Abstract
Chronic kidney disease (CKD) is a chronic renal dysfunction syndrome that is characterized by nephron loss, inflammation, myofibroblasts activation, and extracellular matrix (ECM) deposition. Lipotoxicity and oxidative stress are the driving force for the loss of nephron including tubules, glomerulus, and endothelium. NLRP3 inflammasome signaling, MAPK signaling, PI3K/Akt signaling, and RAAS signaling involves in lipotoxicity. The upregulated Nox expression and the decreased Nrf2 expression result in oxidative stress directly. The injured renal resident cells release proinflammatory cytokines and chemokines to recruit immune cells such as macrophages from bone marrow. NF-κB signaling, NLRP3 inflammasome signaling, JAK-STAT signaling, Toll-like receptor signaling, and cGAS-STING signaling are major signaling pathways that mediate inflammation in inflammatory cells including immune cells and injured renal resident cells. The inflammatory cells produce and secret a great number of profibrotic cytokines such as TGF-β1, Wnt ligands, and angiotensin II. TGF-β signaling, Wnt signaling, RAAS signaling, and Notch signaling evoke the activation of myofibroblasts and promote the generation of ECM. The potential therapies targeted to these signaling pathways are also introduced here. In this review, we update the key signaling pathways of lipotoxicity, oxidative stress, inflammation, and myofibroblasts activation in kidneys with chronic injury, and the targeted drugs based on the latest studies. Unifying these pathways and the targeted therapies will be instrumental to advance further basic and clinical investigation in CKD.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is defined as a decline in renal function [glomerular filtration rate (GFR) <60 mL/min per 1.73 m2] or abnormal markers of kidney injury, or both for at least 3 months.1 Renal fibrosis is the common pathology of CKD and accelerates CKD progressing into end-stage renal disease (ESRD) that is an end-stage with high mortality.2 Nephron loss, inflammation, myofibroblasts activation, and extracellular matrix (ECM) deposition are the main processes of the initial and progression of renal fibrosis.3,4 Lipotoxicity and oxidative stress (OS) drive nephron loss.5 The pathological analysis of fibrotic kidney biopsy showed cellular lipid accumulated in all kinds of resident renal cells, including tubular epithelial cells (TECs), podocytes, macrophages, endothelial cells, and mesangial cells.6 Impaired fatty acid consumption, increased lipid synthesis and uptake, and decreased cholesterol efflux contribute to lipid deposition. The expressions and structures of lipoproteins are also changed in CKD.7 Lipotoxicity and modified lipoprotein drive the loss of renal cells by damaging mitochondrial function, promoting OS and inflammation, and even inducing cell death directly.8 Damaged mesangial cells and injured podocytes cause glomerulosclerosis and proteinuria. And tubular damage is related to renal interstitial fibrosis. The injured tubules produce and secret a lot of fibrotic factors to activate interstitial myofibroblasts which are ECM generation machines.9 The competitive uptake of nutrients and abnormal chemokines secretion of injured resident cells altered the inflammatory microenvironment.3,10 For example, damaged TECs produced granulocyte-macrophage colony-stimulating factor persistently that triggered the activation of macrophage, and then the production of MCP-1 that was a ligand of CCR2 expressed by immune cells.11 Immune cells such as macrophages, T cells, and B cells are recruited and activated to produce many profibrotic cytokines and growth factors that activate myofibroblasts and promote ECM deposition.12 The fibrotic microenvironment triggers renal resident cells such as fibroblasts, pericytes, TECs, and endothelial cells, and bone marrow-derived cells like macrophages, mesenchymal stem cells transdifferentiate into myofibroblasts.13 The activation and proliferation of myofibroblasts produce a large amount of ECM. Interstitial ECM expansion accelerates hypoxia and nephron loss. In this review, we discussed the key signaling pathways mediated by lipotoxicity, OS, inflammation, and myofibroblasts activation of CKD. And the effect of inhibitors targeting these pathways on CKD was introduced.
Signaling pathways related to lipotoxicity in chronic kidney disease
Normal lipid metabolism
Lipids are the core energy source and important constructional components of the human body and are essential for normal physiological activities. They are classified into three types: triglycerides, consisting of one glycerol and three fatty acids; phospholipids, which are the major component of the cell membrane; and lipoids, such as cholesterol. Fatty acids are the substrate for adenosine triphosphate (ATP) and some hormones, such as prostaglandin. Fatty acids are categorized into saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) based on the saturation condition of the carbon chain. Palmitic acid (PA) is a well-known SFA, while omega 3 and omega 6 fatty acids are PUFAs. The intake of SFAs has been demonstrated to be associated with cancer, obesity, myocardial infarction, and insulin resistance by activating the nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3)/apoptosis-associated speck-like protein (ASC) inflammasome.14,15,16,17 However, PUFAs act oppositely to the SFAs and play a preventive role for these diseases.18,19 Phospholipids are a group of lipids containing phosphoric acid. The common shared structure of phospholipids is one hydrophilic head connected with two hydrophobic fatty acyl chains. Phospholipids are divided into sphingolipid and glycerophospholipids that include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine, phosphatidylinositol, and phosphatidic acid.20 The main physiological functions of phospholipids are constituting biological membranes and generating bioactive molecules involved in signal transduction.21 Self-synthesis contributes mostly to the cholesterol pool. Cholesterol can be catalyzed into bile acids which are necessary for the digestion of fats, and multiple hormones released by the adrenal cortex and gonads in normal conditions. Cholesterol is also an important component of the cell membrane. A disorder in cholesterol metabolism leads to atherosclerosis, Alzheimer’s disease, and others.22,23 FFAs and cholesterol derived from food items are usually carried by lipoproteins. Lipoproteins are classified into chylomicrons (CM), very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL) based on their densities. The density of lipoproteins is determined by the ratio of lipids to apolipoproteins. The detailed compositions of these lipoproteins have been reviewed elsewhere.24 Triglycerides are mainly transported by CM and VLDL, while cholesterol is transported by CM, VLDL, and LDL and reverse transported by HDL (Fig. 1).
Normal lipid metabolism in tubular epithelial cells (TECs). Triglycerides and cholesterol derived from the diet are mainly carried by CM, VLDL, and LDL. Triglycerides decompose into three FFAs and one glycerol. Fatty acids enter the cells through CD36 or other transporters. Acyl-CoA enters the mitochondrion with the assistance of CPT1 and CPT2. After a complicated process, the acyl-CoA converts into acetyl-CoA, which takes part in the TCA cycle. Acetyl-CoA is also a substrate for palmitic acid and cholesterol synthesis. CM chylomicrons, VLDL very-low-density lipoproteins, LDL low-density lipoproteins, HDL high-density lipoproteins, LDLR low-density lipoproteins receptor, FATP fatty acid transport protein, FABP fatty acid-binding protein, DGAT1 diacylglycerol acyltransferase 1, AGPAT acylglycerolphosphate acyltransferase, HMGCR 3-hydroxy-3-methylglutaryl coenzyme A reductase, SM squalene monooxygenase, ER endoplasmic reticulum, ATP adenosine triphosphate, ACLY ATP citrate lyase, ACC1 acetyl-CoA carboxylase, FASN fatty acid synthase
The metabolism of triglycerides and cholesterol participates in different kinds of important physiological activities in the human body. They are obtained from the diet and then transported to the blood circulation in the form of lipoproteins, which consist of apolipoprotein and lipids. Following hydrolysis by lipase, the fatty acids and glycerol are released from triglycerides. FFAs can be utilized by TECs as their main fuel through CD36 or other transport proteins, such as fatty acid transport protein (FATP) and fatty acid-binding protein.25,26 FFAs are then activated to acyl-CoA by synthetases to make them permeable for mitochondrion. Acyl-CoA passes through the outer membrane of a mitochondrion with the assistance of carnitine palmitoyltransferase-1 (CPT1) and enters through the inner membrane by the transportation of CPT227 (Fig. 1). The acyl-CoA is converted into acetyl-CoA in the mitochondrial matrix through a series of dehydrogenation and thiolysis reactions. Finally, the acetyl-CoA participants in the tricarboxylic acid cycle (TCA). Nicotinamide adenine dinucleotide and flavin adenine dinucleotide, produced through β-oxidation, provide protons in the process of ATP generation in mitochondrion. Excessive FFAs are esterified into acyl-CoA, which assembles in the glycerol-3-phosphate backbone and is stored as triacylglycerol. Furthermore, lipids can also be derived from glucose metabolism, which is termed as de novo lipogenesis. Glucose produces citrate through the TCA cycle, which is converted into PA through the successive catalyzation of ATP citrate lyase or acetyl-CoA carboxylase (ACC1) and fatty acid synthase (FASN)28 (Fig. 1). Acetyl-CoA, a substrate in the TCA cycle, is also used in cholesterol synthesis, which occurs in almost all mammalian cells, except hepatocytes, adrenal cells, and gonadal cells.29 In the action of a series of enzymes, including two rate-limiting enzymes 3-hydroxy-3-methylglutaryl coenzyme A reductase and squalene monooxygenase, the acetyl-CoA is converted into cholesterol around the endoplasmic reticulum (ER)30 (Fig. 1). To balance the cellular cholesterol level, ATP-binding cassette (ABC) transporter subfamily A member 1 (ABCA1) and ABC subfamily G (ABCG) members 1, 5, and 8 are responsible for the cholesterol efflux.29 The metabolites of glucose, lipid, and amino acid metabolism such as ethanolamine (EA), and choline are substrates for phospholipids synthesis. EA and choline are catalyzed into CDP-EA, and CDP-choline by phosphoethanolamine cytidylyltransferase and phosphocholine cytidylyltransferase respectively.31 And CDP-EA, CDP-choline act with diglyceride to form PC and PE respectively. The catabolism of phospholipids is mediated by phospholipase including A1, A2, B1, B2, C, and D. The phospholipase A2 receptor (PLA2R) was revealed as an antigenic target in autoimmune adult membranous nephropathy and anti-PLA2R antibody was regarded as a specific biomarker of membranous nephropathy.32
Disorder of lipid metabolism in CKD
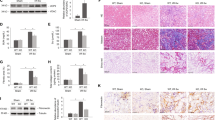
The renal lipid deposition phenomenon has been observed in many animal studies (Table 1). Lipid drops are present in almost all the renal parenchyma cells, suffering from an injury.6 Using large genome-wide transcriptome analysis, an elegant study in 2015 revealed that inflammation and metabolism were the topmost dysregulated pathways in human diseased kidneys.33 Kang et al. found low levels of key enzymes and regulators related to fatty acid oxidation (FAO) and accumulation of lipids in TECs in both the human and mouse fibrotic kidneys. The major fatty acid subtypes, which were deposited in the kidney of the mice with the TEC conditional overexpression of long-chain fatty acid transporter CD36, were stearic, palmitic, linoleic, and docosahexaenoic acids (DHA).33 The deposition of lipids in TECs was also found in angiotensin II-infused rats, high-fat diet (HFD) mice, and cisplatin-induced nephrotoxicity models.34,35,36 More importantly, this phenomenon was also found in human studies. For example, lipids accumulated in mesangial cells, podocytes, and TECs of patients with obesity-related kidney disease and diabetic nephropathy (DN).37,38
Other than lipid accumulation in kidney tissues, CKD patients always have dyslipidemia, which is characterized by high levels of serum triglycerides, LDL-cholesterol (LDL-C), and low HDL-cholesterol (HDL-C) levels. The high levels of LDL-C and oxidative-LDL (ox-LDL) are independent risk factors for CVD, which is the leading cause of death among patients with eGFR <60 mL/min per 1.73 m2.39,40 Dyslipidemia in nephrotic syndrome (NS) is characterized by high LDL and VLDL levels and normal HDL levels, which are different from uremia dyslipidemia.41 Despite the comparable level of HDL in NS patients and healthy people, the reverse cholesterol transportation function of HDL may be impaired.42
Key signaling pathways of renal lipid deposition and altered lipoprotein metabolism
Normal cellular lipid level is maintained by the balance of lipid synthesis, FAO, lipid uptake, and export. Abnormality in any of the normal processes destroys the balance, leading to lipid deposition (Fig. 2). The characteristics of lipid metabolism in CKD are a disturbance in FAO, increased lipid synthesis, enhanced lipid uptake, and obstructed lipid efflux. The levels and structures of lipoproteins are altered in CKD due to proteinuria and the uremic microenvironment.
Mechanisms of renal lipid deposition. TGF-β/Smad3 signaling and Notch1/HES1 signaling mediate the downregulation of PGC-1α, which is a key regulator of the PPAR family and decreases the expression of FAO-related genes. ATF6α and miR-21 transcriptionally decrease the expression levels of PPAR-α. The miR-33 and loss of KLF15 downregulate the expression of FAO-related genes directly. The low level of AMPK activates ACC1, which promotes the formation of monyl-CoA and decreases the expression of CPT1a. The activation of JAML inhibits the SITR1-AMPK axis, resulting in SREBP1 upregulation and lipid synthesis. STK-25 is also involved in lipid synthesis. TNF-α inhibits the nuclear translocation of NFATC1 and blocks the cholesterol efflux by ABCA1. VEGF-β, CD36, and KIM-1 mediate the ectopic uptake of fatty acids in CKD. TGF-β transforming growth factor-β, PPAR peroxisome proliferator-activated receptor, PGC-1α PPARγ coactivator 1α, FAO fatty acid oxidation, ATF6α activating transcription factor 6α, KLF15 Krüppel-like factor 15, AMPK AMP-activated protein kinase, ACC1 acetyl-CoA carboxylase 1, CPT1a carnitine palmitoyltransferase 1a, JAML junctional adhesion molecule-like protein, SREBP element-binding protein, STK-25 serine/threonine-protein kinase 25, TNF-α tumor necrosis factor-α, NFATC1 nuclear factor of activated T cell 1, ABCA1 ATP-binding cassette transporter A1, VEGF-β vascular endothelial growth factor-β, KIM-1 kidney injury molecule-1
Destruction of FAO
The disturbance in the main consumption way of fatty acids, β-oxidation, causes lipid accumulation and direct renal damage. Transcriptomic sequencing analysis showed the downregulation of FAO-related genes, including CPT1a, acyl-coenzyme A oxidase 1 (ACOX1), ACOX2, peroxisome proliferator-activated receptor α (PPARα), and PPARγ coactivator 1α (PPARGC1A, coding PGC-1α) in the human and mice fibrotic kidney.33,43
The mitochondrion is an important organelle, where FAO is carried out and its injury is always associated with FAO disturbance. Transforming growth factor-β (TGF-β) that is the master driving factor of renal fibrosis has been demonstrated to damage FAO by decreasing the expression of mitochondrial biogenesis determinant gene, PGC-1α in the Smad3-dependent pathway.33,44 TECs specifically overexpressing PGC-1α normalized transcript levels of Cpt and other FAO enzymes after TGF-β treatment.33 And PGC-1α modulation correlates directly to altered lipid levels. Hepatic PGC-1α overexpression reduced triacylglycerol (TAG) storage and secretion, resulting in decreased plasma TAG levels.45 Transgenic mice with tubule-specific overexpression of intracellular Notch1 domain showed low renal mitochondrial contents and defective FAO, which were reversed by the overexpression of PGC-1α.46 Mechanistically, the transcription factor Hes1 regulated by Notch signaling decreased the expression of PGC-1α by binding to its promoter.46
PPAR family, especially the PPARα, is known as the master regulator of FAO since the β-oxidation level of long-chain fatty acid in PPARα knockout mice decreased by 70% compared to wild-type mice.47,48 PPAR agonists, such as fenofibrate, play a renal protective role in a series of kidney diseases, including ischemia-reperfusion injury, DN, HFD-induced nephropathy, and hypertensive nephropathy.49,50 The kidneys of aged PPARα-knockout mice showed renal lipid accumulation and decreased expression levels of FAO-related genes, such as CPT1a, ACOX1, medium-chain acyl-CoA dehydrogenase (Mcad), and Acyl-CoA dehydrogenase family member 9 (ACad9).51 Negative regulators of PPARα, miR-21, and transcription factor ATF6α led to successive lipid deposition, mitochondrial dysfunction, and renal fibrosis.52 Another member of the PPAR family, PPAR-γ, which is highly expressed in the adipocytes, is a key regulator of adipogenesis and lipid metabolism.53,54 Decreased expression of PPAR-γ could alter the microenvironment of adipose tissues, which was characterized by white adipocyte hypertrophy and increased inflammation.55 These adipocytes release FFAs and metabolites, including inflammatory factors, such as interleukin-6 (IL-6), and tumor necrosis factor (TNF-α), and hormonal factors, such as leptin, angiotensin II, which mediate kidney injury.56 In other tissues, like the liver, kidney, and heart, PPAR-γ is also expressed at a low level. PPAR-γ regulates many lipid transport- and metabolism-related genes, such as acyl-CoA synthase, FATP, CD36, and lipoprotein lipase (LPL).57 Global PPAR-γ knockout mice showed renal hypertrophy and increased albuminuria in 3rd week. In the 7th week, the mice showed decreased creatinine clearance and developed type 2 diabetes, while the 52 weeks old mice showed server inflammation and renal fibrosis.58 However, to the best of our knowledge, studies demonstrating the correlation between PPAR-γ and renal lipid deposition are limited.
