogen metabolism links glucose homeostasis to thermogenesis in adipocytes. Nature 599, 296–301 (2021).
Fedorenko, A., Lishko, P. V. & Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012).
Tabuchi, C. & Sul, H. S. Signaling pathways regulating thermogenesis. Front. Endocrinol. 12, 595020 (2021).
Schreiber, R. et al. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab. 26, 753–763.e757 (2017).
Shin, H. et al. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab. 26, 764–777 e765 (2017).
Lynes, M. D. et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 23, 631–637 (2017).
Wu, Q. et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 55, 3229–3237 (2006).
Ouellet, V. et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Investig. 122, 545–552 (2012).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
Heine, M. et al. Lipolysis triggers a systemic insulin response essential for efficient energy replenishment of activated brown adipose tissue in mice. Cell Metab. 28, 644–655 e644 (2018).
Fischer, A. W. et al. Lysosomal lipoprotein processing in endothelial cells stimulates adipose tissue thermogenic adaptation. Cell Metab. 33, 547–564 e547 (2021).
Larsson, M. et al. Impaired thermogenesis and sharp increases in plasma triglyceride levels in GPIHBP1-deficient mice during cold exposure. J. Lipid Res. 59, 706–713 (2018).
Bartelt, A. et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat. Commun. 8, 15010 (2017).
O’Mara, A. E. et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 130, 2209–2219 (2020).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Yoneshiro, T. et al. Metabolic flexibility via mitochondrial BCAA carrier SLC25A44 is required for optimal fever. eLife 10, e66865 (2021).
Cannavino, J. et al. Regulation of cold-induced thermogenesis by the RNA binding protein FAM195A. Proc. Natl Acad. Sci. USA 118, e2104650118 (2021).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Lotta, L. A. et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med. 13, e1002179 (2016).
Wang, W. et al. Genetic predisposition to impaired metabolism of the branched chain amino acids, dietary intakes, and risk of type 2 diabetes. Genes Nutr. 16, 20 (2021).
Lynch, C. J. & Adams, S. H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736 (2014).
Salinas-Rubio, D., Tovar, A. R. & Noriega, L. G. Emerging perspectives on branched-chain amino acid metabolism during adipocyte differentiation. Curr. Opin. Clin. Nutr. Metab. Care 21, 49–57 (2018).
Green, C. R. et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 12, 15–21 (2016).
Zaganjor, E. et al. SIRT4 is an early regulator of branched-chain amino acid catabolism that promotes adipogenesis. Cell Rep. 36, 109345 (2021).
Deshmukh, A. S. et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab. 30, 963–975 e967 (2019).
Villarroya, J., Cereijo, R., Giralt, M. & Villarroya, F. Secretory proteome of brown adipocytes in response to cAMP-mediated thermogenic activation. Front. Physiol. 10, 67 (2019).
Ali Khan, A. et al. Comparative secretome analyses of primary murine white and brown adipocytes reveal novel adipokines. Mol. Cell Proteom. 17, 2358–2370 (2018).
Scheja, L. & Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 15, 507–524 (2019).
Nishio, M. & Saeki, K. The remaining mysteries about brown adipose tissues. Cells 9, 1365 (2020).
Fisher, F. M. et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Whittle, A. J. et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012).
Mishra, D. et al. Parabrachial interleukin-6 reduces body weight and food intake and increases thermogenesis to regulate energy metabolism. Cell Rep. 26, 3011–3026 e3015 (2019).
Whittle, A. J. et al. Soluble LR11/SorLA represses thermogenesis in adipose tissue and correlates with BMI in humans. Nat. Commun. 6, 8951 (2015).
Fang, D., Shi, X., Lu, T., Ruan, H. & Gao, Y. The glycoprotein follistatin-like 1 promotes brown adipose thermogenesis. Metabolism 98, 16–26 (2019).
Wang, G. X., Zhao, X. Y. & Lin, J. D. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol. Metab. 26, 231–237 (2015).
Cui, X. et al. Adipose tissue-derived neurotrophic factor 3 regulates sympathetic innervation and thermogenesis in adipose tissue. Nat. Commun. 12, 5362 (2021).
Sun, K. et al. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol. Metab. 3, 474–483 (2014).
During, M. J. et al. Adipose VEGF links the white-to-brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. Endocrinology 156, 2059–2073 (2015).
Rao, R. R. et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291 (2014).
van den Berg, S. M., van Dam, A. D., Rensen, P. C., de Winther, M. P. & Lutgens, E. Immune modulation of brown(ing) adipose tissue in obesity. Endocr. Rev. 38, 46–68 (2017).
Mills, E. L. et al. Cysteine 253 of UCP1 regulates energy expenditure and sex-dependent adipose tissue inflammation. Cell Metab. 34, 140–157 e148 (2022).
Bookout, A. L. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, 1147–1152 (2013).
Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 15, 393–405 (2019).
Caron, A., Lee, S., Elmquist, J. K. & Gautron, L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 19, 153–165 (2018).
Schneeberger, M. et al. Regulation of energy expenditure by brainstem GABA neurons. Cell 178, 672–685 e612 (2019).
Ruan, C. C. et al. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab. 28, 476–489 e475 (2018).
Wang, G. X. et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 20, 1436–1443 (2014).
Shen, H., Jiang, L., Lin, J. D., Omary, M. B. & Rui, L. Brown fat activation mitigates alcohol-induced liver steatosis and injury in mice. J. Clin. Investig. 129, 2305–2317 (2019).