AMP-activated protein kinase (AMPK), a low-energy sensor, is known to be inactive in CKD.59 The low level of AMPK could activate ACC2 and promote the formation of malonyl-CoA, leading to the downregulation of CPT1a.60 The upregulation of AMPK by metformin treatment phosphorylated ACC1 at Ser79 and improved FAO.61 Furthermore, AMPK could also induce mitochondrial biogenesis by promoting the expression of PGC-1α, thereby facilitating FAO.62 The loss of miR-33 could protect mice from unilateral ureteral obstruction (UUO) and folic acid-induced renal fibrosis by recovering the FAO-related factors.63 A recent study revealed another transcriptional factor, Krüppel-like factor 15 (KLF15), which was highly expressed in the normal proximal TECs and could mediate the expression of CPT1a and acetyl-coenzyme A acyltransferase 2 (ACAA2).64 In the kidney of UUO mice, diminishing KLF15 transcriptionally downregulated CPT1a and ACAA2, resulting in compromised FAO and renal fibrosis.64
Lipogenesis
Fat tissues and the liver are the main sites for the synthesis of triglyceride and cholesterol, respectively. However, when the kidney suffers from an injury, lipid metabolism is changed. Sterol regulatory element-binding protein (SREBP) is an important transcription factor, which regulates the biosynthesis of cholesterol, fatty acids, triacylglycerol, and phospholipids.29,65 SREBP1a overexpression transgenic mice showed increased levels of FASN and ACC, resulting in glomerular triglyceride deposition, mesangial expansion, glomerulosclerosis, and proteinuria.66 The upregulation of SREBP in aged kidneys and DN resulted in lipid accumulation in the glomerulus.66,67 Fu et al. revealed that the junctional adhesion molecule-like protein expressed by the damaged podocytes causes an increased lipid synthesis through the SIRT1-AMPK-SREBP1 axis.68 Besides lipotoxicity, SREBP directly mediates renal fibrosis by increasing the expression levels of fibrotic factors, such as TGF-β1, plasminogen activator inhibitor-1 (PAI-1), and COL6A1.69 Transcription factor farnesoid X receptor (FXR), a bile acid-activated nuclear hormone receptor, protects kidneys by inhibiting the SREBP1.70 The mRNA and protein levels of FXR were found to be decreased in human DN and obesity-induced nephropathy.71 Serine/threonine-protein kinase 25 promotes lipid deposition and renal injury by increasing the expression levels of fatty acid de novo synthesis genes, such as FASN and ACC1.72
Enhanced uptake and disturbed export of lipid
CD36 is a major transporter for the uptake of FFAs and is widely expressed in the renal parenchymal cells, including TECs, macrophages, podocytes, mesangial cells, and endothelial cells.26 Immunohistochemical staining of kidney biopsy from the CKD patients showed that the upregulation of CD36 was correlated with lipid deposition.38,73 The tubule-specific overexpression of CD36 in transgenic mice exhibited excessive lipid amassing.33 Falkevall et al. demonstrated that the increased level of vascular endothelial growth factor- β (VEGF-β) in the podocytes of DN mice could promote the uptake of FFAs by upregulating the FATP4.74 FFAs bound albumin in the urine can be reabsorbed by TECs. Transmembrane fatty acid transporter-2 (FATP-2) exclusively expressed on the apical side but not on the basolateral side of tubules mediates the uptake of fatty acid-bound albumin.75 Besides transporting proteins, the TECs can also uptake the fatty acids-bound albumin through endocytosis.27 Kidney injury molecule (KIM)-1 is a well-known tubular injury factor for both acute kidney injury (AKI) and CKD. However, a recent study by Yutaro et al. showed that the increased level of KIM-1 in DN could mediate the endocytosis of PA-bound albumin, leading to tubular DNA damage, cell cycle arrest, inflammation, and fibrosis.76
ABCA1 is a transporter, which mediates the cellular cholesterol and phospholipid efflux in an ATP-dependent pathway.77 A large-scale transcriptional analysis of ABCA1 in the DN patients revealed a decrease in its expression in the early stage of DN.78 Podocytes with the ABCA1 gene knocked out aggravated the podocyte injury in ob/ob mice by inducing the accumulation of cardiolipin and mitochondrial dysfunction. And ABCA1 inducer A30 ameliorated the glomerular sclerosis and proteinuria in db/db mice.79 TNF-α treatment downregulated the expression of nuclear factor of activated T cells 1/ABCA1 axis, leading to the cholesterol-induced podocytes injury.80
Lipoprotein metabolism disorder
The metabolism, structure, and molecular composition of lipoproteins in CKD patients differ from healthy people. CKD patients are always accompanied by low levels of HDL and high levels of LDL. The main apolipoprotein of HDL is ApoA-I. However, the expression of ApoA-I in CKD patients and animals is decreased. The possible reasons are reduced biosynthesis and increased urine excretion.81 The mature process of HDL needs lecithin-cholesterol acyltransferase (LCAT) to catalyze the esterification of the cholesterol. But, the expression of LCAT was downregulated and the catalyzed activity was impaired in patients with CKD. This impaired the maturation of HDL.82 Except for the decreased levels, the composition of HDL has been changed in CKD patients. Uremic solute, symmetric dimethylarginine (SDMA) accumulates in CKD patients due to the decreased urine excretion and forms complex with HDL. SDMA enriched HDL was no longer beneficial for cardiovascular diseases but caused endothelial cell dysfunction.83 Acute phase reaction protein, serum amyloid A (SAA1) was significantly elevated in the serum of CKD patients and accelerated renal interstitial fibrosis by inducing ER stress.84 SAA1 can replace ApoA-I and become the main apolipoprotein of HDL, leading to the proinflammatory effect and reduced cholesterol efflux capacity.24 The ratio of ApoC-III was elevated in CKD patients and resulted in HDL dysfunction.85,86 The uremia microenvironment promotes post-translational modifications such as carbamylation and oxidation of lipoproteins. Carbamylation modification of lysine residues of ApoA-I rendered HDL dysfunction and was associated with increased mortality outcomes in patients with type 2 diabetes.87 The disordered metabolism of LDL in CKD includes increased levels and post-translational modifications. Reduced LDL catabolism and the associated increased LDL residence time contribute to the increased LDL levels and enhance post-translational modifications such as oxidation, carbamylation, and nitration of LDL.39 Oxidized LDL (ox-LDL) impaired the binding ability with LDL receptors, resulting in decreased clearance in the liver. And hepatic LDL receptor dysfunction is also one of the mechanisms of reduced LDL catabolism.88 Increased scavenger receptors, such as lectin-like-oxidized LDL receptor 1 (LOX1), CD36, scavenger receptor-A1 (SR-A1), and SR-A2 in CKD mediate the increased uptake of ox-LDL, leading to the activation of inflammatory signaling pathways.89,90 Increased ApoC-III levels and reduced ApoC-II to ApoC-III ratio block the activity of LPL and hepatic triglyceride lipase that mediate the conversion of VLDL to intermediate-density lipoprotein (IDL) and IDL to LDL, respectively. This process increases the production of LDL.24
Signal pathways mediated lipid nephrotoxicity
Lipids are usually stored in fat tissues in the form of lipid drops. When lipids accumulate in other tissues and cause damaging effects, the phenomenon is known as lipotoxicity.8 Lipid nephrotoxicity was first proposed by Moorhead et al. in 1982.91 The deposition of lipids, including FFAs, cholesterol, and phospholipids, induces mitochondrial dysfunction, OS, inflammation, ER stress, and even apoptosis of renal cells (Fig. 3). Lipid accumulation is associated with increased proteinuria, reduced eGFR, and renal fibrosis. Lipid-lowering drugs, such as statins, attenuate the progression of CKD and reduce proteinuria.92 Altered lipoproteins promote CKD progression by triggering the OS and inflammation process.
Lipotoxicity of tubular epithelial cells and podocytes. a In TEC, the accumulation of FFAs triggers mitochondrial dysfunction directly or by decreasing autophagy. Hydrogen peroxide produced by injured mitochondria activates JNK and cleaves caspase-3, leading to the apoptosis of TEC. Apoptosis caused by FFA is also mediated by the activation of the RAAS system. FFAs induce the production of ROS, which activates inflammasome and damages DNA. DNA damage inhibits the cell cycle and causes cell senescence. The senescent TEC releases cytokines and chemokines, which recruit immune cells, such as macrophages and fibrotic factors, which activate fibroblasts. b In podocytes, the ox-LDL induces loss of nephrin through PI3K/Akt signaling. FFAs cause mitochondrial dysfunction, oxidative stress, and ER stress, resulting in the derangement of cytoskeleton and apoptosis of podocytes. FFA-TSP1 vicious cycle aggravates the lipid damage. TEC tubular epithelial cell, FFA free fatty acid, JNK c-Jun N-terminal kinase, Ang II angiotensin II, RAAS renin-angiotensin system, SASP senescence-associated secretory phenotype, KIM-1 kidney injury molecule-1, ROS reactive oxygen species, PI3K phosphoinositide 3-kinase, MAPK mitogen-activated protein kinases, ER endoplasmic reticulum, TSP1 thrombospondin1
Tubular epithelial cells
Renal tubule cells are the most abundant cells in the kidney and maintain the functions of renal filtration and reabsorption. Tubular injury or apoptosis accelerates the CKD progression by the loss of nephron, thereby aggravating the inflammation and fibrosis. KIM-1 mediates the endocytosis of PA-bound albumin, leading to reduced mitochondrial transmembrane potential and mitophagy.76 Mitochondrial dysfunction and lipid overload increase the production of reactive oxygen species (ROS), which triggers the activation of inflammasome and secretion of IL-1β, IL-6, and TNF-α. ROS also induces DNA damage and G2/M cell cycle arrest in TECs, resulting in the cell senescence and partial epithelial to mesenchymal transition (partial EMT).5,76 Damaged tubules exhibit senescence-associated secretory phenotype that is characterized by secreting profibrotic factors, such as TGF-β1, Wnt, and sonic hedgehog. These factors promote the activation and proliferation of fibroblast and the production of ECM in renal interstitial fibrosis (Fig. 3).2 Another study revealed that the increase of fatty acid-bound albumin but not the albumin in TECs impaired the mitochondrial function and over oxidated the peroxiredoxin 2 (PRDX2). Then, the increased peroxide levels activated the p- c-Jun N-terminal kinase (JNK) /caspase-3 apoptotic pathway, resulting in the apoptosis of cells.93 HFD induces tubular lysosomal acidification and decreases autophagy, thereby exaggerating the mitochondrial dysfunction and inflammasome activation.94 These studies demonstrate that mitochondrial dysfunction plays a key role in the FFA-induced damage to the TECs. The mechanism of mitochondrial damage caused by lipids has not been clarified yet. The mitochondrial injury might be induced by mitochondrial FFA or ox-LDL overload due to the presence of their receptor CD36 on the mitochondrial membrane.26 PA treatment also induced the expression of angiotensin II. And the activation of the renin-angiotensin system (RAAS) caused ER stress.95 Although much evidence showed FFA impaired TECs directly, Kang et al. demonstrated that the transgenic mice with tubular-specific overexpression of CD36 showed tubular lipid accumulation but not renal fibrosis. Transgenic mice showed higher levels of ECM-related genes at 20 weeks old, but the difference was insignificant as compared to the wild-type mice.33 It was suspected that obvious statistical differences might be observed in elder mice.
As FAO is the preferred energy source of TECs, defective FAO causes tubular damage naturally.33 CPT1a is a key rate-limiting enzyme for the β-oxidation of FFAs. Tubular-specific knockout of CPT1a gene in mice showed elevated levels of serum creatine, proteinuria, tubular damage, and tubular interstitial fibrosis at the age of 18–20 months.96 Among the CKD patients, renal CPT1a level was negatively correlated with fibrosis score.97 Furthermore, lower oxygen consumption rates and the impaired mitochondrial complex I and V of primary TECs isolated from the 9-month-aged CPT1a knocked out transgenic mice indicated that the disturbance in β-oxidation caused mitochondrial damage and then renal injury.96 And tubular-specific overexpression CPT1a mice showed attenuated renal fibrosis, inflammation, and tubular injury when suffering from UUO, folic acid nephropathy, and adenine-induced nephrotoxicity compared to wild-type mice.97 CPT1a inhibitor etomoxir could aggravate folic acid nephropathy by inhibiting the FAO.33
Podocytes
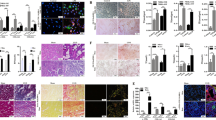
The podocyte foot processes effacement and podocyte apoptosis are the leading cause of proteinuria and subsequent glomerular sclerosis. Clinical and animal studies have revealed lipid biology as a key determinant of podocyte function.98 As compared to TECs, the podocytes are highly susceptible to saturated FFAs, but not to monounsaturated FFAs.99 PA causes mitochondrial dysfunction and release of calcium ions from ER, leading to the derangement of podocyte cytoskeleton and apoptosis.99,100,101 The increased uptake of FFA mediated by CD36 induces the upregulation of thrombospondin1 (TSP1) which is also a ligand of CD36 in a p38 mitogen-activated protein kinase (MAPK) signaling-dependent way. And TSP1 treatment induces podocyte apoptosis.102 The TSP1-deficient mice showed the attenuation of obesity, diabetes, and adriamycin-associated podocytes apoptosis.102,103,104 The vicious cycle of FFA and TSP1 is an important mechanism of podocyte injury (Fig. 3). PA also inhibits the phosphorylation of insulin receptors, such as IRS1 and PKB, and blocks insulin-stimulated glucose uptake in human podocytes.105 The insulin resistance of podocytes is associated with podocytes damage and loss of podocytes foot processes.106 Although fatty acids are probably not the preferred energy source of podocytes, FAO disturbance causes podocytes’ injury. The stimulation of FAO by adiponectin, which is an activator of AMPK or aminoimidazole-4-carboxamide-1-D-ribofuranoside (AICAR), protects the podocytes from PA injury, while the CPT1a inhibitor augments PA toxicity and abolishes the beneficial effects of AICAR.60 Besides FFAs, other lipids damage the podocytes too. The peroxidation of mitochondrial cardiolipin mediated by ABCA1 promotes podocytes injury in DN.79 The ox-LDL inhibits the phosphorylation of phosphatidylinositol 3-kinase (PI3K) and Akt, leading to the redistribution and loss of nephrin.107 The G1 and G2 renal-risk variants of the apolipoprotein L1 gene (APOL1) have caused glomerulosclerosis in many African Americans.108
Mesangial cells
Mesangial cells in normal glomerulus secrete minimal matrix to support capillary loops. However, the FFAs and ox-LDL cause the direct injury of mesangial cells. PA treatment-induced monocyte chemoattractant protein-1 (MCP-1) expression of mesangial cells by inhibiting the phosphorylation of AMPK.109 Increased MCP-1 recruited macrophages, dendritic cells, and T cells from bone marrow by arising the expression of intercellular adhesion molecule 1 (ICAM-1) and enhancing monocyte adhesion, resulting in severe inflammation of CKD.11,110 Furthermore, PA could also increase the expression of protein arginine methyltransferase 1 and induce mesangial apoptosis by activating the ER stress.111 The inhibition of FAO by etomoxir increased the PA-induced mesangial apoptosis.112 The glycation of apolipoprotein B reduces the binding affinity of LDL to LDL receptors in mesangial cells, leading to the trapping of LDL in the ECM.113 The uremic microenvironment modifies LDL into ox-LDL. The uptake of ox-LDL mediated by scavenger receptors activates the PAI-1 transcription through TGF-β signaling.114 The increased uptake of cholesterol and triglycerides transforms mesangial cells into foam cells.37,115 Injured mesangial cells lose contractile function and produce excessive ECM, leading to glomerular sclerosis.