Thomou, T. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455 (2017).
Sponton, C. H. et al. The regulation of glucose and lipid homeostasis via PLTP as a mediator of BAT-liver communication. EMBO Rep. 21, e49828 (2020).
Stanford, K. I. et al. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 27, 1111–1120 e1113 (2018).
Kong, X. et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 28, e633 (2018).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Prospective Studies, C. et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373, 1083–1096 (2009).
Tomiyama, A. J. Stress and obesity. Annu Rev. Psychol. 70, 703–718 (2019).
Chooi, Y. C., Ding, C. & Magkos, F. The epidemiology of obesity. Metabolism 92, 6–10 (2019).
Bluher, M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298 (2019).
Gadde, K. M., Martin, C. K., Berthoud, H. R. & Heymsfield, S. B. Obesity: pathophysiology and management. J. Am. Coll. Cardiol. 71, 69–84 (2018).
Yang, J. et al. Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat. Genet. 47, 1114–1120 (2015).
Rohde, K. et al. Genetics and epigenetics in obesity. Metabolism 92, 37–50 (2019).
Huvenne, H., Dubern, B., Clement, K. & Poitou, C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes. Facts 9, 158–173 (2016).
Pan, R., Zhu, X., Maretich, P. & Chen, Y. Combating obesity with thermogenic fat: current challenges and advancements. Front. Endocrinol. 11, 185 (2020).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 123, 3404–3408 (2013).
Boucher, J. M. et al. Pathological conversion of mouse perivascular adipose tissue by notch activation. Arterioscler Thromb. Vasc. Biol. 40, 2227–2243 (2020).
Sakamoto, T. et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am. J. Physiol. Endocrinol. Metab. 310, E676–E687 (2016).
Peterson, K. R., Flaherty, D. K. & Hasty, A. H. Obesity alters B cell and macrophage populations in brown adipose tissue. Obesity 25, 1881–1884 (2017).
Yao, J. et al. Macrophage IRX3 promotes diet-induced obesity and metabolic inflammation. Nat. Immunol. 22, 1268–1279 (2021).
Kazak, L. et al. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat. Metab. 1, 360–370 (2019).
Hussain, M. F., Roesler, A. & Kazak, L. Regulation of adipocyte thermogenesis: mechanisms controlling obesity. FEBS J. 287, 3370–3385 (2020).
Lee, P. et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309 (2014).
Yuan, Y. et al. Exercise-induced alpha-ketoglutaric acid stimulates muscle hypertrophy and fat loss through OXGR1-dependent adrenal activation. EMBO J. 39, e103304 (2020).
Wu, T. et al. Feeding-induced hepatokine, Manf, ameliorates diet-induced obesity by promoting adipose browning via p38 MAPK pathway. J. Exp. Med. 218, e20201203 (2021).
Li, H., Qi, J. & Li, L. Phytochemicals as potential candidates to combat obesity via adipose non-shivering thermogenesis. Pharm. Res. 147, 104393 (2019).
Chen, S. et al. The phytochemical hyperforin triggers thermogenesis in adipose tissue via a Dlat-AMPK signaling axis to curb obesity. Cell Metab. 33, 565–580 e567 (2021).
Bhadada, S. V., Patel, B. M., Mehta, A. A. & Goyal, R. K. beta(3) receptors: role in cardiometabolic disorders. Ther. Adv. Endocrinol. Metab. 2, 65–79 (2011).
Vasconcelos, J., Freire, E., Almendra, R., Silva, G. L. & Santana, P. The impact of winter cold weather on acute myocardial infarctions in Portugal. Environ. Pollut. 183, 14–18 (2013).
Cheng, W. et al. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano 13, 8537–8565 (2019).
Wang, X. et al. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 81, 114–124 (2016).
Li, Y. et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell 185, 949–966 e919 (2022).
Cho, N. H. et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 138, 271–281 (2018).
Zimmet, P., Shi, Z., El-Osta, A. & Ji, L. Epidemic T2DM, early development and epigenetics: implications of the Chinese Famine. Nat. Rev. Endocrinol. 14, 738–746 (2018).
Viner, R., White, B. & Christie, D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet 389, 2252–2260 (2017).
Magliano, D. J. et al. Young-onset type 2 diabetes mellitus—implications for morbidity and mortality. Nat. Rev. Endocrinol. 16, 321–331 (2020).
Chatterjee, S., Khunti, K. & Davies, M. J. Type 2 diabetes. Lancet 389, 2239–2251 (2017).
Zhu, Y. et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: patient outcomes research to advance learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care 42, 2211–2219 (2019).
Magkos, F. Metabolically healthy obesity: what’s in a name? Am. J. Clin. Nutr. 110, 533–539 (2019).
Guilherme, A., Virbasius, J. V., Puri, V. & Czech, M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 (2008).
Bartelt, A. & Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36 (2014).
Chondronikola, M. et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 (2014).
Hanssen, M. J. et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 21, 863–865 (2015).
van der Lans, A. A. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 123, 3395–3403 (2013).
Kaisanlahti, A. & Glumoff, T. Browning of white fat: agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 75, 1–10 (2019).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 14, 272–279 (2011).
Olsen, J. M. et al. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J. Cell Biol. 207, 365–374 (2014).
Dallner, O. S., Chernogubova, E., Brolinson, K. A. & Bengtsson, T. Beta3-adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology 147, 5730–5739 (2006).