Macrophages and endothelial cells
A small number of macrophages are renal resident cells. However, the lipotoxicity of other renal cells promotes the release of chemokines and cytokines, which recruit and activate macrophages from blood circulation. Activated macrophages produce much more inflammatory cytokines, ROS, and nitric oxide (NO), leading to further renal damage. The upregulated expression of ICAM-1 in glomerular endothelial cells by ox-LDL treatment increases the adhesion and infiltration of monocytes within the glomerulus.116 Lipid also acts directly on macrophages and mediates the progression of CKD and its cardiovascular complications. The uptake of ox-LDL and modified lipoproteins by macrophages aggravate glomerulosclerosis and increase the risk of atherosclerosis.117 Ox-LDL stimulates the activation of caspase-3 and promotes the apoptosis of macrophages in both the CD36 and Toll-like receptors (TLR2)-dependent pathways, leading to plaque necrosis in advanced atherosclerosis.118,119 And atherosclerosis aggravation can elevate the mortality of CKD. The knockout of CD36 in macrophages ameliorated renal lipid deposition, followed by preserved renal function and reduced fibrosis.120
The intact endothelial cells in tubular interstitial space guarantee the transportation of serum oxygen. Besides oxygen, serum lipids are also carried by vessels. VEGF-β specifically controls the FFA uptake of endothelial cells by upregulating vascular FATPs.121 Therefore, the expression of VEGFs determines the tissue distribution of serum lipids. Ablation of VEGF-β reduced renal lipid accumulation in db/db mice.74
Therapeutic opportunities of CKD based on lipid metabolism
The above-mentioned studies showed a strong association between lipotoxicity and CKD progression. Therefore, the lipid-lowering strategy might be a potential therapy for CKD. The possible lipid-lowering drugs and their effects on preserving eGFR and reducing proteinuria and the risk of ESRD are summarized in Table 2. Alternative drugs for reducing the lipid accumulation of DN have been reviewed elsewhere.8
Statin
Statin is the inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) that is a rate-limiting enzyme of cholesterol synthesis and is regarded as the primary lipid-lowering drug for CKD patients. SHARP (Study of Heart and Renal Protection) studies revealed that the CKD patients, who did not undergo dialysis and were treated with simvastatin (20 mg daily) and ezetimibe (10 mg daily), showed a reduction in major atherosclerotic events, nonhemorrhagic stroke, and arterial revascularization by 17%, 25%, and 21%, respectively, as compared to the placebo group. However, for the CKD patients, receiving either hemodialysis or peritoneal dialysis (PD), the statin treatment had little or no beneficial effect on CVD.122,123 A similar conclusion was also revealed by the clinical trials of 4D (German Diabetes Dialysis Study) and AURORA (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events).124,125 Besides reducing the CVD risk, statins, such as atorvastatin, also showed a modest beneficial effect on eGFR.126 Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease (PLANET I) trial indicated that the treatment of 80-mg atorvastatin decreased urine protein to creatinine ratio by 13% after 52 weeks as compared to baseline.127 Statins play a protective role for both cardiovascular and renal diseases in CKD patients. The molecular mechanisms for the renal protective role of statins include not only the renal advantageous effect of reducing lipid deposition, but also include its direct anti-inflammatory, antioxidant, pro-apoptotic, and antifibrotic effects on renal parenchymal cells.128,129 Simvastatin restricted the proliferation of mesangial cells by reducing the expression of cell cycle-dependent kinase and reduced proteinuria of spontaneously hypertensive rats fed with HFD by protecting endothelial cells from OS.130,131 Lovastatin reduces the expression of MCP-1 and macrophage infiltration in the glomerulus.132 Different statins can inhibit podocyte injury and apoptosis by activating the PI3K/AKT-signaling pathway.107 It is worth noting that the high dose of lovastatin treatment might induce apoptosis and inhibit the proliferation of TECs.133,134
PPAR agonist
The transcription factor, PPAR-α, is primarily involved in the regulation of FAO. Fenofibrate is a selective agonist of PPAR-α. Fenofibrate prevented the FFA-induced lipid accumulation and OS of mesangial cells and TECs in vitro.49 Consistently, fenofibrate ameliorated glomerular injury, inflammation, and renal fibrosis in vivo.33,49 Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study enrolled the elder type 2 diabetic patients with moderate eGFR (≥30 mL/min/1.73 m2) to investigate the effects and safety of fenofibrate on CVD and ESRD. The result after a 5-year follow-up showed fenofibrate reduced the total cardiovascular events and preserved renal function without any adverse effects.135 However, lowering the triglyceride levels might increase the serum creatine levels in the patients with eGFR <30 mL/min/1.73 m2,.136 Another agonist of PPARα, clofibric acid, acted to improve cardiac function and decrease the excretion of urine albumin but not serum creatine in 5/6 nephrectomy rats.137,138
PPARγ agonists, the thiazolidinedione (TZD) family, not only regulate lipid metabolism, but also protect the kidney from multiple damaging processes, such as inflammation, aging, and OS.139 A randomized controlled trial (RCT) results showed rosiglitazone inhibited the increase in albumin/creatinine ratio (ACR) as compared to metformin and preserved eGFR as compared to glyburide in type 2 diabetic patients.140 The prevention of CKD progression by TZDs has also been demonstrated in various animal studies.141 For example, pioglitazone preserved mitochondrial function and attenuated renal fibrosis in 5/6 nephrectomy rats and aging rats.142,143
CD36 antagonist
CD36 plays a key role in mediating the renal lipid uptake. Souza et al. revealed that apoA I-mimetic peptide 5A could attenuate renal fibrosis and reduce macrophage infiltration in the UUO mice. The peptide 5A treatment did not show fewer renal lesions than the CD36 knockout mice. This indicated that the peptide 5A might play a renal protective role by antagonizing CD36.144 The anti-CD36 antibody could inhibit the secretion of TGF-β1 induced by the advanced oxidation protein products (AOPPs) treatment.145 CD36 peptide (p93-110) that specifically inhibited the interaction of CD36 and TSP1 restored the FFA-induced apoptosis of podocytes.102
Mitochondrial protective drugs
Mitochondrion is a major place for FAO in TECs. Thus, protecting mitochondrion facilitates lipid metabolism keeping balance. A promising mitochondria-targeting drug, Szeto-Schiller peptide 31 (SS-31) specifically targeted cytochrome c and kept mitochondrial membrane potential abolished lipotoxicity, loss of podocytes and endothelial cells, glomerulosclerosis, and inflammation in HFD mice.146 Besides its role in HFD mice, the renal protective effects of SS-31 have also been demonstrated in various models, including warm ischemia-reperfusion, UUO, postischemic CKD, and aging mice.147 Mitochondria-targeting anti-oxidation drug, MitoQ, prevented the glomerular hypertrophy and mesangial expansion in db/db mice.148 A Chinese traditional medicine, berberine, could improve mitochondrial function and reduce lipid deposition in podocytes by promoting the PGC-1α expression.149 The PPAR and AMPK agonists discussed in other sections in this review could also promote mitochondrial biogenesis.62 The mitochondria-targeting drugs are well-reviewed elsewhere.62,147,150
PUFA
We have discussed the disadvantages of SFAs, however, not all FAs are bad for kidneys. PUFAs, especially, the long-chain n-3 PUFA, including DHA and eicosapentaenoic acid, are the good FAs. Various clinical trials and animal experiments have demonstrated that PUFAs could reduce albuminuria and ameliorate hypertension, inflammation, and glomerulosclerosis in DN.151 However, recent studies draw different conclusions. A meta-analysis revealed that the supplementation of n-3 PUFA caused a decrease in the cardiovascular death of CKD patients with hemodialysis and prevented ESRD among the CKD patients, who did not receive renal replacement.152 But, the evidence certainty of these conclusions was very low due to the small sample size, low event rates, and presence of moderate heterogeneity of the trials included in the analysis. An RCT study tested the effects of n-3 PUFA on renal injury in type 2 diabetic patients. The results suggested that, as compared to placebo, the n-3 PUFA treatment (4 g/day) had no effects on the excretion of urine albumin, serum markers of kidney function, and eGFR. Only urine neutrophil gelatinase-associated lipocalin levels decreased in the n-3 PUFA treated group.153 The results were consistent with the Vitamin D and Omega-3 Trial (VITAL), which concluded that the supplementation of vitamin D3 or omega-3 fatty acids could not preserve kidney function in type 2 diabetic patients.154 Although its monotherapy did not prevent CKD progression, the n-3 PUFA enhanced the effects of RAAS inhibitors in decreasing proteinuria and renal injury markers.153
Bile acid receptors agonist
The activation of bile acid receptors, including FXR and takeda G protein-coupled receptor 5 (TGR5) which increase FAO are promising therapeutic targets for CKD.155 FXR agonist GW4064 decreased glomerulosclerosis and interstitial fibrosis by inhibiting the SREBP1 expression.156 Selective FXR agonist INT-747 modulated lipid accumulation and improved renal injury, such as proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis in streptozotocin (STZ)-induced DN mice.70 The possible mechanism might be inhibiting the transcriptional activity of NF-κB. A selective TGR5 agonist INT-777 induced mitochondrial biogenesis and then increased the FAO in obesity-associated nephropathy and DN.157 A dual FXR/TGR5 agonist INT-767 played a renal protective role in diabetes- and obesity-related kidney diseases by affecting multiple pathways related to the lipid metabolism, such as the upregulation of AMPK, SIRT1, PGC-1a, and Nrf-1.71
Other drugs targeting lipid metabolism
AMPK plays an important role in multiple lipid metabolism activities including enhancing FAO by regulating CPT1a expression and mitochondrial biogenesis, and inhibiting lipid synthesis. AMPK agonist A-769662 (A76) ameliorated renal fibrosis in 5/6 nephrectomy mice fed a high-protein (HP) diet.59 AICAR improved lipid accumulation, mitochondrial and lysosomal dysfunction, and renal fibrosis in HFD mice.158 Anthocyanins and resveratrol decreased albuminuria and improved the expansion of the glomerular matrix and inflammation in db/db mice via the phosphorylation of AMPK.159,160 A recent study demonstrated that a small molecule TW-37 could inhibit the uptake of KIM-1-mediated PA-albumin and ameliorate renal inflammation and fibrosis in vivo.76 ABCA1 inducer A30 ameliorates podocyte injury and CKD by reducing the cardiolipin oxidation.79
Nonpharmacological therapy
Besides artificial drug therapy, lifestyle modification is also important for preventing CKD progression. A meta-analysis summarized 17 studies that observed the effects of body weight loss through lifestyle, pharmacological or surgical intervention on the CKD patients, who were overweight or obese. The results showed a decrease in the level of LDL-cholesterol due to weight loss. However, the total cholesterol, HDL-cholesterol, and C-reactive protein had no significant changes. The effects of weight loss on the reduction of proteinuria, blood pressure, and cardiovascular death are uncertain.161 An RCT study investigated the additive anti-proteinuria effects of weight loss on angiotensin II receptor blockers (ARBs) in hypertensive CKD patients.162 Authors revealed that weight loss was beneficial for proteinuria reducing the effect of ARBs. Another trial also observed a significant reduction of serum creatinine and cystatin C levels in a short-term intensive weight reduction intervention group of DN patients.163
Signaling pathways and targeted therapies in oxidative stress
Reactive oxygen is a side product of oxidative phosphorylation in the mitochondrion. And it can be cleared by antioxidants such as superoxide dismutase (SOD), and glutathione. However, when the balance between oxidants and antioxidants is disturbed, excessive oxidants including ROS and reactive nitrogen species (RNS) lead to renal injury. And the process is regarded as the OS of the kidney. As high oxygen consume organ, the kidney is vulnerable to OS damage. In this part, we will discuss the signaling pathways of oxidants generation, OS, and the targeted therapies for CKD (Fig. 4).
Key signaling pathways mediated oxidative stress. NOXs are core enzymes mediated oxidants production. Nox4 is upregulated in the kidney by Ang II and TGF-β pathways or by oxidation end products AOPP. Upregulated Nox4 induces excessive ROS accumulation and activates downstream pathways including ERK, mTORC, PKB, and MAPK signaling. Excessive ROS activates the NF-κB pathway, inducing chronic inflammation and renal fibrosis by upregulating the expression of CXCR4 and β-catenin. As positive feedback, NF-κB activation increased the expression of NOX and NOS to create more ROS. Downregulation of the SIRT family inhibits Nrf2, which is a positive regulator of antioxidant proteins and an inhibitor of NF-κB. The reduction of antioxidants increases the expression of ASK1 that activates the p38 and JNK. Red fonts in brackets are inhibitors. NOX nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, NOS nitric oxide synthase, Ang II angiotensin II, TGF-β transforming growth factor-β, ROS reactive oxygen species, AOPP advanced oxidation protein products RAGE receptor of advanced glycation end products, ERK extracellular regulated protein kinases, mTORC mammalian target of rapamycin complex, PKB protein kinase B, MAPK mitogen-activated protein kinase, NF-κB nuclear factor kappa-B, SIRT sirtuins, Nrf2 nuclear factor erythroid 2-related factor 2
Signaling pathways of oxidative stress in CKD
ROS, which includes the superoxide anion (O2–), hydrogen peroxide (H2O2), and hydroxyl radicals (OH), are mainly catalyzed by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and xanthine oxidase (XO) in mitochondria.164 Among NOX family members, Nox4 has been best characterized in the kidney. Nox4 was proved to be upregulated in most renal resident cells including TECs, podocytes, mesangial cells, and endothelial cells after renal injury, and promoted the progression of kidney disease.165 Smad3 and extracellular-signal-regulated kinase (ERK1/2) signaling activation triggered by TGF-β increased the expression of Nox4.166,167 Ang II treatment also induced the upregulation of Nox4, and the successive ROS accumulation activated ERK, Akt, PKB, and eGFR signaling.165,168,169 Podocyte-specific induction of Nox4 in vivo stimulated ER stress, and expression of hypoxia-inducible factor-1α (HIF-1α) and TGF-β, leading to glomerulosclerosis and albuminuria.170,171 And podocyte-specific Nox4 deletion attenuated albuminuria, glomerulosclerosis, mesangial expansion, as well as glomerular basement membrane thickness by inhibiting renal ROS, glomerular MCP-1, and protein kinase C alpha (PKC-α).172 The upregulated Nox4 and H2O2 activated mTORC1, which contributed to salt-induced hypertension and renal injury in the SS rat model.173 Excessive ROS triggered inflammatory NF-κB signaling and promoted the progression of renal fibrosis.174,175 Circularly, NF-κB activation increased the expression of NOX and nitric oxide synthases (NOS) which were core enzymes that mediated oxidants production.175 However, Nox4 knockout mice enhanced renal fibrosis and OS after UUO injury.176 It indicated too much or too less ROS can be harmful to the kidney. Other Nox members were also studied in the kidney. Nox2 and Nox5 were upregulated in Ang II treated podocytes.165 Consistently, Ang II receptor blockers inhibited Nox2 expression and were accompanied by increased SOD expression, decreased OS, and reduced renal and cardiac fibrosis.177 Specific overexpression of human Nox5 in vascular smooth muscle cells or mesangial cells led to glomerulosclerosis, inflammation, and renal fibrosis in diabetic mice.178
NOS is necessary for the synthesis of RNS that principally includes NO, peroxynitrite (ONOO−), nitrotyrosine, and nitrosothiols. NOS has three isoforms. Neuronal NOS (nNOS) and endothelial NOS (eNOS) are constitutively expressed by central and peripheral neurons and endothelial cells, respectively. However, inducible NOS (iNOS) is evoked by inflammation.179 In a healthy kidney, nNOS is expressed in cortical tubules but not in glomeruli, whereas eNOS is expressed in glomeruli but not in tubules.180 Increased iNOS in injured kidneys was associated with inflammation in CKD animal models.181 Dual CB1 receptor/iNOS antagonist, MRI-1867 decreased renal inflammation, fibrosis, and OS of obesity-related kidney disease.182 However, the studies about NOS in CKD are not well understood currently.
Except for the direct damage to the kidney, oxidants modified proteins, lipids, and DNA to form oxidation end products. AOPP bound to the receptor of advanced glycation end products and triggered the activation of Nox2, leading to the production of ROS. The accumulated ROS activated p65 NF-κB, increasing the generation of Wnt ligands and subsequent cytoplasmic β-catenin accumulation. The nuclear translocation of β-catenin due to excessive cytoplasmic storage triggered podocyte injury and albumin urine.183 The podocyte injury caused by AOPP treatment was also mediated by C-X-C chemokine receptor type 4 via p65 NF-κB signaling.184 Lipid peroxidation products cause DNA damage by synthesizing readily diffusible biofilm species such as aldehydes such as malondialdehyde or 4-hydroxynonenal. Membrane lipid peroxidation disrupted cellular and mitochondrial membrane barriers, interfering with electron transport, which in turn reduced ATP production.185
OS is not only due to the accumulation of ROS, but also the decrease of antioxidants. In OS, without the repression of antioxidant protein thioredoxin, apoptosis signal-regulating kinase 1 (ASK1) was phosphorylated and activated the downstream MAPKs p38 and JNK, leading to progressive kidney injury.186 Nuclear factor erythroid 2-related factor 2 (Nrf2) is a central redox-sensitive transcription factor that positively regulates antioxidant proteins including glutathione S-transferase, heme oxygenase-1, catalase, γ-glutamylcysteine synthetase, and NAD(P)H quinone oxidoreductase.187 Kelch-like ECH-associated protein-1 (Keap1) is a core repressor of Nrf2. Keap1 binds to Nrf2 and then leads to the polyubiquitination and subsequent proteasomal degradation of Nrf2. The renal protective role of Nrf2 was proved in multiple CKD animal models.188 Nrf2 deletion aggravated renal fibrosis induced by UUO, OS, and glomerulosclerosis of STZ-induced DN, renal damage of lupus nephritis.189,190,191 Besides regulating antioxidants, inhibiting inflammation is another mechanism of Nrf2 for protecting renal from injury. Nrf2 blocked the activation of the master driver of the inflammatory response, the NF-κB signaling pathway, via multiple different mechanisms. For example, Keap1 decreased the activation of upstream kinase IKKβ. And Nrf2 competed with NF-κB for binding to transcriptional coactivator CBP, resulting in the reduction of downstream genes of NF-κB.187
Sirtuins (SIRT) family is crucial to negatively regulate OS. Podocyte-specific SIRT1 overexpression attenuated podocyte loss and reduced OS in diabetes-induced nephropathy.192 Increased cyclooxygenase-2 (COX2) and its derivative PGE2 regulated by SIRT1 protected mouse renal medullary interstitial cells from OS.193 The natural antioxidant, resveratrol (RSV), improved manganese-superoxide dismutase (Mn-SOD) dysfunction in AMPK/SIRT1 dependent way and prevented the progression of diabetic kidney disease.194 RSV also prevented the aging pathologic changes in kidneys by elevating the level of Nrf2 and SIRT1.195 SIRT3 protected TECs from apoptosis by inhibiting ROS accumulation and ROS-sensitive Akt/FoxO signaling pathway.196 Overexpression of SIRT6 significantly inhibited Ang II-induced vascular endothelial cells apoptosis and ROS generation by upregulating the expression of the Nrf2 and its antioxidative downstreams.197 And SIRT6 played a protective role in OS by other pathways, such as decreasing the phosphorylation of AKT, increasing the phosphorylation of AMPK, and the levels of FoxO3α.198
The antioxidative stress therapies
Supplementing antioxidants is no doubt a direct approach to ameliorating OS. Pyridoxamine dihydrochloride (Pyridorin) that is a derivative of vitamin B6 is supposed to be a candidate for CKD treatments by scavenging ROS and inhibiting the formation of advanced glycation end products.199 However, a double-blind, randomized, placebo-controlled trial of Pyridorin failed to show the serum creatinine decreasing effect compared to the placebo group in type 2 DN patients with proteinuria.200 A phase 3 clinical trial (the PIONEER trial, NCT02156843) was conducted to evaluate the safety and efficacy of oral Pyridorin in reducing the risk of progression of DN. And the results are not reported yet.
We have discussed the important role of Nox, especially Nox4 in the OS and progression of kidney disease. The inhibitors of the Nox family have been tested in clinical trials and animals to evaluate the renoprotective effect. GKT136901, a Nox1/4 inhibitor treatment reduced albuminuria and the development of nephropathy in db/db (diabetic) mice by decreasing renal ERK1/2 phosphorylation and oxidative damage.201 Another Nox1/4 inhibitor, GKT137831 also exhibited the renal beneficial effect.171,202 Since GKT137831 achieved satisfactory renal protection in various CKD animal models, a multicenter, randomized, placebo-controlled trial about GKT137831 was conducted in type 1 diabetes-related kidney disease patients. The primary outcome of this trial was comparing the urine albumin to creatinine ratio and eGFR in different groups.203 The outcome of this not ended trial will provide strong evidence for the efficacy of Nox inhibitors in CKD.
Bardoxolone methyl is an inducer of Nrf2 which acts to release Nrf2 from the depression of Keap1. Elegant clinical trials were conducted to evaluate the effect of Bardoxolone methyl on CKD patients. The BEAM study (NCT00811889) showed that bardoxolone methyl improved the eGFR of CKD patients.204 However, proteinuria and blood pressure were elevated. A phase 3 clinical trial, the BEACON study (NCT01351675), confirmed the results and revealed that the risk of ESRD or death from cardiovascular causes was not reduced even higher in the bardoxolone methyl-treated group than in the placebo group.205 The other phase 3 clinical trials about bardoxolone methyl are undergoing in patients with autosomal dominant polycystic kidney disease (ADPKD) (NCT03918447) and Alport Syndrome (NCT03019185). The renal unfriendly effect of Nrf2were also repeated in animal studies. Tan et al. showed that Keap1 deficiency in vivo elevated Nrf2 signaling and aggravated proteinuria evoked by adriamycin, Ang II, or protein overload. And Nrf2 inducer CDDO-Im significantly increased the proteinuria of Ang II treated mice. Conversely, Nrf2 knockout mice ameliorated proteinuria induced by Ang II.206
GS-444217 was designed to inhibit ASK1 and was proved to halt the decline of renal function and renal fibrosis. Furthermore, GS-444217 strengthened the anti-glomerulosclerosis and albuminuria effects of the angiotensin-converting enzyme inhibitor (ACEi).186 Another ASK1 inhibitor, selonsertib was evaluated in moderate-to-advanced diabetic kidney disease.207 Randomized, dose-ranging, placebo-controlled phase 2 trial results showed selonsertib had no dose-dependent adverse effects over 48 weeks. And the rate of eGFR decline was reduced compared to placebo between 4 and 48 weeks after excluding confounding factors. However, acute reductions in creatinine secretion at weeks 0–4 in the high-dose group prevented the trial from meeting the primary endpoint. Urine ACRs also did not differ between the selonsertib and placebo groups. From the above clinical trial results of drugs for OS, it can be concluded that signaling pathways of OS are potential targets of CKD, but new targets and treatments remain to be discovered.
Signaling pathways of chronic inflammation
Inflammation plays a critical role in the initiation and progression of renal fibrosis. Proinflammatory and profibrotic cytokines and inflammatory cells which include immune cells from bone marrow and locally damaged resident renal cells constitute the inflammatory microenvironment. Macrophages are the best-characterized immune cells in renal inflammation.208 M1 and M2 are two extreme phenotypes of macrophages. M1 proinflammatory macrophages produce ROS, TNF-α, and IL-1β to amplify inflammation and mediate acute kidney inflammation. While the repairing phase, the majority of macrophages convert into reparative M2 phenotype that acts to resist acute inflammation by secreting anti-inflammatory cytokines like IL-10 and insulin-like growth factor-1.209 However, the persistent existence of M2 macrophages triggers immoderate repair which is characterized by excessive ECM deposition. And M1 macrophages also participate in chronic inflammation and renal fibrosis.12,210,211 Mechanistically, macrophages produce a large number of cytokines and growth factors like TGF-β1 to activate myofibroblasts from multiple sources such as fibroblasts, TECs, and pericytes.212 Macrophages contribute to the pool of myofibroblasts directly through macrophage to mesenchymal transition (MMT).213,214 And macrophages damage vascular endothelial cells and enhance hypoxia injury.12 The role of T cells in renal disease has been revealed extensively.215 For example, CD28− T cells accumulate in patients with CKD and anti-immune disease. Senescent CD28− T cells secret lots of inflammatory cytokines such as IFNγ and TNF-α, and express cytotoxic effector molecules such as granzyme B and perforin to promote tissue inflammation and injury.216 Helper CD4+ T lymphocytes (Th) deletion reduced renal fibrosis and reconstitution. CD4+ T cells, especially Th2 cells contributed to the progression of renal fibrosis in UUO mice by secreting profibrotic Th2 cytokines such as IL-4 and IL-13.217,218 Cytotoxic CD8+ T lymphocytes enter into glomeruli and kill podocytes only when Bowman’s capsule is disrupted.219 However, CD8+ T cells play a renal protective role in ADPKD.220 Fibrocytes are the important collagen production immune cells.221 The mechanisms of fibrocytes promoting renal inflammation and fibrosis are producing inflammatory cytokines such as TNF-α and MCP-1 and profibrotic cytokines like TGF-β, and converting into myofibroblasts directly.12 NF-κB pathway, JAK-STAT signaling, inflammasome, TLR signaling, and other signalings such as cGAS-STING signaling participate in the activation of inflammatory cells and the generation of inflammatory cytokines (Fig. 5). In this part, we will discuss major signaling pathways that mediated the activation of immune cells and the promising therapies for CKD based on inflammation.
Signaling pathways regulated inflammation in CKD. NF-κB is a central transcriptional factor of inflammation. TNF-α binds to the TNF receptor and activates NF-κB in Tim-3 dependent way. TLR4 involves in the activation of NF-κB induced by Ang II. mtDNA released from mitochondria caused by TFAM deletion triggers cGAS-STING DNA-sensing signaling activation and the STING-dependent NF-κB activation. DAMPs like HMGB1 and HSPs bind to TLR2 or TLR4 and attract dimerization. Activated TLR4 recruits adaptor proteins including TIRAP, MyD88, TRAM, IRAK, TRIF to activate NF-κB. TLR signaling regulates the production of NLRP3, pro-IL-1β, and pro-IL-18 that is the priming process of NLRP3 inflammasome formation in NF-κB-dependent way. NLRP3 inflammasome is assembled by NLRP3, the adaptor protein ASC and active caspase 1. ROS, K+ efflux, oxidized mitochondrial DNA release, and ion fluxes activate NLRP3 inflammasome and the successive caspase 1. Activated caspase 1 cut pro-IL-1 and pro-IL-18 to produce mature IL-1 and IL-18. It also cleaves and activates the cytoplasmic gasdermin proteins, and the activated gasdermin proteins are translocated to the membrane to constitute holes for IL-1 and IL-18 release. ApoC3 induced the activation of NRLP3 inflammasome in caspase-8-dependent way and the subsequent IL-1β release. IL-6 binds to the membrane IL-6 receptor (IL-6R), and recruits and homodimerizes signal transducer glycoprotein 130 (gp130). gp130 mediates the activation of the JAK and leads to the phosphorylation of STAT. SOCS can be upregulated by JAK-STAT signaling activation and acts as the negative feedback of JAK-STAT signaling. Red fonts in brackets are inhibitors. NF-κB nuclear factor kappa-B, JAK Janus kinase, STAT signal transducer and activator of transcription, NRLP3 NOD-like receptor protein 3, TLR toll-like receptor, TNF-α tumor necrosis factor-α, Ang II angiotensin II, IL interleukin, DAMPs danger-associated molecular patterns, ROS reactive oxygen species, TFAM mitochondrial transcription factor A, ASC apoptosis-associated speck-like protein containing a CARD, SOCS suppressor of cytokine signaling, TIRAP TIR domain-containing adaptor protein, MyD88 myeloid differentiation factor 88, TRAM TRIF-related adaptor molecule, IRAK interleukin receptor-associated kinase, TRIF TIR domain-containing adaptor inducing interferon, HSPs heat shock proteins, HMGB1 high-mobility group box 1
Major signaling pathways participate in the activation of immune cells
NF-κB pathway
As transcription factors, the NF-κB family regulates the induction and resolution of numerous inflammatory genes which are crucial to kidney disease by binding to the κB elements in promotor and enhancer. RelA (also called p65), RelB, c-Rel, p50 (also called NF-κB1), and p52 (also called NF-κB2) are five members of the NF-κB family.222 The shared Rel homology domain is the binding domain for Rel proteins to form the heterodimers of NF-κB. RelA, RelB, and c-Rel contain a DNA-binding domain that is necessary for the transcription of target genes. p50 and p52 that lack DNA-binding domain form heterodimers with other Rel proteins, and modulate the DNA-binding activity of NF-κB.223 The p65/p50 dimer is the most studied heterodimer in kidney disease. In healthy kidneys, NF-κB is anchored in the cytoplasm with the assistance of Inhibitors of κB (IκB) proteins including IκBα, IκBβ, p100, p105, and IκBε. The inhibitor of the κB kinase (IKK) complex is composed of two catalytic subunits, IKKα (IKK1) and IKKβ(IKK2), and a regulatory subunit, IKKγ. When stimulus triggers the cascade of NF-κB signaling, IKKs are phosphorylated by TGFβ-activated kinase 1 (TAK1), and then promote the ubiquitination and degradation of IκB.224 Then, NF-κB is released from IκB and transfers into the nucleus to regulate the transcription of target genes. The canonical NF-κB pathway is mainly mediated by IκBα. In the activation of the noncanonical NF-κB pathway, NF-κB inducing kinase (NIK) and IKKα phosphorylate p100 to release RelB.223 NF-κB signaling has been widely investigated in CKD. It is concluded that NF-κB signaling is a key mediator of renal inflammation both in CKD animals and CKD patients. The role of NF-κB signaling in CKD has been reviewed elsewhere.223,224,225 In this part, we update the regulation and crosstalk of NF-κB signaling in CKD with the latest research.
TNF-α and angiotensin II are the strong stimuli of NF-κB signaling. TNF-α binds to the TNF receptor and activates NF-κB. The increased transcription of TNF-α and SMYD2 can be triggered by NF-κB. SMYD2 promoted methylation and phosphorylation of p65 at K221 and K218, leading to the progression of ADPKD.226 The positive feedback loops between SMYD2 and NF-κB enhanced renal inflammation. Tim-3 mediated the activation of NF-κB induced by TNF-α.227 TNF receptor-1 inhibitor, R-7050 reduced renal damage and fibrosis in wild-type mice and mice with Krüppel-like factor 4 (KLF4)-deficient macrophages.210 Another TNF superfamily (TNFSF) member, TWEAK was proved to worsen the phenotype of ADPKD mice and renal fibrosis of UUO mice by activating NF-κB signaling.228,229 TWEAK is also an activator of nonclassical NF-κB signaling, and is the only cytokine demonstrated to activate nonclassical NF-κB signaling in tubules currently. NIK deficiency disturbed TWEAK-induced CCL19, CCL21, and CXCL10 generation in TECs.230 As a strong inflammatory cytokine, TNF-α plays a prominent role in renal inflammation and fibrosis by activating other multiple pathways, not only the NF-κB pathway. For example, TNF-α induced the expression of retinoic acid receptor responder protein 1 (RARRES1). And the soluble RARRES1 activated p53 and induced podocytes apoptosis by binding with and inhibiting RIO kinase 1 (RIOK1).231
Angiotensin II binds to angiotensin II type 1 receptor (AT1R) and activates AT1R, which promotes phosphorylation of p65 at Ser536, and then triggers the transcription of inflammatory factors like MCP-1, IL-8, IL-6, TNFα, and IL-17A.232 However, AT1R deficiency did not reverse inflammation completely due to the compensation of AT2R. Only both AT1R and AT2R antagonist applications blocked renal monocyte infiltration and NF-κB activation of UUO mice.233 The other signaling pathways are involved in the regulation of NF-κB. The receptor activator of NF-κB (RANK) upregulation induced by high glucose activated NF-κB and then increased the transcription of inflammatory cytokines, and OS-associated genes, Nox4 and its obligate partner, P22phox, resulting in the podocyte injury in DN.234 NF-κB is negatively regulated by some proteins. Cytoplastic annexin A1 (ANXA1) bound to NF-κB subunit p65 and inhibited its nucleus translocation. Thus, ANXA1 overexpression ameliorated kidney injuries, albuminuria, glomerulosclerosis, tubulointerstitial fibrosis, and kidney inflammation by inhibiting NF-κB signaling.235 The consistent results were obtained from ANXA1 mimetic peptide, Ac2-26 treated diabetic kidney disease mice. MG53 (also named TRIM72) was also proved to inhibit the activation of NF-κB and attenuated renal fibrosis by binding to p65 and blocking its nucleus translocation.236 The activation of NF-κB decreased the level of TYRO3 which was a podocyte protective factor in glomerular disease.237 Protein S inhibited the TNF-α induced NF-κB activation in a TYRO3 receptor-dependent way.238
Epigenetic regulation like microRNAs (miRNAs) that are a class of small noncoding RNAs that regulate gene expression through binding target mRNAs plays an important role in NF-κB activation. Angiotensin II treatment increased circulating miR-103a-3p expression, resulting in the reduction of serine/threonine-protein kinase of glomerular endothelial cells that exerted anti-inflammatory effects by inhibiting NF-κB/p65 activation.239 Methyltransferase-like 3 promoted m6A modification of miR-21-5p. The maturation of miR-21-5p activated NF-κB via SPRX1/ERK signaling and enhanced renal fibrosis and inflammation of UUO mice.240 TECs derived miR-19b-3p abundant exosome potentiated NF-κB signaling of macrophages by abolishing the repression of suppressor of cytokine signaling-1 (SOCS1).241,242 miR-802 inhibited the expression of NF-κB repressing factor and promoted the renal injury of obesity-associated nephropathy by activating NF-κB.243 miR-373 induced by TGF-β targeted to SIRT1 which inhibited NF-κB and enhanced renal fibrosis.244 miR-146a inhibited classical and nonclassical NF-κB signaling pathways and ameliorated lupus nephropathy and DN.245,246
JAK-STAT signaling
The Janus kinase (JAK)-signal transducer of activators of transcription (STAT) pathways was discovered in the anti-viral process of interferons. The phosphorylation of JAK activates STAT directly and then the transcription factor STAT regulates targeted genes in the nucleus.247 This two-step pathway delivers transmembrane signals to the nucleus rapidly. JAK family has four members including JAK1, JAK2, JAK3, and receptor tyrosine kinase 2 (TYK2). And there are seven STATs such as STAT1 to 4, 5a, 5b, and 6. More than 50 cytokines and growth factors are regulated by JAK-STAT signaling. All members of JAK-STAT signaling have been found in the damaged kidney.248,249 In human kidney biopsy of high inflammatory kidney diseases such as lupus nephritis, DN, IgA nephropathy, and FSGS, JAK, and STAT, and the inflammatory factors including IL4R, IL6R, IL-13, and interferon-gamma that involved in JAK-STAT signaling were upregulated.250,251,252 Peripheral blood monocytes (PBMCs) from patients with IgA nephropathy have upregulated STAT production after cytokines stimulation.251 JAK-STAT upregulation in podocytes, TECs, and mesangial cells was associated with the progression of DN.253 Podocyte-specific JAK2 overexpression aggravated albuminuria, mesangial expansion, glomerular basement membrane thickening, and tubulointerstitial fibrosis of Akita diabetic mice. Oral JAK1/2 inhibitor, LN3103801 treatment alleviated the pathology changes of JAK2 overexpression in diabetic mice.254 The overexpression of E3 ubiquitin ligase casitas B-lineage lymphoma (Cbl) degraded JAK2, and inactivated the successive STAT4 and the targeted Runx3 transcription, resulting in the inhibition of endothelial dysfunction by increasing NO production.255 STAT6 was increased in TECs and STAT6 deficiency attenuated UUO-induced renal fibrosis. A natural carotenoid extracted from the seeds of Bixa Orellana, bixin, ameliorated kidney interstitial fibrosis by suppressing the phosphorylation and stability of STAT6.256 JAK3-STAT6 signaling pathway mediated monocytes from bone marrow to myofibroblasts transition. Wild-type mice engrafted with STAT6(-/-) bone marrow cells displayed fewer myofibroblasts infiltration and ECM deposition in the obstructed kidneys.257 And UUO mice treated with the inhibitor of JAK3, CP690,550 showed similar results. However, the JAK/STAT inhibitor, tofacitinib, did not attenuate uric acid crystal-induced inflammation and CKD progression.250