Peirce, V. & Vidal-Puig, A. Regulation of glucose homoeostasis by brown adipose tissue. Lancet Diabetes Endocrinol. 1, 353–360 (2013).
Bennett, J. E. et al. NCD Countdown 2030: pathways to achieving sustainable development goal target 3.4. Lancet 396, 918–934 (2020).
Kivimaki, M. & Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 15, 215–229 (2018).
Zhao, D., Liu, J., Wang, M., Zhang, X. & Zhou, M. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 16, 203–212 (2019).
Joseph, P. et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ. Res. 121, 677–694 (2017).
Leong, D. P. et al. Reducing the global burden of cardiovascular disease, part 2: prevention and treatment of cardiovascular disease. Circ. Res. 121, 695–710 (2017).
Andersson, C. & Vasan, R. S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 15, 230–240 (2018).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation 143, e984–e1010 (2021).
Piche, M. E., Tchernof, A. & Despres, J. P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 126, 1477–1500 (2020).
Neeland, I. J. et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 7, 715–725 (2019).
Van Gaal, L. F., Mertens, I. L. & De Block, C. E. Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880 (2006).
Ortega, F. B., Lavie, C. J. & Blair, S. N. Obesity and cardiovascular disease. Circ. Res. 118, 1752–1770 (2016).
Katta, N., Loethen, T., Lavie, C. J. & Alpert, M. A. Obesity and coronary heart disease: epidemiology, pathology, and coronary artery imaging. Curr. Probl. Cardiol. 46, 100655 (2021).
Ruiz, J. R. et al. Role of human brown fat in obesity, metabolism and cardiovascular disease: strategies to turn up the heat. Prog. Cardiovasc Dis. 61, 232–245 (2018).
Chen, H. J., Meng, T., Gao, P. J. & Ruan, C. C. The role of brown adipose tissue dysfunction in the development of cardiovascular disease. Front. Endocrinol. 12, 652246 (2021).
Singh, R., Barrios, A., Dirakvand, G. & Pervin, S. Human brown adipose tissue and metabolic health: potential for therapeutic avenues. Cells 10, 3030 (2021).
Takx, R. A. et al. Supraclavicular brown adipose tissue 18F-FDG uptake and cardiovascular disease. J. Nucl. Med. 57, 1221–1225 (2016).
Xiong, S. et al. Peroxisome proliferator-activated receptor gamma coactivator-1alpha is a central negative regulator of vascular senescence. Arterioscler Thromb. Vasc. Biol. 33, 988–998 (2013).
Nisoli, E. et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299, 896–899 (2003).
Chang, L., Milton, H., Eitzman, D. T. & Chen, Y. E. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ. J. 77, 11–18 (2013).
Chang, L. et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126, 1067–1078 (2012).
Mu, W. et al. BMP4-mediated browning of perivascular adipose tissue governs an anti-inflammatory program and prevents atherosclerosis. Redox Biol. 43, 101979 (2021).
Xu, A., Wang, Y., Lam, K. S. & Vanhoutte, P. M. Vascular actions of adipokines molecular mechanisms and therapeutic implications. Adv. Pharm. 60, 229–255 (2010).
Wojcicka, G. et al. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharm. Res. 63, 68–76 (2011).
Gao, Y. J., Lu, C., Su, L. Y., Sharma, A. M. & Lee, R. M. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br. J. Pharm. 151, 323–331 (2007).
Lee, Y. C. et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation 124, 1160–1171 (2011).
Packer, M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J. Am. Coll. Cardiol. 71, 2360–2372 (2018).
Villasante Fricke, A. C. & Iacobellis, G. Epicardial adipose tissue: clinical biomarker of cardio-metabolic risk. Int. J. Mol. Sci. 20, 5989 (2019).
Nalliah, C. J. et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J. Am. Coll. Cardiol. 76, 1197–1211 (2020).
Chechi, K. et al. UCP1 expression-associated gene signatures of human epicardial adipose tissue. JCI Insight 4, 8 (2019).
Perez-Belmonte, L. M. et al. Expression of epicardial adipose tissue thermogenic genes in patients with reduced and preserved ejection fraction heart failure. Int. J. Med. Sci. 14, 891–895 (2017).
Chechi, K. et al. Functional characterization of the Ucp1-associated oxidative phenotype of human epicardial adipose tissue. Sci. Rep. 7, 15566 (2017).
Cittadini, A. et al. Cardiovascular abnormalities in transgenic mice with reduced brown fat: an animal model of human obesity. Circulation 100, 2177–2183 (1999).
Thoonen, R. et al. Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J. Mol. Cell Cardiol. 84, 202–211 (2015).
da Silva, J. S. et al. Adenosine A2A receptor agonist prevents cardiac remodeling and dysfunction in spontaneously hypertensive male rats after myocardial infarction. Drug Des. Devel Ther. 11, 553–562 (2017).
Bordicchia, M. et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 122, 1022–1036 (2012).
Gonzalez, N., Moreno-Villegas, Z., Gonzalez-Bris, A., Egido, J. & Lorenzo, O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol. 16, 44 (2017).
Wagner, R. et al. Metabolic implications of pancreatic fat accumulation. Nat. Rev. Endocrinol. 18, 43–54 (2022).
Dumas, J. F. & Brisson, L. Interaction between adipose tissue and cancer cells: role for cancer progression. Cancer Metastasis Rev. 40, 31–46 (2021).