IL-6 is a key proinflammatory factor in innate and adaptive immune responses. IL-6 binds to the membrane IL-6 receptor (IL-6R), and recruits and homodimerizes signal transducer glycoprotein 130 (gp130). Membrane proximal domains (Box domains 1 and 2) of gp130 mediate the activation of the JAK and leads to the phosphorylation of STAT.258 Recombinant gp130 linked with Fc competitively binds to IL-6/IL-6R complex with endogenous gp130 and abolishes the transduction of IL-6 signaling. Fc-gp130 administration prevented renal fibrosis by inhibiting the activation of STAT3.259 Post-transcriptional modification such as acetylation of STAT enhanced the dimerization and nucleus translocation of STAT. Inhibited STAT3 acetylation reduced albuminuria and kidney injury of diabetic db/db mouse.260 The SOCS is a prominent negative feedback protein of JAK-STAT signaling. SOCS can be upregulated by JAK-STAT signaling activation. The kinase inhibitory region of SOCS1 binds to the substrate-binding groove of JAK with high specificity and acts as a pseudo substrate for JAK kinases.261 Overexpressing SOCS1 or SOCS3 with adenovirus mitigated interstitial fibrosis, macrophage infiltration, and proteinuria of DN by suppressing STAT1 and STAT3 activation.262 KLF4 that was a DNA-binding transcription regulator suppressed STAT3 transcriptional activity by binding to pSTAT3 (Tyr705). Podocyte-specific knockout KLF4 activated STAT signaling, resulting in podocytes injury, and parietal epithelial cells activation.263,264
The activation of the NLRP3 inflammasome
The inflammasome is a multi-protein complex assembled by cytoplasmic pattern recognition receptors (PRRs) that sense pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) and is an important part of the innate immune system. NOD-like receptors (NLRs), TLRs, Rig-I-like receptors, absent in melanoma 2 (AIM2)-like receptors (ALRs), and C-type lectin receptors are the five families of PRR.265 Only proteins from the NLR and ALR families form inflammasomes. The expression of inflammasome components in the kidney was reviewed elsewhere.266 NLRP3 inflammasome is the best-characterized inflammasome in CKD.267 NLRP3 inflammasome is assembled by NLRP3, the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and active caspase 1. ROS, K+ efflux, oxidized mitochondrial DNA release, and ion fluxes due to lysosomal disruption activate NLRP3 inflammasome and the successive caspase 1 which executes inflammatory factors production and cell death.268 Activated caspase 1 cut pro-IL-1 and pro-IL-18 to produce mature IL-1 and IL-18. It also cleaves and activates the cytoplasmic gasdermin proteins, and the activated gasdermin proteins are translocated to the membrane to constitute holes, leading to cell swelling, cytoplasmic outflow, cell membrane rupture, and pyroptosis finally.269 The inflammatory factors such as IL-1, and IL-18 outflow from the holes composed of gasdermin.270
The proinflammatory and profibrotic effect of NLRP3 inflammasome has been confirmed in multiple animal models of kidney diseases such as UUO, angiotensin II-induced hypertensive kidney injury, DN, glomerulonephritis, and 5/6 nephrectomy.266 Then we summarize the regulation of NLRP3 inflammasome and the following pyroptosis in CKD. IL-36 cytokines facilitated NLRP3 inflammasome activation in an IL-36 receptor-dependent way and contributed to renal inflammation and fibrosis of UUO mice.271 Apolipoprotein C3 (ApoC3) activated human monocytes and promoted renal injury from unilateral ureter obstruction in humanized mice.272 Mechanically, ApoC3 activated Syk through TLR2 or TLR4 in the assistant of transmembrane protein SCIMP which contains immunoreceptor tyrosine-based activation motifs. Syk-mediated calcium influx through TRPM2 evoked NAPDH oxidase that catalyzed the generation of ROS. Finally, ApoC3 induced the activation of NRLP3 inflammasome in caspase-8 dependent way and the subsequent IL-1β release. ApoC3 also induced the cascade of NF-κB signaling and MAPK signaling. The pyroptosis can be triggered not only by caspase 1 but also by caspase-3. Caspase-3 and GSDME were elevated in TECs and promoted the progression of obstructive nephropathy by enhancing TECs’ pyroptosis. And this process could be induced by TNF-α and caused the release of high-mobility group box 1 (HMGB1) that was a nuclear protein acting as a DAMP.273 The free HMGB1 recruits macrophages and neutrophils from bone marrow and triggers NLRP3 inflammasome activation of these immune cells.274 The upregulated NLRP3 inflammasome/IL-1β cascade induced NF-κB p65 activation, mitochondrial ROS generation, and lipid accumulation of podocytes.275 Thioredoxin (TRX)-interacting protein (TXNIP) dissociates from TRX in a ROS-sensitive manner, and binds to and activates NLRP3 inflammasome.276 Mitochondria ROS (mtROS) generated in TECs under the treatment of high glucose decreased TRX expression and released TXNIP to activate NLRP3 inflammasome, resulting in tubular damage in the kidneys of patients with DN.277 And the NLRP3 activation and mtROS generation were regulated by increased CD36 expression in TECs.278 Pim-1 elevated in renal lysates from mice with lupus nephritis, PBMCs from patients with SLE, and renal biopsy tissue from patients with lupus nephritis. The upregulated Pim-1 increased the intracellular Ca2+ of podocytes which modulated NLRP3 inflammasome activation. And Pim-1 inhibitor AZD1208 ameliorated proteinuria, glomerulonephritis, renal immune complex deposits, and serum anti-dsDNA antibody levels in mice with lupus nephritis.279 Post transcription modification like miR-10a/b inhibited the formation of the NLRP3 inflammasome by binding to the 3’-untranslated region of NLRP3 mRNA.280 mir-486a-3p and miR-141-3p showed similar suppression effects of NLRP3 and protected kidneys from chronic injury.281,282
Toll-like receptor signaling
TLRs are one of the members of PRRs. Human TLRs have 10 isoforms including TLR1-10. Different from NLR that we discussed above, TLRs including TLR1, 2, 4, 5, and 6 are transmembrane proteins with various leucine-rich repeats which are the recognition domain of PAMPs and DAMPs. TLRs are predominantly expressed by immune cells like macrophages, T cells, B cells, NK cells, and dendritic cells.283 Resident renal cells such as TECs, podocytes, mesangial cells, and endothelial cells are also expressed TLRs when suffering from striking.284 DAMPs like HMGB1, and heat shock proteins (HSPs) bind to TLR2 or TLR4 and attract dimerization. Activated TLR4 recruits adaptor proteins including TIR domain-containing adaptor protein, myeloid differentiation factor 88, TIR domain-containing adaptor inducing interferon (TRIF)-related adaptor molecule, interleukin receptor-associated kinase, TRIF to mediate the production of ROS and the activation of NF-κB.253,285 TLR signaling induces NF-κB activation and regulates the production of NLRP3, pro-IL-1β, and pro-IL-18 which is the priming process of NLRP3 inflammasome formation.
TLRs play an important role in renal inflammation and progressive renal fibrosis. The research on TLRs in kidney diseases has been reviewed elsewhere.286,287 In summary, TLRs (mainly TLR4 and TLR2) activation promotes resident renal cells injury, immune cells derived from bone marrow recruitment, and the expression of inflammatory factors such as MCP-1, IL-6, IL-8, IL-1β in NF-κB/NLRP3 inflammasome signaling and MAPK signaling-dependent way. Here, we update the effect and regulation of TLRs in CKD. TLR4 deficiency in vivo or in vitro dampened NF-κB activation caused by angiotensin II treatment, thus decreasing renal inflammation and OS.288 AT1R blocker losartan reduced the expression of TLR4.289 STAT3 also mediated the activation of TLR4 induced by angiotensin II in renal proximal tubular cells, and STAT3 inhibitor S31-201 normalized renal fibrosis and dysfunction of hypertensive kidney disease.290 Free light chains such as κ and λ chains induced proximal tubule cell injury by promoting HMGB1 release and STAT1 upregulation, causing the expression of TLR2, TLR4, TLR6, and downstream proinflammatory cytokines.291 The proinflammatory TLR4/IFN-γ/STAT1 pathways transactivated the miR-214 gene which inhibited TLR4 directly in turn.292 The upregulated long noncoding RNA H19 in the injured renal tubular cells caused by calcium oxalate (CaOx) crystals countered miR-216b which inhibited HMGB1 expression by directly binding its 3’-untranslated region.293 Thus, an RNA H19 induced the expression of HMGB1, and the following TLR4 and NF-κB. The role of the co-receptor of TLR4 in innate immunity, MD2 had been revealed in DN. MD2 deficiency mice or MD2 inhibitor L6H9 abolished the activation of MAPK signaling and the downstream ACE, angiotensin receptors, and angiotensin II expression in proximal tubular cells.294 The co-receptors of TLR4 are diverse and mediate different even opposite outcomes. Biglycan evoked the cooperation of CD44 and TLR4, increased M1 macrophages autophagy and renal M2 macrophages infiltration, and reduced tubular damage after ischemia/reperfusion injury.295 PD is one of the renal replaced therapies for ESRD patients; however, peritoneal membrane fibrosis causes dialysis failure. TLR2 and TLR4 mediated profibrotic and proinflammatory responses of peritoneal membrane fibrosis. And the harmful effect of TLR2/4 was blocked by the TLRs inhibitor, soluble TLR2.296,297
Other signaling pathways
The cyclic GMP-AMP synthase (cGAS) – stimulator of interferon genes (STING) signaling plays a key role in innate immune defense. DNA sensor, cGAS is motivated by pathogenic or endogenous DNA and activates STING and the downstream type I interferon (IFN) gene transcription.298 mtDNA released from mitochondria caused by TFAM deletion triggered cGAS-STING DNA-sensing signaling activation and the STING-dependent NF-κB activation. TECs specific knockout STING or STING specific inhibitor C-176 treatment attenuated inflammation and the functional and structural changes of renal fibrosis.299 mtDNA release can be triggered by the loss of disulfide-bond A oxidoreductase-like protein (DsbA-L) as well. DsbA-L deficiency promoted inflammation and insulin resistance by activating the cGAS-STING pathway in adipose tissue.300 However, proximal tubular-specific knockout DsbA-L mice showed mild renal fibrosis caused by UUO surgery. The mechanism was DsbA-L activated the Hsp90 /Smad3 and p53/CTGF axis.301 The relationship between DsbA-L and cGAS-STING pathway under CKD needs further investigation. And the gene expression of cGAS and STING were positively related to renal fibrosis in CKD patients. APOL1 risk allele G2 is associated with high kidney disease risk in African Americans. STING and NLRP3 expression were elevated in podocytes of transgenic mice with APOL1 G2 risk variant and patients with high-risk APOL1 genotypes. Deletion or inhibition of STING and NLRP3 respectively lowered albuminuria and improved kidney function of G2APOL1 mice.302,303
A study enrolled 1538 hospitalized patients with renal injury and tested the level of several inflammatory factors in urine. The relationship between inflammatory factors and the progression of CKD was assessed after a median of 4.3 years of follow-up.304 The results showed MCP-1/CCL2 and YKL-40 (CHI3L1) levels were negatively associated with eGFR, and uromodulin (UMOD) level was positively associated with eGFR. The renal hostile role of CCL2 and CHI3L1 and the renal protective role of UMOD were also proved in animal studies. This study provided biomarkers of inflammation for renal disease progression. IL23 signaling activated in TECs increased the expression of calcium/calmodulin-dependent protein kinase IV (CaMK4) which suppressed the level of arginine-hydrolyzing enzyme arginase 1 (ARG1), resulting in the release of arginine. Sufficient arginine promoted T cells proliferation and inflammatory cytokines production.305 Anti-inflammatory cytokine, IL-10 deficiency aggravated renal fibrosis and inflammation in obesity-related nephrology mice.306
Anti-inflammation therapeutics of CKD
We have reviewed the important signaling pathways and cytokines mediated inflammation in CKD. The targeted drugs are promising for attenuating CKD progression. We introduce drugs in clinical trials first (Table 3).
Sodium-glucose cotransporter 2 (SGLT2) inhibitors inhibit glucose reabsorption by targeting SGLT2 proteins which are mainly expressed by proximal TECs. The mechanisms for the renoprotective effect of SGLT2 inhibitor are not only due to glycemic control, but also inflammation and OS depression. The NLRP3 inflammasome activation and ATP-induced secretion of IL-1β were inhibited in the macrophages isolated in type 2 diabetes mellitus (T2DM) patients by SGLT2 inhibitor, empagliflozin treatment.307 Empagliflozin inhibited the renal expression of MCP‐1, TGF‐β, NF‐κB, and IL‐6, and circulating levels of MCP‐1, IL‐6, and TNF‐α in DN animals.308 Transcriptomic analysis of plasma biomarkers showed canagliflozin reduced the mRNA level of TNF receptor 1, IL-6, and matrix metalloproteinase 7 (MMP7) in patients with T2DM compared with glimepiride treated group.309 A phase 3 trial (NCT02065791) results showed the relative risk of ESRD in the canagliflozin group was 30% lower than the placebo group at a median follow-up of 2.62 years.310 Canagliflozin also decreased the risk of cardiovascular events in patients with DN. Based on the positive results, further clinical trials about SGLT2 inhibitors were conducted. The effect of empagliflozin on proteinuria and kidney disease progression in patients with non-diabetic glomerulonephritis is under investigation (Phase 3 clinical trial, NCT05283057). And the recruitment is completed, but the results are not reported yet. A phase 4 clinical trial (NCT05225077) that evaluates the effect of short-term dapagliflozin on renal function after heart catheterization or percutaneous coronary intervention in patients with CKD is recruited. Exciting clinical trial results provide strong evidence for the use of SGLT2 inhibitors in patients with DN, even in CKD patients without diabetes. A phase 2 trial (RESCUE, NCT03926117) evaluated the effect of IL-6 inhibitor, ziltivekimab on the percentage change of high-sensitivity CRP in adult patients with moderate to severe CKD and high-sensitivity CRP of at least 2 mg/L.311 After 12 weeks, ziltivekimab reduced the levels of high-sensitivity CRP significantly in a dose-dependent way. In the highest 30 mg dosage group, high-sensitivity CRP decreased by 92% compared with 4% for the placebo group. It did not affect the total cholesterol to HDL-cholesterol ratio and was well tolerated. The conclusion of the RESCUE trial provided a promising drug for lowing the risk of atherosclerosis in CKD patients by inhibiting inflammation. And further studies are needed to assess whether ziltivekimab attenuates CKD progression and reduced the risk of cardiovascular events. The renal protective role of IL-6 inhibitor has been demonstrated in animal experiments. Experiments in animal models showed IL-6 specific inhibitor, Fc-gp130 attenuated renal fibrosis and immune cells infiltration.259 The reducing cardiovascular event rates and renal progression effects of canakinumab, a human monoclonal antibody neutralizing IL-1β, were evaluated on CKD patients with stable post-myocardial infarction and high-sensitivity C-reactive protein ≥2 mg/L. The phase 2 clinical trial (NCT01327846) results showed canakinumab reduced major adverse cardiovascular event rates compared with placebo treatment over a median follow-up period of 3.7 years. However, renal function reflected by eGFR, creatinine, and the urine ACR were not influenced by canakinumab.312 The effect on decreasing albuminuria and inflammatory biomarkers of JAK1/2 inhibitor, baricitinib, was evaluated in elder patients with diabetic kidney disease (NCT01683409). After 24 weeks of treatment, baricitinib decreased morning ACR by 41% compared with placebo. And the level of uric inflammatory biomarkers including CXCL10, CCL2, soluble TNFR1 and 2, SAA, and cell adhesion molecule 1 was decreased by barcitinib. However, about 10 folds of anemia occurred in the baricitinib group compared with the placebo. The conclusion was not so convincing due to it was drawn from the results of less than 30 patients.313 Further study is needed to evaluate the safety of barcitinib. Multicenter preclinical randomized controlled trials (pRCT) evaluated the effect of baricitinib on lupus nephritis. MRL/MpJ-Faslpr mice were randomized to oral administration with baricitinib or vehicle at four dependent centers. Data analysis was performed at an independent fifth site. The results showed baricitinib did not decrease proteinuria and renal injury.314 pRCT predicted patients with lupus nephritis would not benefit from baricitinib.