Lengyel, E., Makowski, L., DiGiovanni, J. & Kolonin, M. G. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer 4, 374–384 (2018).
Grabner, G. F., Xie, H., Schweiger, M. & Zechner, R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 3, 1445–1465 (2021).
Anderson, L. J. et al. Whole-body and adipose tissue metabolic phenotype in cancer patients. J. Cachexia Sarcopenia Muscle 13, 1124–1133 (2022).
Laforest, S. et al. Associations between markers of mammary adipose tissue dysfunction and breast cancer prognostic factors. Int. J. Obes. 45, 195–205 (2021).
Di Franco, S. et al. Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery. Nat. Commun. 12, 5006 (2021).
Nigro, E. et al. Adiponectin and leptin exert antagonizing effects on proliferation and motility of papillary thyroid cancer cell lines. J. Physiol. Biochem 77, 237–248 (2021).
Xu, L. et al. Adipocytes affect castration-resistant prostate cancer cells to develop the resistance to cytotoxic action of NK cells with alterations of PD-L1/NKG2D ligand levels in tumor cells. Prostate 78, 353–364 (2018).
Tiwari, P. et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J. Exp. Med. 216, 1345–1358 (2019).
Cozzo, A. J., Fuller, A. M. & Makowski, L. Contribution of adipose tissue to development of cancer. Compr. Physiol. 8, 237–282 (2017).
Brindley, D. N., Tang, X., Meng, G. & Benesch, M. G. K. Role of adipose tissue-derived autotaxin, lysophosphatidate signaling, and inflammation in the progression and treatment of breast cancer. Int. J. Mol. Sci. 21, 5938 (2020).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 4, 17105 (2018).
Muscaritoli, M., Rossi Fanelli, F. & Molfino, A. Perspectives of health care professionals on cancer cachexia: results from three global surveys. Ann. Oncol. 27, 2230–2236 (2016).
Dolly, A., Dumas, J. F. & Servais, S. Cancer cachexia and skeletal muscle atrophy in clinical studies: what do we really know? J. Cachexia Sarcopenia Muscle 11, 1413–1428 (2020).
Baazim, H., Antonio-Herrera, L. & Bergthaler, A. The interplay of immunology and cachexia in infection and cancer. Nat. Rev. Immunol. 22, 309–321 (2022).
Kir, S. & Spiegelman, B. M. Cachexia & brown fat: a burning issue in cancer. Trends Cancer 2, 461–463 (2016).
Dong, M., Lin, J., Lim, W., Jin, W. & Lee, H. J. Role of brown adipose tissue in metabolic syndrome, aging, and cancer cachexia. Front. Med. 12, 130–138 (2018).
Tsoli, M. et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res. 72, 4372–4382 (2012).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Kliewer, K. L. et al. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol. Ther. 16, 886–897 (2015).
Han, J., Meng, Q., Shen, L. & Wu, G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 17, 14 (2018).
Elattar, S., Dimri, M. & Satyanarayana, A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 32, 4727–4743 (2018).
Kir, S. et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513, 100–104 (2014).
Kir, S. et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab. 23, 315–323 (2016).
Hu, W. et al. Extracellular vesicles-released parathyroid hormone-related protein from Lewis lung carcinoma induces lipolysis and adipose tissue browning in cancer cachexia. Cell Death Dis. 12, 134 (2021).
Di, W. et al. Colorectal cancer prompted adipose tissue browning and cancer cachexia through transferring exosomal miR-146b-5p. J. Cell Physiol. 236, 5399–5410 (2021).
Arora, G. K. et al. Cachexia-associated adipose loss induced by tumor-secreted leukemia inhibitory factor is counterbalanced by decreased leptin. JCI Insight 3, 14 (2018).
Suriben, R. et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med. 26, 1264–1270 (2020).
Xie, H. et al. An immune-sympathetic neuron communication axis guides adipose tissue browning in cancer-associated cachexia. Proc. Natl Acad. Sci. USA 119, e2112840119 (2022).
Essa, N., O’Connell, F., Prina-Mello, A., O’Sullivan, J. & Marcone, S. Gold nanoparticles and obese adipose tissue microenvironment in cancer treatment. Cancer Lett. 525, 1–8 (2022).
Fuster, J. J., Ouchi, N., Gokce, N. & Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 118, 1786–1807 (2016).
Brown, K. A. Metabolic pathways in obesity-related breast cancer. Nat. Rev. Endocrinol. 17, 350–363 (2021).
de Candia, P. et al. The pleiotropic roles of leptin in metabolism, immunity, and cancer. J. Exp. Med. 218, e20191593 (2021).
Deng, T., Lyon, C. J., Bergin, S., Caligiuri, M. A. & Hsueh, W. A. Obesity, inflammation, and cancer. Annu Rev. Pathol. 11, 421–449 (2016).
Liu, Z. et al. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappaB/GSDMD signal in mice adipose tissue. J. Pineal Res. 63, e12414 (2017).
Lindhorst, A. et al. Adipocyte death triggers a pro-inflammatory response and induces metabolic activation of resident macrophages. Cell Death Dis. 12, 579 (2021).
Schulz, M., Salamero-Boix, A., Niesel, K., Alekseeva, T. & Sevenich, L. Microenvironmental regulation of tumor progression and therapeutic response in brain metastasis. Front. Immunol. 10, 1713 (2019).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Dieli-Conwright, C. M. et al. Adipose tissue inflammation in breast cancer survivors: effects of a 16-week combined aerobic and resistance exercise training intervention. Breast Cancer Res. Treat. 168, 147–157 (2018).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Liu, J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 (2009).
Talukdar, S. et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 (2012).
Jackaman, C. et al. Aging and cancer: the role of macrophages and neutrophils. Ageing Res. Rev. 36, 105–116 (2017).
Arendt, L. M. et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 73, 6080–6093 (2013).
Wang, S. et al. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J. Hematol. Oncol. 11, 82 (2018).
Incio, J. et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med. 10, eaag0945 (2018).
Cao, R., Brakenhielm, E., Wahlestedt, C., Thyberg, J. & Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl Acad. Sci. USA 98, 6390–6395 (2001).
Tomita, T., Kato, M. & Hiratsuka, S. Regulation of vascular permeability in cancer metastasis. Cancer Sci. 112, 2966–2974 (2021).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Incio, J. et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–869 (2016).
Sia, D., Villanueva, A., Friedman, S. L. & Llovet, J. M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 152, 745–761 (2017).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7, 7 (2021).
Ringelhan, M., Pfister, D., O’Connor, T., Pikarsky, E. & Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 19, 222–232 (2018).
Islami, F. et al. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J. Clin. 67, 273–289 (2017).
Li, X. et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 21, 541–557 (2021).
Marengo, A., Rosso, C. & Bugianesi, E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 67, 103–117 (2016).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
European Association for the Study of the, L. European association for the study of, D. & European Association for the Study of, O. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes. Facts 9, 65–90 (2016).
Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184, 2537–2564 (2021).
Powell, E. E., Wong, V. W.-S. & Rinella, M. Non-alcoholic fatty liver disease. Lancet 397, 2212–2224 (2021).
Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 158, 1851–1864 (2020).
Liu, Z. et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J. Hepatol. 73, 263–276 (2020).
Crane, J. D. et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 21, 166–172 (2015).
Gallardo-Montejano, V. I. et al. Perilipin 5 links mitochondrial uncoupled respiration in brown fat to healthy white fat remodeling and systemic glucose tolerance. Nat. Commun. 12, 3320 (2021).
Li, L. et al. Interleukin-6 mediated exercise-induced alleviation of adiposity and hepatic steatosis in mice. BMJ Open Diabetes Res. Care 9, e001431 (2021).
Roberts, L. D. et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 19, 96–108 (2014).
Kim, Y. et al. Platycodon grandiflorus root extract attenuates body fat mass, hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue. Nutrients 8, 532 (2016).
Raffaele, M. et al. Cold press pomegranate seed oil attenuates dietary-obesity induced hepatic steatosis and fibrosis through antioxidant and mitochondrial pathways in obese mice. Int. J. Mol. Sci. 21, 5469 (2020).
Shin, M. R., Shin, S. H. & Roh, S. S. Diospyros kaki and Citrus unshiu mixture improves disorders of lipid metabolism in nonalcoholic fatty liver disease. Can. J. Gastroenterol. Hepatol. 2020, 8812634 (2020).
Cruz, M. M. et al. Palmitoleic acid decreases non-alcoholic hepatic steatosis and increases lipogenesis and fatty acid oxidation in adipose tissue from obese mice. Front. Endocrinol. 11, 537061 (2020).
Pei, L. et al. Cyanidin-3-O-beta-glucoside regulates the activation and the secretion of adipokines from brown adipose tissue and alleviates diet induced fatty liver. Biomed. Pharmacother. 105, 625–632 (2018).
Tian, D. et al. Therapeutic effect and mechanism of polysaccharides from Anoectochilus Roxburghii (Wall.) Lindl. in diet-induced obesity. Phytomedicine 99, 154031 (2022).
Pittala, S., Krelin, Y., Kuperman, Y. & Shoshan-Barmatz, V. A mitochondrial VDAC1-based peptide greatly suppresses steatosis and NASH-associated pathologies in a mouse model. Mol. Ther. 27, 1848–1862 (2019).
Tsagkaraki, E. et al. CRISPR-enhanced human adipocyte browning as cell therapy for metabolic disease. Nat. Commun. 12, 6931 (2021).
Ghoshal, S. et al. Whole body Ip6k1 deletion protects mice from age-induced weight gain, insulin resistance and metabolic dysfunction. Int. J. Mol. Sci. 23, 2059 (2022).
Poekes, L. et al. Defective adaptive thermogenesis contributes to metabolic syndrome and liver steatosis in obese mice. Clin. Sci. 131, 285–296 (2017).
Wang, Q. et al. Deletion of the feeding-induced hepatokine TSK ameliorates the melanocortin obesity syndrome. Diabetes 70, 2081–2091 (2021).
Mills, E. L. et al. UCP1 governs liver extracellular succinate and inflammatory pathogenesis. Nat. Metab. 3, 604–617 (2021).
Ye, J., Wu, S., Pan, S., Huang, J. & Ge, L. Risk scoring based on expression of long noncoding RNAs can effectively predict survival in hepatocellular carcinoma patients with or without fibrosis. Oncol. Rep. 43, 1451–1466 (2020).
Hsieh, J. J. et al. Renal cell carcinoma. Nat. Rev. Dis. Primers 3, 1–19 (2017).
Capitanio, U. et al. Epidemiology of renal cell carcinoma. Eur. Urol. 75, 74–84 (2019).