Except for drugs in clinical trials discussed above, a large number of novel CKD treatments that inhibit inflammation have been tested on animal models. NF-κB is no doubt an important target. Arctigenin (ATG) reduced proteinuria in diabetic patients and renal fibrosis of UUO mice by inhibiting the NF-κB pathway in phosphatase 2A (PP2A) dependent way. PP2A also dephosphorylated the actin cytoskeleton-related proteins Drebrin-1 (DBN1) on the T335 site, and then increased adhesion and reduced the migration of podocytes.315 An NF-κB inhibitor, pyrrolidine dithiocarbamate, was revealed to attenuate renal inflammation and fibrosis in multiple CKD animal models, such as gentamicin-treated rats, 5/6 nephrectomy rats, and aldosterone and salt-induced renal disease.229 Zinc supplementation ameliorated vascular calcification of CKD by depressing the NF-κB pathway.316 The possible mechanism was that Zinc bound to zinc-sensing receptor ZnR/GPR39 and upregulated the expression of zinc-finger protein TNF-α-induced protein 3 (TNFAIP3, also known as A20) which is a suppressor of NF-κB. The major stimulus of NF-κB, TNF-α, can be inhibited by infliximab or etanercept. The TNF-α inhibition decreased proteinuria and attenuated CKD progression in various CKD animal models. Adalimumab which was a neutralizing antibody of TNFα was applied to patients with primary focal segmental glomerulosclerosis (FSGS) in the FONT trial (NCT00814255).317 However, the trial ultimately ended due to under-enrollment. A case report showed 33-year-old man treated with adalimumab for 7 years appeared with renal dysfunction, nephrotic proteinuria, and erythrocyte uria. The albuminuria and renal function were back to normal when adalimumab was abandoned.318 And similar case reports were described.319,320 The effects and safety of adalimumab should be evaluated in further study. PLK1 inhibitor (BI-2536) was predicted to be the top candidate for depressing 15 genes expression related to DN with a connectivity mapping approach and Integrated Network-based Cellular Signatures (LINCS) L1000 data set querying.321 Indeed BI-2536 ameliorated proteinuria and kidney injury of DN mice by dampening NF-κB and Smad3 signaling.
The inhibitors of JAK-STAT signaling targeting each process including receptors, JAK activation, STAT activation, and STAT binding to DNA have been explored extensively.322 Here, we list some inhibitors that have been tested on CKD. Nifuroxazide was found to inhibit the activation of STAT3 with the STAT3-dependent luciferase reporter cell line. Further research identified STAT1, STAT5 and JAK2 also can be blocked by nifuroxazide.323 Nifuroxazide treatment significantly reduced renal inflammation and improved glomerular filtration function and diabetic kidney disease-related renal structural alterations.324 Similar results were observed in UUO mice treated with nifuroxazide.325 Small molecule S3I-201 and Stattic protected kidneys from injury in multiple animal models with CKD by inhibiting the SH2 domain of STAT3.322 JAK1/2 inhibitor, ruxolitinib, protected podocytes from high glucose injury.326 JAK2/3 inhibitor, AG490 reduced inflammation, tubular apoptosis, and interstitial fibrosis of UUO mice and proteinuria of DN mice.199,327
MCC950 inhibited the NLRP3 activation inflammasome formation by binding to the nucleotide-binding and oligomerization domain and altering the protein structure of NLRP3.328,329 MCC950 reduced NLRP3 inflammasome activation and the following caspase 1 activation and IL-1β release in podocytes. Thus, MCC950 attenuated proteinuria and renal lesions of mice with lupus nephritis.330 Luteolin, a natural flavonoid compound, prevented podocyte injury from high glucose injury by inhibiting NLRP3 inflammasome activation.331 Xenon, an inert anesthetic gas decreased serum levels of anti-double-stranded DNA autoantibody and neutrophil chemokines, thereby improving kidney function and renal histology by blunting NF-κB/NLRP3 inflammasome activation.332 IL-6 receptor inhibitor, tocilizumab, suppressed inflammatory response and OS of kidney in diabetic mice.333 β-hydroxybutyrate precursor, 1,3-butanediol, protected mice from nephrocalcinosis-related CKD by inhibiting NLRP3 inflammasome activation.334 The similar renoprotective effect of β-hydroxybutyrate was revealed by another study. The results showed that β-hydroxybutyrate administration decreased the expression of caspase 1 and pyroptosis of proximal tubular cells after ischemia-reperfusion injury.335 Anakinra, a recombinant interleukin-1 receptor antagonist attenuated kidney progression in mice with DN and IgA nephropathy, but not in lupus nephritis.336 Caspase inhibitor, M-920 inhibited caspase 1-dependent inflammasome activation and then ameliorated DN.337
HSP70-TLR4 axis contributed to the albuminuria, renal inflammation, and progression of STZ-induced diabetic mice, and HSP70 inhibitors, VER-155008 and pifithrin-μ protected the kidney from injury.338 TLR4 antagonist, CRX-526, inhibited the upregulation of osteopontin which mediated the crosstalk between TECs and fibroblasts and negatively correlated with kidney function, and NF-κB nuclear translocation, thus significantly reducing albuminuria and renal injury.339,340 TAK-242 was another inhibitor of TLR4 and proved to inhibit M2 macrophage polarization and renal inflammation after UUO surgery.341
We have summarized the effects and regulation of core inflammatory signaling pathways and the targeted drugs tested in clinical trials and animal models of CKD. Several anti-inflammatory drugs perform exhilaratingly in preventing CKD progression, reducing proteinuria or the risk of cardiovascular disease. And further clinical trials are needed to specify the application of these drugs. As we discussed above, various anti-inflammatory inhibitors are investigated on animal models of CKD and show satisfactory results. The possible reason could be inflammatory signaling pathways are crosslinking with each other. Thus, one process inhibition arises amplified dominoes. However, this is also the reason for the unsatisfactory effects of some inhibitors due to the compensation from another signaling. Finding the key factors that control inflammation is important for discovering effective anti-inflammatory inhibitors.
Key pathways mediated myofibroblasts activation and ECM deposition
Myofibroblasts are regarded as the key producers of ECM. The capacity for generating collagen fibers of myofibroblasts is much greater than fibroblasts. α-smooth muscle actin (α-SMA) is a classical but not specific marker of myofibroblasts. Platelet-derived growth factor receptor β, fibroblast-specific protein 1, CD73, and vimentin are also non-specific markers of myofibroblasts.342 Thus, more than two markers are used to identify myofibroblasts usually. With the development of signal-cell RNA sequencing technology, more markers such as naked cuticle homolog 2 were discovered.212 Myofibroblasts are hard to detect in healthy kidneys but increase greatly in the fibrotic kidney. Proliferation and activation are two pathways to obtain numerous myofibroblasts. Renal resident fibroblasts, pericytes, TECs, and endothelial cells, and bone-marrow-derived cells such as macrophages, mesenchymal stem cells were reported to be the precursors of myofibroblasts.343,344 Developmental pathways including TGF-β signaling, Wnt/β-catenin pathway, Notch signaling, and RAAS signaling play an important role in the activation and proliferation of myofibroblasts and the production of ECM (Fig. 6).
Key pathways mediated myofibroblasts activation and ECM deposition. In TGF-β signaling, TGF-β1 released from LTBP binds with TGFBR2, which recruits TGFBR1, inducing the downstream phosphorylation of Smad2 and Smad3. P-Smad2 and P-Smad3 form a complex with Smad4 and translocate into the nucleus, promoting the transcription of profibrotic genes and inhibiting the antifibrotic factors. In activated Wnt/β-catenin signaling, Wnt ligands bind with Frizzled receptor and co-receptor LRP5/6 to inhibit the assembling of the β-catenin destruction complex. Cytoplasm accumulated β-catenin translocates into the nuclear and binds to TCF/LEF to promote downstream target genes transcription. Notch signaling is triggered by the interaction between ligands and receptors, inducing the cleavage by ADAM protease and γ-secretase complex. The product NICD is transported into the nucleus to regulate the transcription of downstream genes with the assistance of Rbpj and MAML. The cascade of RAAS is that renin converts angiotensinogen to angiotensin I. Under the action of ACE, Ang I is converted into Ang II, which can bind to AT1R to activate multiple pathways such as NF-κB signaling. Red fonts in brackets are inhibitors. TGF-β transforming growth factor-beta, LTBP latent TGF-β binding protein, TGFBR1 TGF-β receptor 1, TGFBR2 TGF-β receptor 2, TCF T-cell factor, LEF lymphoid enhancer-binding factor, LRP low-density lipoprotein-related protein, NICD Notch intracellular domain, MAML Mastermind-like Protein, RAAS renin-angiotensin-aldosterone system, Ang I angiotensin I, ACE angiotensin-converting enzyme, AT1R angiotensin II receptor type 1
TGF-β signaling
TGF-β, a member of the transforming growth factor superfamily consists of three isoforms including TGF-β1, TGF-β2, and TGF-β3. TGF-β1 is the most well-studied isoform in renal fibrosis. TGF-β1 is synthesized as a precursor form. And the precursor of TGF-β1 interacts with latency-associated peptide and latent TGF-β-binding protein to form the complex that keeps TGF-β1 in an inactive state.345 Overexpression of latent TGF-β reduced the expression of active TGF-β1 and ECM-related proteins such as collagens after UUO injury compared to wild-type mice.346 However, the complex is cleaved in the injured kidney by proteases including plasmin, MMP2 and MMP9, and thrombospondin to release active TGF-β1.347,348 The active TGF-β1 triggers classical and nonclassical pathways. The classical TGF-β cascade is TGF-β1 binds to TGFBR2 directly and phosphorylates TGFBR2. And the phosphorylated TGFBR2 recruits and activates TGFBR1. The active TGFBR1 then phosphorylates Smad2 and Smad3. Phosphorylated Smad2 and Smad3 translocate into the nucleus with the assistance of Smad4. The phosphorylated Smad3 directly binds to the promoter of targeted genes to modify the transcription.349 The transcription of Smad7 is also increased by phosphorylated Smad3. And Smad7 acts as a negative regulator of the TGF-β/Smad3 pathway by competing with Smad2 and Smad3 for the binding sites on activated TGFBR1.350 The binding of TGF-β1 and TGFBR2 also triggers Smad independent pathways including JNK, MAPK, and ERK.349
The expression of components of TGF-β signaling is upregulated in fibrotic kidneys of patients and animals with CKD. Classical and nonclassical pathways of TGF-β are activated to promote the activation and proliferation of myofibroblasts, and the generation of ECM.349 Phosphorylated Smad3 induced by TGF-β directly regulates the expression of α-SMA which is the classical marker of myofibroblasts, and ECM proteins such as Collagens, fibronectin.351 The upregulation of TGF-β breaks the balance between ECM synthesis and degradation. TGF-β1 treatment inhibited the expression of PAs and induced the production of their inhibitor PAI-1, leading to the reduced degradation of ECM.352 Tissue inhibitor of metalloproteinase that was an inhibitor of MMP was upregulated by TGF-β/Smad signaling in CKD and promoted age-related renal fibrosis.353 Precursors like TECs, endothelial cells, and macrophages convert into myofibroblasts through partial epithelial-mesenchymal transition (partial EMT), endothelial-mesenchymal transition (EndMT), and MMT, respectively. Runt-related transcription factor 1 (RUNX1) mediated partial EMT in a Smad3-dependent way.354 Brain-specific homeobox/POU domain protein Pou4f1 (Brn3a) is also the target of Smad3. TGF-β/Smad3 upregulated the expression of Pou4f1 which was a key mediator of MMT.214 The upregulated thioredoxin domain containing 5 (TXNDC5) enforced TGF-β signaling activity and promoted fibroblasts activation by post-translational stabilizing and upregulating TGFBR1.355 TGF-β/Smad3 signaling also regulates the expression of multiple noncoding RNAs such as miR-21 which is a well-known profibrotic miRNA.351 miR-21 inhibitor, lademirsen (SAR339375), has been conducted in phase 2 clinical trial (NCT02855268) which assesses the efficacy in reducing the decline in renal function of patients with Alport syndrome. Long noncoding RNA (lnc-TSI) blocked the interaction of Smad3 with TGFBR1 by binding with the MH2 domain of Smad3 and attenuated renal fibrosis.356
Due to the strong profibrotic effects, TGF-β signaling has been considered to be a targeted pathway to attenuate renal fibrosis. And the therapies that targeted TGF-β signaling have been widely studied in the past few decades. Pirfenidone is a synthetic small molecule inhibiting TGF-β synthesis. A phase 2 clinical trial (NCT00001959) of pirfenidone in patients with FSGS showed eGFR decreased during the baseline period and pirfenidone treatment inhibited the eGFR decline. Another clinical trial showed pirfenidone elevated the mean eGFR compared with placebo in patients with DN.357 The positive results of these clinical trials make pirfenidone a promising drug for attenuating the progression of CKD. Fresolimumab, a monoclonal anti-TGF-β antibody was applied to patients with steroid-resistant focal segmental glomerulosclerosis (SR-FSGS). Although the clinical trial (NCT01665391) did not meet the endpoint, at day 112, the mean percent of urinary protein/creatinine ratio decreased significantly in patients treated with fresolimumab 1 mg/kg.358 However, TGF-β1-specific, humanized, neutralizing monoclonal antibody (TGF-β1 mAb) did not decrease SCr compared to placebo in patients with DN.359 And similar results were concluded from a phase 2 clinical trial of LY2382770 (NCT01113801). Limitations of the trial confounded drawing definitive conclusions that TGF-β had no role in the pathogenesis of DN. And another possible reason is other pathways are also important for the progression of renal fibrosis. Bardoxolone, an Nrf2 activator, protected the kidney from injury by increasing Smad7 levels and inhibiting TGF-β/Smad expression.360 The novel inhibitors of TGF-β signaling are under investigation. Klotho was an anti-aging protein that was discovered in 1977 by Japanese scientists.361 Secreted α-Klotho inhibited TGF-β signaling by directly binding to TGFBR2 and ameliorated renal fibrosis.362 However, recombinant full-length Klotho supplement is difficult to apply to CKD patients due to the high cost and difficulties to produce active recombinant full-length Klotho. Liu et al. found the core sequence of α-Klotho for binding to TGFBR2 and inhibited the activation of classical and nonclassical TGF-β signaling. The Klotho-derived peptide, KP1, mimicked the renal-protected function of full-length Klotho and ameliorated renal fibrosis of UUO and UIRI mice.363 SIS3 is a specific inhibitor abolishing Smad3 but not Smad2 phosphorylation and attenuates renal fibrosis in diabetic and obstructive nephropathy.364 GQ5 inhibited the phosphorylation of Smad3 by blocking the interaction of Smad3 with TGFBR1 and attenuated fibrotic lesions in obstructive nephropathy.365
Wnt/β-catenin signaling
The Wnt/β-catenin pathway is evolutionarily conserved and regulates embryonic development, carcinogenesis, and tissue injury and repair.366 Wnt proteins are a conserved family of secreted lipid-modified glycoproteins, which includes 19 different members in mammalian species: Wnt1, 2, 2b, 3, 3a, 4, 5a, 5b, 6, 7a, 7b, 8a, 8b, 9a, 9b, 10a, 10b, 11, and 16. As a type of glycolipoprotein, Wnt proteins are modified before secreting. The N-terminal cysteine-rich residues of Wnts are glycosylated in the ER and the C-terminal serine 209 residue is palmitoylated by a membrane-bound acyltransferase, known as porcupine.367 Transport of the lipid-modified Wnt ligands is regulated by Wntless (Wls). After being released from cargo receptor, extracellular Wnt ligands bind to plasma membrane receptors, Frizzled receptor family of proteins, and co-receptors, low-density lipoprotein-related protein 5 and 6 (LRP5/6). The (pro)renin receptor is necessary for the activation of Wnt signaling.368 Then the signal transmits to cytoplasmic phosphoprotein, disheveled (Dsh/Dvl). Wnt ligands activate the canonical, β-catenin-dependent pathway, and the noncanonical, β-catenin-independent pathway. In a healthy kidney, Wnt signaling is in an inactive state. β-catenin is constitutively phosphorylated by casein kinase 1 (CK1) and glycogen synthase kinase-3β (GSK-3β), and degraded by ubiquitin-mediated proteolysis in the E3 ubiquitin ligase β-TrCP dependent way.369 However, the upregulated Wnt ligands in fibrotic kidney bind to Frizzled and LRP5/6 and inhibit the binding of APC, CK1, GSK-3β, and β-catenin to the scaffold protein, Axin1. The destruction complex of β-catenin collapses. Cytoplasm accumulated β-catenin translocates into the nuclear and binds to T-cell factor/lymphoid enhancer-binding factor to promote downstream target genes transcription.370
Wnt/β-catenin signaling is important signaling mediated renal fibrosis. Wnt/β-catenin signaling is activated in AKI and CKD. The increased Wnt ligands and β-catenin promoted renal repair in AKI. However, the sustained activation of Wnt/β-catenin signaling aggravated renal fibrosis.371 Loss of α-Klotho amplified Wnt/β-catenin signaling in patients and animals with CKD.372 ECM proteins such as fibronectin, partial EMT driver Snail-1, ECM degradation mediators MMP7 and PAI-1, and all the components of the RAAS are downstream targets of β-catenin.373,374 Wnt3a exacerbated IL-4- or TGF-β1-induced macrophage alternative M2 polarization. Deletion of β-catenin in macrophages attenuated the ECM deposition and renal inflammation by limiting M2 macrophage polarization.375 Overexpression of Wnt9a drove tubular senescence and produce TGF-β1, which promoted proliferation and activation in normal rat kidney fibroblasts.376 Tubule-derived Wnts but not fibroblasts-derived Wnts were necessary for fibroblasts activation and renal fibrosis.377 Moreover, β-catenin expressed by TECs controlled the release of osteopontin (OPN) enriched exosome which promoted fibroblast proliferation and activation by binding to CD44.340
Blockade of Wnt/β-catenin signaling is a potential therapy for ameliorating renal fibrosis. α-Klotho abolished the interaction between Wnt ligands and the receptors and then inhibited the activation of Wnt/β-catenin signaling. Secreted α-Klotho supplement suppressed myofibroblast activation, reduced matrix expression, and ameliorated renal fibrosis.372 Paricalcitol hampered activation of renal myofibroblasts and suppressed expression of the fibrogenic factors. Mechanistically, paricalcitol blunted β-catenin-mediated gene transcription by inducing the interaction between vitamin D receptors and β-catenin.378 Small molecule ICG-001 inhibited the binding of β-catenin and CREB binding protein (CBP) and abolished β-catenin-driven gene transcription, resulting in the attenuated fibrotic lesions in obstructive nephropathy.379 Dickkopf (DKK) families including DKK1-4 are natural antagonists of Wnt/β-catenin signaling by binding to LRP6 and blocking the interaction between Wnt ligands and LRP6.380 Moreover, DKK1 and DKK2 induce endocytosis of LRP6. Various studies showed DKKs reduced myofibroblasts activation and counteracted kidney fibrosis.373,381 A phase 2 clinical trial (NCT01337752) evaluating the effect of anti- DKK1 monoclonal antibody (BHQ880) compared with placebo on time to first skeletal-related event in patients with untreated multiple myeloma and renal insufficiency in combination with bortezomib and dexamethasone is recruiting. However, DKK1 promotes mesangial matrix accumulation and proteinuria in mice with DN.382 The effects of DKKs may differ from diseases. Although the renal protective role of Wnt/β-catenin signaling inhibition has been proved in multiple CKD animal models, there are no clinical trials of drugs targeting Wnt/β-catenin signaling in patients with CKD currently.