Jonasch, E., Walker, C. L. & Rathmell, W. K. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat. Rev. Nephrol. 17, 245–261 (2021).
Khene, Z. E. et al. Adherent perinephric fat affects perioperative outcomes after partial nephrectomy: a systematic review and meta-analysis. Int. J. Clin. Oncol. 26, 636–646 (2021).
Shah, P. H. et al. Prognostic evaluation of perinephric fat, renal sinus fat, and renal vein invasion for patients with pathological stage T3a clear-cell renal cell carcinoma. BJU Int. 123, 270–276 (2019).
Okhunov, Z. et al. Evaluation of perirenal fat as a predictor of cT 1a renal cortical neoplasm histopathology and surgical outcomes. J. Endourol. 26, 911–916 (2012).
Xiao, W. et al. Melatonin/PGC1A/UCP1 promotes tumor slimming and represses tumor progression by initiating autophagy and lipid browning. J. Pineal Res. 67, e12607 (2019).
Kundu, A. et al. PRDM16 suppresses HIF-targeted gene expression in kidney cancer. J. Exp. Med. 217, 6 (2020).
Wei, G. et al. The thermogenic activity of adjacent adipocytes fuels the progression of ccRCC and compromises anti-tumor therapeutic efficacy. Cell Metab. 33, 2021–2039 e2028 (2021).
Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J. Clin. 66, 115–132 (2016).
Connor, A. A. & Gallinger, S. Pancreatic cancer evolution and heterogeneity: integrating omics and clinical data. Nat. Rev. Cancer 22, 131–142 (2022).
Mizrahi, J. D., Surana, R., Valle, J. W. & Shroff, R. T. Pancreatic cancer. Lancet 395, 2008–2020 (2020).
Andersen, D. K. et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes 66, 1103–1110 (2017).
Sah, R. P. et al. Phases of metabolic and soft tissue changes in months preceding a diagnosis of pancreatic ductal adenocarcinoma. Gastroenterology 156, 1742–1752 (2019).
Sagar, G. et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 65, 1165–1174 (2016).
Hart, P. A. et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 1, 226–237 (2016).
Pereira, S. P. et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 5, 698–710 (2020).
Cheng, L. et al. Brown and beige adipose tissue: a novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 10, 48–65 (2021).
Wang, H., Maitra, A. & Wang, H. Obesity, intrapancreatic fatty infiltration, and pancreatic cancer. Clin. Cancer Res. 21, 3369–3371 (2015).
Takahashi, M. et al. Fatty pancreas: a possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 109, 3013–3023 (2018).
Fan, L. et al. Breast cancer in China. Lancet Oncol. 15, e279–e289 (2014).
DeSantis, C. E. et al. Breast cancer statistics, 2019. CA Cancer J. Clin. 69, 438–451 (2019).
Miller, K. D. et al. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 70, 443–459 (2020).
Britt, K. L., Cuzick, J. & Phillips, K. A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 20, 417–436 (2020).
Kothari, C., Diorio, C. & Durocher, F. The importance of breast adipose tissue in breast cancer. Int. J. Mol. Sci. 21, 5760 (2020).
Singh, R. et al. Increased expression of beige/brown adipose markers from host and breast cancer cells influence xenograft formation in mice. Mol. Cancer Res. 14, 78–92 (2016).
Pare, M. et al. Breast cancer mammospheres secrete Adrenomedullin to induce lipolysis and browning of adjacent adipocytes. BMC Cancer 20, 784 (2020).
Gantov, M. et al. Beige adipocytes contribute to breast cancer progression. Oncol. Rep. 45, 317–328 (2021).
Ginzac, A. et al. A decrease in brown adipose tissue activity is associated with weight gain during chemotherapy in early breast cancer patients. BMC Cancer 20, 96 (2020).
Thrift, A. P. & El-Serag, H. B. Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 18, 534–542 (2020).
Joshi, S. S. & Badgwell, B. D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 71, 264–279 (2021).
Yeoh, K. G. & Tan, P. Mapping the genomic diaspora of gastric cancer. Nat. Rev. Cancer 22, 71–84 (2022).
Tan, P. & Yeoh, K. G. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology 149, 1153–1162 e1153 (2015).
Gu, L. et al. Prognostic value of pretreatment overweight/obesity and adipose tissue distribution in resectable gastric cancer: a retrospective cohort study. Front. Oncol. 11, 680190 (2021).
Matsui, R., Inaki, N. & Tsuji, T. Impact of visceral adipose tissue on compliance of adjuvant chemotherapy and relapse-free survival after gastrectomy for gastric cancer: a propensity score matching analysis. Clin. Nutr. 40, 2745–2753 (2021).
Lee, J. W. et al. Significance of CT attenuation and F-18 fluorodeoxyglucose uptake of visceral adipose tissue for predicting survival in gastric cancer patients after curative surgical resection. Gastric Cancer 23, 273–284 (2020).
Akutagawa, T. et al. Cancer-adipose tissue interaction and fluid flow synergistically modulate cell kinetics, HER2 expression, and trastuzumab efficacy in gastric cancer. Gastric Cancer 21, 946–955 (2018).
Han, J. et al. Subcutaneous, but not visceral, adipose tissue as a marker for prognosis in gastric cancer patients with cachexia. Clin. Nutr. 40, 5156–5161 (2021).
Zhang, H. et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int. J. Cancer 144, 2501–2515 (2019).