Notch signaling
The Notch pathway is a phylogenetically conserved pathway, which controls the signal communication between adjacent cells. The ligands of Notch signaling, Jagged 1, 2, and Delta 1, 3, 4 are expressed on the cell membranes. In the interaction of the ligands with the receptors, Notch 1-4 initiates the successive cleavage of the receptors mediated by ADAM protease and γ-secretase complex. Finally, the Notch intracellular domain is transported to the nucleus and regulates the transcription of downstream genes with the assistance of transcriptional regulators, such as Rbpj and MAML.383
Both ligands and receptors of Notch signaling are upregulated in the injured kidney of patients and animals with DN, FSGS, minimal change disease, and lupus nephritis, and of animals with folic acid nephropathy, puromycin aminonucleoside-induced nephropathy, and UUO.384 The degree of glomerulosclerosis was positively related to the levels of cleaved Notch1 expressed by podocytes, while the severity of tubulointerstitial fibrosis and the estimated GFR were positively related to the levels of cleaved Notch1 in the tubulointerstitium.385 Tubular overexpressing cleaved Notch1 genetic mice aggravated renal fibrosis induced by folic acid. However, tubular-specific deletion of Rbpj, an important transcriptional regulator of Notch signaling, reduced the transcription of ECM proteins, and myofibroblasts markers including vimentin and α-SMA.386 Notch3 is also a critical isoform involved in kidney fibrosis. Notch3 deficiency protected UUO mice from tubular damage and cell loss with significantly reduced interstitial collagen deposition.387 Loss of histone deacetylase Sirt6 increased the transcription of Notch1 and Notch4 of podocytes.388 The activation of Notch signaling exacerbated podocyte injury, proteinuria, and glomerulosclerosis in mice with DN and adriamycin-induced nephropathy. Aberrant activation of Notch1 signaling in glomerular endothelium induced severe albuminuria which was an independent risk factor of glomerulosclerosis.389
We have introduced the critical role of Notch signaling in promoting the progression of CKD. Notch signaling inhibition is a probable solution for CKD. γ-secretase inhibitor DBZ treatment diminished the transcript levels of fibrosis markers, including collagen 1a1, collagen 3a1, fibronectin, and decreased the activation of myofibroblasts reflected by α-SMA and vimentin in mouse models of folic acid and UUO- induced kidney disease.386
The activation of RAAS
RAAS is important for regulating blood pressure homeostasis, vascular injury, and repair responses. Renin is mainly produced by renal juxtaglomerular cells, and in circulation, renin converts liver-derived angiotensinogen to angiotensin I (Ang I). Under the action of ACE, Ang I is converted into the biologically active Ang II, which can bind to angiotensin II receptor type 1 (AT1) to initiate the downstream cascade reaction.390,391 There is no classical pathway that mediated the signal transduction after Ang II binding to AT1R. Traditionally recognition is RAAS activated in circulation due to the components being produced in different organs. However, local RAAS activation evokes attention. All the components of RAAS are upregulated in the fibrotic kidney. The activation of AT1 triggers multiple signaling pathways, for example, kinase activation including ERK, PI3K, AKT, and JAK-STAT, activation of transcription factors including Smad, NF-κB, and Nrf2, OS, and cell cycle-related factors.392 The activation of RAAS promotes the progression of renal fibrosis through various pathways. RAAS also mediates blood pressure elevation and cardiac fibrosis.391,393
ACEis and ARBs are the prime therapies for CKD patients. The CAPTOPRIL trial showed ACEi, captopril, reduced the proteinuria of patients with DN and proteinuria by 30%; decreased the risk of double serum creatine levels by 43%; decreased the risk of death or dialysis or renal transplantation by 50% compared to placebo.394 The exciting results drove ACEi to an effective and classical drug for CKD patients with serum creatine less than 2.5 mg/dL. An impressive clinical trial expanded the range of ACEis applied to CKD patients. The results showed ACEi, benazepril, reduced the risk of doubling the serum creatinine level, ESRD, or death by 43% compared to placebo in CKD patients with serum creatinine levels of 3.1 to 5.0 mg/dL.395 Although ACEis are effective and widely used, some patients would have the side effects such as cough. And ARBs such as irbesartan can be alternative drugs. The physiological effect of aldosterone is controlling sodium reabsorption and potassium secretion through binding to the mineralocorticoid receptor (MR). The activation of MR in CKD leads to OS, inflammation, fibrotic remodeling, and hemodynamic alterations.396,397 Finerenone is a nonsteroidal, selective MR antagonist. A clinical trial conducted in 2015 demonstrated finerenone decreased the urinary ACR in patients with DN receiving ACEis or ARBs in a dose-dependent way.398 A further study evaluated the long-term outcome of finerenone on patients with CKD and type 2 diabetes. The results showed that 17.8% of patients in the finerenone group occurred a sustained decrease of at least 40% in the eGFR and the ratio was 21.1% in the placebo group. 13.0% of patients in the finerenone group occurred cardiovascular events, and 14.8% in the placebo group.399 And the P values were both lower than 0.05. It was concluded that finerenone lowered the risks of CKD progression and cardiovascular events in patients with DN.
Conclusions
Lipotoxicity, OS, inflammation, and fibrosis play an important role in promoting the progression of CKD. We have discussed the key signaling pathways that regulated these primary pathologic changes and the therapies targeted to these pathways. The ectopic lipid and lipoprotein metabolism not only is the result of CKD but also accelerates CKD progression. Lowering lipid therapies such as stains preserve renal function and decrease proteinuria and cardiovascular events for CKD patients. However, the available lipid-lowering drugs are limited, especially for CKD patients undergoing dialysis. The possible reason might be that, although the serum lipid levels are controlled, the genetic disorders of the kidney cannot be rectified easily. The signaling pathways mediated OS and inflammation form a complex network. The generation of ROS activates inflammatory factors such as NLRP3 inflammasome and NF-κB. And the reinforced inflammation enhances the production of ROS. Anti-inflammation drugs and anti-OS drugs get great achievements for CKD treatment recently. SGLT2 inhibitors are novel available drugs for preventing the decline of renal function. Anti-fibrosis drugs perform satisfying in CKD animals and patients. ACEi and ARB are classical drugs for CKD treatment. Inhibitors targeting TGF-β signaling are promising drugs for CKD patients. However, the signaling pathways of fibrosis have been studied for several decades, and targeted drugs in clinical trials are limited. Further investigations on the signaling pathways that regulated CKD and the targeted therapies are needed.
References
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic kidney disease. Lancet 389, 1238–1252 (2017).
Yuan, Q., Tan, R. J. & Liu, Y. Myofibroblast in kidney fibrosis: origin, activation, and regulation. Adv. Exp. Med. Biol. 1165, 253–283 (2019).
Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7, 684–696 (2011).
Humphreys, B. D. Mechanisms of renal fibrosis. Annu Rev. Physiol. 80, 309–326 (2018).
Zhou, D. & Liu, Y. Renal fibrosis in 2015: understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 12, 68–70 (2016).
Du, X. G. & Ruan, X. Z. Lipid metabolism disorder and renal fibrosis. Adv. Exp. Med. Biol. 1165, 525–541 (2019).
Speer, T., Ridker, P. M., von Eckardstein, A., Schunk, S. J. & Fliser, D. Lipoproteins in chronic kidney disease: from bench to bedside. Eur. Heart J. 42, 2170–2185 (2021).
Opazo-Ríos, L. et al. Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int. J. Mol. Sci. 21, 2632 (2020).
Liu, B. C., Tang, T. T., Lv, L. L. & Lan, H. Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 93, 568–579 (2018).
Kedia-Mehta, N. & Finlay, D. K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 10, 2123 (2019).
Xu, L., Sharkey, D. & Cantley, L. G. Tubular GM-CSF promotes late MCP-1/CCR2-mediated fibrosis and inflammation after ischemia/reperfusion injury. J. Am. Soc. Nephrol. 30, 1825–1840 (2019).
Meng, X. M. Inflammatory mediators and renal fibrosis. Adv. Exp. Med. Biol. 1165, 381–406 (2019).
Boor, P. & Floege, J. The renal (myo-)fibroblast: a heterogeneous group of cells. Nephrol. Dial. Transplant. 27, 3027–3036 (2012).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Ali, A. et al. Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non-small cell lung cancer. EMBO Mol. Med. 10, e8313 (2018).
Eguchi, K. et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 15, 518–533 (2012).
Yamagishi, K. et al. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: the JPHC Study. Eur. Heart J. 34, 1225–1232 (2013).
Schulze, M. B., Minihane, A. M., Saleh, R. N. M. & Risérus, U. Intake and metabolism of omega-3 and omega-6 polyunsaturated fatty acids: nutritional implications for cardiometabolic diseases. Lancet Diabetes Endocrinol. 8, 915–930 (2020).
Hilgendorf, K. I. et al. Omega-3 fatty acids activate ciliary FFAR4 to control adipogenesis. Cell 179, 1289–1305.e1221 (2019).
Wang, B. & Tontonoz, P. Phospholipid remodeling in physiology and disease. Annu Rev. Physiol. 81, 165–188 (2019).
Bohdanowicz, M. & Grinstein, S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 93, 69–106 (2013).
Di Paolo, G. & Kim, T. W. Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat. Rev. Neurosci. 12, 284–296 (2011).
Yu, X. H., Zhang, D. W., Zheng, X. L. & Tang, C. K. Cholesterol transport system: an integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res. 73, 65–91 (2019).
Noels, H., Lehrke, M., Vanholder, R. & Jankowski, J. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat. Rev. Nephrol. 17, 528–542 (2021).
Gai, Z. et al. Lipid accumulation and chronic kidney disease. Nutrients 11, 722 (2019).
Yang, X. et al. CD36 in chronic kidney disease: novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 13, 769–781 (2017).
Jang, H. S., Noh, M. R., Kim, J. & Padanilam, B. J. Defective mitochondrial fatty acid oxidation and lipotoxicity in kidney diseases. Front. Med. (Lausanne) 7, 65 (2020).
Morigny, P., Boucher, J., Arner, P. & Langin, D. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 17, 276–295 (2021).
Luo, J., Yang, H. & Song, B. L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 21, 225–245 (2020).
Cerqueira, N. M. et al. Cholesterol biosynthesis: a mechanistic overview. Biochemistry 55, 5483–5506 (2016).
Tavasoli, M., Lahire, S., Reid, T., Brodovsky, M. & McMaster, C. R. Genetic diseases of the Kennedy pathways for membrane synthesis. J. Biol. Chem. 295, 17877–17886 (2020).
Ronco, P. & Debiec, H. Pathophysiological advances in membranous nephropathy: time for a shift in patient's care. Lancet 385, 1983–1992 (2015).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Saito, K. et al. Lipid accumulation and transforming growth factor-beta upregulation in the kidneys of rats administered angiotensin II. Hypertension 46, 1180–1185 (2005).
Kume, S. et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 18, 2715–2723 (2007).
Portilla, D. et al. Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int. 69, 2194–2204 (2006).
de Vries, A. P. et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2, 417–426 (2014).
Herman-Edelstein, M., Scherzer, P., Tobar, A., Levi, M. & Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 55, 561–572 (2014).
Soppert, J., Lehrke, M., Marx, N., Jankowski, J. & Noels, H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 159, 4–33 (2020).
Thompson, S. et al. Cause of death in patients with reduced kidney function. J. Am. Soc. Nephrol. 26, 2504–2511 (2015).
Agrawal, S., Zaritsky, J. J., Fornoni, A. & Smoyer, W. E. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat. Rev. Nephrol. 14, 57–70 (2018).
Zhou, H., Tan, K. C., Shiu, S. W. & Wong, Y. Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes Metab. Res. Rev. 24, 617–623 (2008).
Li, L. et al. Metabolomics reveal mitochondrial and fatty acid metabolism disorders that contribute to the development of DKD in T2DM patients. Mol. Biosyst. 13, 2392–2400 (2017).
Fontecha-Barriuso, M. et al. The role of PGC-1α and mitochondrial biogenesis in kidney diseases. Biomolecules 10, 347 (2020).
Morris, E. M. et al. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G979–G992 (2012).
Han, S. H. et al. PGC-1α protects from Notch-induced kidney fibrosis development. J. Am. Soc. Nephrol. 28, 3312–3322 (2017).
Nakamura, M. T., Yudell, B. E. & Loor, J. J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 53, 124–144 (2014).
Lefebvre, P., Chinetti, G., Fruchart, J. C. & Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Invest. 116, 571–580 (2006).
Tanaka, Y. et al. Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int. 79, 871–882 (2011).
Speeckaert, M. M., Vanfraechem, C., Speeckaert, R. & Delanghe, J. R. Peroxisome proliferator-activated receptor agonists in a battle against the aging kidney. Ageing Res. Rev. 14, 1–18 (2014).
Chung, K. W. et al. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J. Am. Soc. Nephrol. 29, 1223–1237 (2018).
Jao, T. M. et al. ATF6α downregulation of PPARα promotes lipotoxicity-induced tubulointerstitial fibrosis. Kidney Int. 95, 577–589 (2019).
Fajas, L. et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J. Biol. Chem. 272, 18779–18789 (1997).
Hiraike, Y. et al. NFIA co-localizes with PPARγ and transcriptionally controls the brown fat gene program. Nat. Cell Biol. 19, 1081–1092 (2017).
El Ouarrat, D. et al. TAZ is a negative regulator of PPARγ activity in adipocytes and TAZ deletion improves insulin sensitivity and glucose tolerance. Cell Metab. 31, 162–173.e165 (2020).
Zhu, Q. & Scherer, P. E. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat. Rev. Nephrol. 14, 105–120 (2018).
Guan, Y. Peroxisome proliferator-activated receptor family and its relationship to renal complications of the metabolic syndrome. J. Am. Soc. Nephrol. 15, 2801–2815 (2004).
Toffoli, B. et al. Nephropathy in Pparg-null mice highlights PPARγ systemic activities in metabolism and in the immune system. PLoS One 12, e0171474 (2017).
Kikuchi, H. et al. Failure to sense energy depletion may be a novel therapeutic target in chronic kidney disease. Kidney Int. 95, 123–137 (2019).
Kampe, K., Sieber, J., Orellana, J. M., Mundel, P. & Jehle, A. W. Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am. J. Physiol. Ren. Physiol. 306, F401–F409 (2014).
Lee, M. et al. Phosphorylation of acetyl-CoA carboxylase by AMPK reduces renal fibrosis and is essential for the anti-fibrotic effect of metformin. J. Am. Soc. Nephrol. 29, 2326–2336 (2018).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 13, 629–646 (2017).
Price, N. L. et al. Genetic deficiency or pharmacological inhibition of miR-33 protects from kidney fibrosis. JCI Insight 4, e131102 (2019).
Piret, S. E. et al. Loss of proximal tubular transcription factor Krüppel-like factor 15 exacerbates kidney injury through loss of fatty acid oxidation. Kidney Int. 100, 1250–1267 (2021).
Eberlé, D., Hegarty, B., Bossard, P., Ferré, P. & Foufelle, F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86, 839–848 (2004).
Sun, L., Halaihel, N., Zhang, W., Rogers, T. & Levi, M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J. Biol. Chem. 277, 18919–18927 (2002).
Jiang, T., Liebman, S. E., Lucia, M. S., Li, J. & Levi, M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 68, 2608–2620 (2005).
Fu, Y. et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell Metab. 32, 1052–1062.e1058 (2020).
Dorotea, D., Koya, D. & Ha, H. Recent insights into SREBP as a direct mediator of kidney fibrosis via lipid-independent pathways. Front. Pharm. 11, 265 (2020).
Wang, X. X. et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59, 2916–2927 (2010).
Wang, X. X. et al. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J. Am. Soc. Nephrol. 29, 118–137 (2018).
Cansby, E. et al. Depletion of protein kinase STK25 ameliorates renal lipotoxicity and protects against diabetic kidney disease. JCI Insight 5, e140483 (2020).
Hua, W. et al. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS One 10, e0127507 (2015).
Falkevall, A. et al. Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab. 25, 713–726 (2017).
Khan, S. et al. Kidney proximal tubule lipoapoptosis is regulated by fatty acid transporter-2 (FATP2). J. Am. Soc. Nephrol. 29, 81–91 (2018).