Liu, Y. et al. Exosomal miR-155 from gastric cancer induces cancer-associated cachexia by suppressing adipogenesis and promoting brown adipose differentiation via C/EPBbeta. Cancer Biol. Med. 19, 8 (2022).
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394, 1467–1480 (2019).
Kanth, P. & Inadomi, J. M. Screening and prevention of colorectal cancer. BMJ 374, n1855 (2021).
Biller, L. H. & Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: a review. J. Am. Med. Assoc. 325, 669–685 (2021).
Akimoto, N. et al. Rising incidence of early-onset colorectal cancer—a call to action. Nat. Rev. Clin. Oncol. 18, 230–243 (2021).
Man, K. et al. A thermogenic fat-epithelium cell axis regulates intestinal disease tolerance. Proc. Natl Acad. Sci. USA 117, 32029–32037 (2020).
Alnabulsi, A. et al. The expression of brown fat-associated proteins in colorectal cancer and the relationship of uncoupling protein 1 with prognosis. Int. J. Cancer 145, 1138–1147 (2019).
Zheng, Y. & Rudensky, A. Y. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 8, 457–462 (2007).
Park, J., Kim, S. W. & Cho, M. C. The role of LIM kinase in the male urogenital system. Cells 11, 78 (2021).
Alnabulsi, A. et al. Identification of a prognostic signature in colorectal cancer using combinatorial algorithm-driven analysis. J. Pathol. Clin. Res. 8, 245–256 (2022).
Yang, J. et al. Molecular imaging of brown adipose tissue mass. Int. J. Mol. Sci. 22, 9436 (2021).
Cavo, M. et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 18, e206–e217 (2017).
Cohade, C., Osman, M., Pannu, H. K. & Wahl, R. L. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J. Nucl. Med. 44, 170–176 (2003).
Steinberg, J. D., Vogel, W. & Vegt, E. Factors influencing brown fat activation in FDG PET/CT: a retrospective analysis of 15,000+ cases. Br. J. Radiol. 90, 20170093 (2017).
Chen, K. Y. et al. Brown adipose reporting criteria in imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab. 24, 210–222 (2016).
Santhanam, P., Solnes, L., Hannukainen, J. C. & Taieb, D. Adiposity-related cancer and functional imaging of brown adipose tissue. Endocr. Pr. 21, 1282–1290 (2015).
Pace, L. et al. Brown adipose tissue in breast cancer evaluated by [(18)F] FDG-PET/CT. Mol. Imaging Biol. 22, 1111–1115 (2020).
Cao, Q. et al. A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer 14, 126 (2014).
Chu, K., Bos, S. A., Gill, C. M., Torriani, M. & Bredella, M. A. Brown adipose tissue and cancer progression. Skelet. Radiol. 49, 635–639 (2020).
Bos, S. A., Gill, C. M., Martinez-Salazar, E. L., Torriani, M. & Bredella, M. A. Preliminary investigation of brown adipose tissue assessed by PET/CT and cancer activity. Skelet. Radiol. 48, 413–419 (2019).
Hu, H. H., Smith, D. L. Jr., Nayak, K. S., Goran, M. I. & Nagy, T. R. Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J. Magn. Reson Imaging 31, 1195–1202 (2010).
Gu, J., Wang, X., Yang, H., Li, H. & Wang, J. Preclinical in vivo imaging for brown adipose tissue. Life Sci. 249, 117500 (2020).
Yaligar, J. et al. Dynamic contrast-enhanced MRI of brown and beige adipose tissues. Magn. Reson Med. 84, 384–395 (2020).
Yu, Q., Huang, S., Xu, T. T., Wang, Y. C. & Ju, S. Measuring brown fat using MRI and implications in the metabolic syndrome. J. Magn. Reson Imaging 54, 1377–1392 (2021).
Wu, M., Junker, D., Branca, R. T. & Karampinos, D. C. Magnetic resonance imaging techniques for brown adipose tissue detection. Front. Endocrinol. 11, 421 (2020).
Michaelis, K. A. et al. Establishment and characterization of a novel murine model of pancreatic cancer cachexia. J. Cachexia Sarcopenia Muscle 8, 824–838 (2017).
Zhang, Y. et al. MRI assessment of associations between brown adipose tissue and cachexia in murine pancreatic ductal adenocarcinoma. Intern. Med. Open Access 9, 1 (2019).
Luan, Y. et al. Development of ovarian tumour causes significant loss of muscle and adipose tissue: a novel mouse model for cancer cachexia study. J. Cachexia Sarcopenia Muscle 13, 1289–1301 (2022).
Abdullahi, A. & Jeschke, M. G. Nutrition and anabolic pharmacotherapies in the care of burn patients. Nutr. Clin. Pr. 29, 621–630 (2014).
Abdullahi, A. & Jeschke, M. G. Taming the flames: targeting white adipose tissue browning in hypermetabolic conditions. Endocr. Rev. 38, 538–549 (2017).
Abdullahi, A. & Jeschke, M. G. White adipose tissue browning: a double-edged sword. Trends Endocrinol. Metab. 27, 542–552 (2016).
Cheong, L. Y. & Xu, A. Intercellular and inter-organ crosstalk in browning of white adipose tissue: molecular mechanism and therapeutic complications. J. Mol. Cell Biol. 13, 466–479 (2021).
Sakers, A., De Siqueira, M. K., Seale, P. & Villanueva, C. J. Adipose-tissue plasticity in health and disease. Cell 185, 419–446 (2022).
Okamatsu-Ogura, Y. et al. UCP1-dependent and UCP1-independent metabolic changes induced by acute cold exposure in brown adipose tissue of mice. Metabolism 113, 154396 (2020).
Wang, Z. et al. Chronic cold exposure enhances glucose oxidation in brown adipose tissue. EMBO Rep. 21, e50085 (2020).
Lee, P. et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63, 3686–3698 (2014).
De Sousa, R. A. L. et al. Physical exercise consequences on memory in obesity: a systematic review. Obes. Rev. 22, e13298 (2021).
Aldiss, P. et al. Exercise-induced ‘browning’ of adipose tissues. Metabolism 81, 63–70 (2018).
Cao, L. et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 14, 324–338 (2011).
Saito, M., Matsushita, M., Yoneshiro, T. & Okamatsu-Ogura, Y. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: from mice to men. Front. Endocrinol. 11, 222 (2020).
Li, G. et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 26, 672–685.e674 (2017).
Horvath, C. & Wolfrum, C. Feeding brown fat: dietary phytochemicals targeting non-shivering thermogenesis to control body weight. Proc. Nutr. Soc. 79, 338–356 (2020).
Murugan, D. D., Balan, D. & Wong, P. F. Adipogenesis and therapeutic potentials of antiobesogenic phytochemicals: Insights from preclinical studies. Phytother. Res. 35, 5936–5960 (2021).
White, J. D., Dewal, R. S. & Stanford, K. I. The beneficial effects of brown adipose tissue transplantation. Mol. Asp. Med. 68, 74–81 (2019).
Lee, C. W., Hsiao, W. T. & Lee, O. K. Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet. Transl. Res. 182, 61–74 e68 (2017).
Morigny, P., Boucher, J., Arner, P. & Langin, D. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 17, 276–295 (2021).
Hu, W. et al. Adipose tissue browning in cancer-associated cachexia can be attenuated by inhibition of exosome generation. Biochem Biophys. Res. Commun. 506, 122–129 (2018).
Han, Y. H. et al. The extract of Arctium lappa L. Fruit (Arctii Fructus) improves cancer-induced cachexia by inhibiting weight loss of skeletal muscle and adipose tissue. Nutrients 12, 3195 (2020).
Vancura, A., Bu, P., Bhagwat, M., Zeng, J. & Vancurova, I. Metformin as an anticancer agent. Trends Pharm. Sci. 39, 867–878 (2018).
Li, M., Li, X., Zhang, H. & Lu, Y. Molecular mechanisms of metformin for diabetes and cancer treatment. Front. Physiol. 9, 1039 (2018).
Oliveira, A. G. & Gomes-Marcondes, M. C. Metformin treatment modulates the tumour-induced wasting effects in muscle protein metabolism minimising the cachexia in tumour-bearing rats. BMC Cancer 16, 418 (2016).
Auger, C. et al. Metformin prevents the pathological browning of subcutaneous white adipose tissue. Mol. Metab. 29, 12–23 (2019).
Koenen, M., Hill, M. A., Cohen, P. & Sowers, J. R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 128, 951–968 (2021).
Molfino, A. et al. Evaluation of browning markers in subcutaneous adipose tissue of newly diagnosed gastrointestinal cancer patients with and without cachexia. Cancers 14, 1948 (2022).
Straughn, A. R. & Kakar, S. S. Withaferin A ameliorates ovarian cancer-induced cachexia and proinflammatory signaling. J. Ovarian Res. 12, 115 (2019).
Hamada, Y. & Hayashi, N. Chewing increases postprandial diet-induced thermogenesis. Sci. Rep. 11, 23714 (2021).
Scotney, H. et al. Glucocorticoids modulate human brown adipose tissue thermogenesis in vivo. Metabolism 70, 125–132 (2017).
Maushart, C. I. et al. Resting energy expenditure and cold-induced thermogenesis in patients with overt hyperthyroidism. J. Clin. Endocrinol. Metab. 107, 450–461 (2022).
Pathak, K., Woodman, R. J., James, A. P. & Soares, M. J. Fasting and glucose induced thermogenesis in response to three ambient temperatures: a randomized crossover trial in the metabolic syndrome. Eur. J. Clin. Nutr. 72, 1421–1430 (2018).
Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933 (2022).
Sun, W. et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102 (2020).
Sarvari, A. K. et al. Plasticity of epididymal adipose tissue in response to diet-induced obesity at single-nucleus resolution. Cell Metab. 33, 437–453.e435 (2021).
Acknowledgements
This study was supported by the CAMS Innovation Fund For Medical Sciences (2021, 2021-1-I2M-002, to Y.Z.), the National Natural Science Foundation of China (2021, 82102810, to C.W.), and the fellowship of the China Postdoctoral Science Foundation (2021, 2021M700501, to C.W.).
Author information
Authors and Affiliations
Contributions
X.Y., Y.C., R.R., and Q.X. were involved in the conception and design of the review. X.Y., Y.C., and R.R. wrote the manuscript. C.W., R.X., J.S., and Q.X. critically reviewed and edited the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors agree to publish the article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, X., Chen, Y., Ruze, R. et al. The evolving view of thermogenic fat and its implications in cancer and metabolic diseases. Sig Transduct Target Ther 7, 324 (2022). https://doi.org/10.1038/s41392-022-01178-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-01178-6
This article is cited by
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases
Signal Transduction and Targeted Therapy (2023)