Mori, Y. et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 33, 1042–1061.e1047 (2021).
Ducasa, G. M., Mitrofanova, A. & Fornoni, A. Crosstalk between lipids and mitochondria in diabetic kidney disease. Curr. Diab Rep. 19, 144 (2019).
Merscher-Gomez, S. et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes 62, 3817–3827 (2013).
Ducasa, G. M. et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J. Clin. Invest. 129, 3387–3400 (2019).
Pedigo, C. E. et al. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J. Clin. Invest. 126, 3336–3350 (2016).
Jacobs-Cachá, C. et al. A specific tubular ApoA-I distribution is associated to FSGS recurrence after kidney transplantation. J. Clin. Med. 10, 2174 (2021).
Vaziri, N. D. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat. Rev. Nephrol. 12, 37–47 (2016).
Zewinger, S. et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur. Heart J. 38, 1597–1607 (2017).
Zhang, F. et al. SAA1 is transcriptionally activated by STAT3 and accelerates renal interstitial fibrosis by inducing endoplasmic reticulum stress. Exp. Cell Res. 408, 112856 (2021).
Shao, B. et al. A cluster of proteins implicated in kidney disease is increased in high-density lipoprotein isolated from hemodialysis subjects. J. Proteome Res. 14, 2792–2806 (2015).
Valleix, S. et al. D25V apolipoprotein C-III variant causes dominant hereditary systemic amyloidosis and confers cardiovascular protective lipoprotein profile. Nat. Commun. 7, 10353 (2016).
Lui, D. T. W. et al. Carbamylated HDL and mortality outcomes in type 2 diabetes. Diabetes Care 44, 804–809 (2021).
Han, S., Vaziri, N. D., Gollapudi, P., Kwok, V. & Moradi, H. Hepatic fatty acid and cholesterol metabolism in nephrotic syndrome. Am. J. Transl. Res. 5, 246–253 (2013).
Liu, H. et al. Interleukin-1β promotes Ox-LDL uptake by human glomerular mesangial cells via LOX-1. Int. J. Med. Sci. 17, 1056–1061 (2020).
Podrez, E. A. et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 105, 1095–1108 (2000).
Moorhead, J. F., Chan, M. K., El-Nahas, M. & Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2, 1309–1311 (1982).
Epstein, M. & Vaziri, N. D. Statins in the management of dyslipidemia associated with chronic kidney disease. Nat. Rev. Nephrol. 8, 214–223 (2012).
Ruggiero, C. et al. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am. J. Physiol. Ren. Physiol. 306, F896–F906 (2014).
Yamamoto, T. et al. High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J. Am. Soc. Nephrol. 28, 1534–1551 (2017).
Li, C. et al. Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney. Am. J. Physiol. Ren. Physiol. 310, F351–F363 (2016).
Kruger, C. et al. Proximal tubular cell-specific ablation of carnitine acetyltransferase causes tubular disease and secondary glomerulosclerosis. Diabetes 68, 819–831 (2019).
Miguel, V. et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Invest. 131, e140695 (2021).
Fornoni, A., Merscher, S. & Kopp, J. B. Lipid biology of the podocyte–new perspectives offer new opportunities. Nat. Rev. Nephrol. 10, 379–388 (2014).
Sieber, J. et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Ren. Physiol. 299, F821–F829 (2010).
Xu, S. et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis. 6, e1976 (2015).
Lee, E., Choi, J. & Lee, H. S. Palmitate induces mitochondrial superoxide generation and activates AMPK in podocytes. J. Cell Physiol. 232, 3209–3217 (2017).
Cui, W. et al. Interaction of thrombospondin1 and CD36 contributes to obesity-associated podocytopathy. Biochim Biophys. Acta 1852, 1323–1333 (2015).
Maimaitiyiming, H., Zhou, Q. & Wang, S. Thrombospondin 1 deficiency ameliorates the development of adriamycin-induced proteinuric kidney disease. PLoS One 11, e0156144 (2016).
Daniel, C., Schaub, K., Amann, K., Lawler, J. & Hugo, C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes 56, 2982–2989 (2007).
Lennon, R. et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol. Dial. Transplant. 24, 3288–3296 (2009).
Artunc, F. et al. The impact of insulin resistance on the kidney and vasculature. Nat. Rev. Nephrol. 12, 721–737 (2016).
Bussolati, B. et al. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J. Am. Soc. Nephrol. 16, 1936–1947 (2005).
Freedman, B. I., Limou, S., Ma, L. & Kopp, J. B. APOL1-associated nephropathy: a key contributor to racial disparities in CKD. Am. J. Kidney Dis. 72, S8–S16 (2018).
Declèves, A. E., Mathew, A. V., Cunard, R. & Sharma, K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J. Am. Soc. Nephrol. 22, 1846–1855 (2011).
Giunti, S. et al. The MCP-1/CCR2 system has direct proinflammatory effects in human mesangial cells. Kidney Int. 69, 856–863 (2006).
Park, M. J., Han, H. J. & Kim, D. I. Lipotoxicity-induced PRMT1 exacerbates mesangial cell apoptosis via endoplasmic reticulum stress. Int. J. Mol. Sci. 18, 1421 (2017).
Mishra, R. & Simonson, M. S. Saturated free fatty acids and apoptosis in microvascular mesangial cells: palmitate activates pro-apoptotic signaling involving caspase 9 and mitochondrial release of endonuclease G. Cardiovasc Diabetol. 4, 2 (2005).
Schlondorff, D. Cellular mechanisms of lipid injury in the glomerulus. Am. J. Kidney Dis. 22, 72–82 (1993).
Song, C. Y., Kim, B. C., Hong, H. K. & Lee, H. S. Oxidized LDL activates PAI-1 transcription through autocrine activation of TGF-beta signaling in mesangial cells. Kidney Int. 67, 1743–1752 (2005).
Li, J., Li, H., Wen, Y. B. & Li, X. W. Very-low-density lipoprotein-induced triglyceride accumulation in human mesangial cells is mainly mediated by lipoprotein lipase. Nephron Physiol. 110, p1–p10 (2008).
Kamanna, V. S., Pai, R., Ha, H., Kirschenbaum, M. A. & Roh, D. D. Oxidized low-density lipoprotein stimulates monocyte adhesion to glomerular endothelial cells. Kidney Int. 55, 2192–2202 (1999).
Prieur, X., Roszer, T. & Ricote, M. Lipotoxicity in macrophages: evidence from diseases associated with the metabolic syndrome. Biochim Biophys. Acta 1801, 327–337 (2010).
Wintergerst, E. S., Jelk, J., Rahner, C. & Asmis, R. Apoptosis induced by oxidized low density lipoprotein in human monocyte-derived macrophages involves CD36 and activation of caspase-3. Eur. J. Biochem. 267, 6050–6059 (2000).
Seimon, T. A. et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 12, 467–482 (2010).
Pennathur, S. et al. The macrophage phagocytic receptor CD36 promotes fibrogenic pathways on removal of apoptotic cells during chronic kidney injury. Am. J. Pathol. 185, 2232–2245 (2015).
Hagberg, C. E. et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464, 917–921 (2010).
Palmer, S. C. et al. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann. Intern. Med. 157, 263–275 (2012).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011).
Wanner, C. et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353, 238–248 (2005).
Fellström, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360, 1395–1407 (2009).
Colhoun, H. M. et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am. J. Kidney Dis. 54, 810–819 (2009).
de Zeeuw, D. et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol. 3, 181–190 (2015).
Amann, K. & Benz, K. Statins–beyond lipids in CKD. Nephrol. Dial. Transplant. 26, 407–410 (2011).
Krane, V. & Wanner, C. Statins, inflammation and kidney disease. Nat. Rev. Nephrol. 7, 385–397 (2011).
Yoshimura, A. et al. Simvastatin suppresses glomerular cell proliferation and macrophage infiltration in rats with mesangial proliferative nephritis. J. Am. Soc. Nephrol. 9, 2027–2039 (1998).
Knight, S. F., Yuan, J., Roy, S. & Imig, J. D. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am. J. Physiol. Ren. Physiol. 298, F86–F94 (2010).
Park, Y. S. et al. Lovastatin reduces glomerular macrophage influx and expression of monocyte chemoattractant protein-1 mRNA in nephrotic rats. Am. J. Kidney Dis. 31, 190–194 (1998).
Vrtovsnik, F., Couette, S., Prié, D., Lallemand, D. & Friedlander, G. Lovastatin-induced inhibition of renal epithelial tubular cell proliferation involves a p21ras activated, AP-1-dependent pathway. Kidney Int. 52, 1016–1027 (1997).
Iimura, O., Vrtovsnik, F., Terzi, F. & Friedlander, G. HMG-CoA reductase inhibitors induce apoptosis in mouse proximal tubular cells in primary culture. Kidney Int. 52, 962–972 (1997).
Ting, R. D. et al. Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: the FIELD Study. Diabetes Care 35, 218–225 (2012).
Ferro, C. J. et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 14, 727–749 (2018).
Kasiske, B. L., O'Donnell, M. P., Garvis, W. J. & Keane, W. F. Pharmacologic treatment of hyperlipidemia reduces glomerular injury in rat 5/6 nephrectomy model of chronic renal failure. Circ. Res. 62, 367–374 (1988).
Chuppa, S. et al. MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in Cardiorenal Syndrome Type 4. Kidney Int. 93, 375–389 (2018).
Zhang, R. & Zheng, F. PPAR-gamma and aging: one link through klotho? Kidney Int. 74, 702–704 (2008).
Lachin, J. M. et al. Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin. J. Am. Soc. Nephrol. 6, 1032–1040 (2011).
Agrawal, S., He, J. C. & Tharaux, P. L. Nuclear receptors in podocyte biology and glomerular disease. Nat. Rev. Nephrol. 17, 185–204 (2021).
Sun, L. et al. Pioglitazone improves mitochondrial function in the remnant kidney and protects against renal fibrosis in 5/6 nephrectomized rats. Front. Pharm. 8, 545 (2017).
Yang, H. C. et al. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J. Am. Soc. Nephrol. 20, 2380–2388 (2009).
Souza, A. C. et al. Antagonism of scavenger receptor CD36 by 5A peptide prevents chronic kidney disease progression in mice independent of blood pressure regulation. Kidney Int. 89, 809–822 (2016).
Iwao, Y. et al. CD36 is one of important receptors promoting renal tubular injury by advanced oxidation protein products. Am. J. Physiol. Ren. Physiol. 295, F1871–F1880 (2008).
Szeto, H. H. et al. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 90, 997–1011 (2016).
Szeto, H. H. Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J. Am. Soc. Nephrol. 28, 2856–2865 (2017).
Xiao, L. et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 11, 297–311 (2017).
Qin, X. et al. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharm. 177, 3646–3661 (2020).
Clark, A. J. & Parikh, S. M. Targeting energy pathways in kidney disease: the roles of sirtuins, AMPK, and PGC1α. Kidney Int. 99, 828–840 (2021).
Shapiro, H., Theilla, M., Attal-Singer, J. & Singer, P. Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nat. Rev. Nephrol. 7, 110–-121 (2011).
Saglimbene, V. M. et al. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 39, 358–368 (2020).
Miller, E. R. III et al. The effects of n-3 long-chain polyunsaturated fatty acid supplementation on biomarkers of kidney injury in adults with diabetes: results of the GO-FISH trial. Diabetes Care 36, 1462–1469 (2013).
de Boer, I. H. et al. Effect of vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA 322, 1899–1909 (2019).
Herman-Edelstein, M., Weinstein, T. & Levi, M. Bile acid receptors and the kidney. Curr. Opin. Nephrol. Hypertens. 27, 56–62 (2018).
Jiang, T. et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 (2007).
Wang, X. X. et al. G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J. Am. Soc. Nephrol. 27, 1362–1378 (2016).
Declèves, A. E. et al. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int. 85, 611–623 (2014).
Koh, E. S. et al. Anthocyanin-rich Seoritae extract ameliorates renal lipotoxicity via activation of AMP-activated protein kinase in diabetic mice. J. Transl. Med. 13, 203 (2015).
Kim, M. Y. et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia 56, 204–217 (2013).
Conley, M. M. et al. Interventions for weight loss in people with chronic kidney disease who are overweight or obese. Cochrane Database Syst. Rev. 3, Cd013119 (2021).
Ahn, S. Y. et al. Weight loss has an additive effect on the proteinuria reduction of angiotensin II receptor blockers in hypertensive patients with chronic kidney disease. Kidney Res. Clin. Pract. 37, 49–58 (2018).
Friedman, A. N., Chambers, M., Kamendulis, L. M. & Temmerman, J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin. J. Am. Soc. Nephrol. 8, 1892–1898 (2013).
Daenen, K. et al. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 34, 975–991 (2019).
Yang, Q. et al. Nox4 in renal diseases: an update. Free Radic. Biol. Med. 124, 466–472 (2018).
Zhou, B. et al. Brd4 inhibition attenuates unilateral ureteral obstruction-induced fibrosis by blocking TGF-β-mediated Nox4 expression. Redox Biol. 11, 390–402 (2017).
Das, R. et al. Upregulation of mitochondrial Nox4 mediates TGF-β-induced apoptosis in cultured mouse podocytes. Am. J. Physiol. Ren. Physiol. 306, F155–F167 (2014).
Chen, J., Chen, J. K. & Harris, R. C. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol. Cell Biol. 32, 981–991 (2012).
Gorin, Y. et al. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am. J. Physiol. Ren. Physiol. 285, F219–F229 (2003).
You, Y. H., Quach, T., Saito, R., Pham, J. & Sharma, K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J. Am. Soc. Nephrol. 27, 466–481 (2016).
Jha, J. C. et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1237–1254 (2014).
Jha, J. C. et al. Podocyte-specific Nox4 deletion affords renoprotection in a mouse model of diabetic nephropathy. Diabetologia 59, 379–389 (2016).
Kumar, V., Kurth, T., Zheleznova, N. N., Yang, C. & Cowley, A. W. Jr NOX4/H(2)O(2)/mTORC1 pathway in salt-induced hypertension and kidney injury. Hypertension 76, 133–143 (2020).
Jin, H., Wang, Y., Wang, D. & Zhang, L. Effects of Qingshen granules on the oxidative stress-NF/kB signal pathway in unilateral ureteral obstruction rats. Evid. Based Complement Alternat. Med. 2018, 4761925 (2018).
Morgan, M. J. & Liu, Z. G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21, 103–115 (2011).
Nlandu Khodo, S. et al. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 23, 1967–1976 (2012).
Sedeek, M., Nasrallah, R., Touyz, R. M. & Hébert, R. L. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J. Am. Soc. Nephrol. 24, 1512–1518 (2013).
Jha, J. C. et al. Endothelial or vascular smooth muscle cell-specific expression of human NOX5 exacerbates renal inflammation, fibrosis and albuminuria in the Akita mouse. Diabetologia 62, 1712–1726 (2019).
Förstermann, U. & Sessa, W. C. Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837 (2012). 837a-837d.
Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 17, 575–590 (2021).
Yoo, K. H., Thornhill, B. A., Forbes, M. S. & Chevalier, R. L. Inducible nitric oxide synthase modulates hydronephrosis following partial or complete unilateral ureteral obstruction in the neonatal mouse. Am. J. Physiol. Ren. Physiol. 298, F62–F71 (2010).
Udi, S. et al. Dual inhibition of cannabinoid CB(1) receptor and inducible NOS attenuates obesity-induced chronic kidney disease. Br. J. Pharm. 177, 110–127 (2020).
Zhou, L. et al. Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int. 95, 830–845 (2019).
Mo, H. et al. C-X-C chemokine receptor type 4 plays a crucial role in mediating oxidative stress-induced podocyte injury. Antioxid. Redox Signal 27, 345–362 (2017).
Tejchman, K., Kotfis, K. & Sieńko, J. Biomarkers and mechanisms of oxidative stress-last 20 years of research with an emphasis on kidney damage and renal transplantation. Int. J. Mol. Sci. 22, 8010 (2021).
Liles, J. T. et al. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J. Clin. Invest. 128, 4485–4500 (2018).
Guerrero-Hue, M. et al. Protective role of Nrf2 in renal disease. Antioxidants (Basel) 10, 39 (2020).
Nezu, M. & Suzuki, N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int. J. Mol. Sci. 21, 2951 (2020).
Kong, W. et al. Nrf2 deficiency promotes the progression from acute tubular damage to chronic renal fibrosis following unilateral ureteral obstruction. Nephrol. Dial. Transplant. 33, 771–783 (2018).
Jiang, T. et al. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59, 850–860 (2010).
Jiang, T. et al. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 85, 333–343 (2014).
Hong, Q. et al. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 93, 1330–1343 (2018).
He, W. et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Invest. 120, 1056–1068 (2010).
Kitada, M., Kume, S., Imaizumi, N. & Koya, D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 60, 634–643 (2011).
Kim, E. N. et al. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging (Albany NY) 10, 83–99 (2018).
Hong, Y. A., Kim, J. E., Jo, M. & Ko, G. J. The role of sirtuins in kidney diseases. Int. J. Mol. Sci. 21, 6686 (2020).
Yang, Y. et al. SIRT6 protects vascular endothelial cells from angiotensin II-induced apoptosis and oxidative stress by promoting the activation of Nrf2/ARE signaling. Eur. J. Pharm. 859, 172516 (2019).
Yang, X. et al. Roles of SIRT6 in kidney disease: a novel therapeutic target. Cell Mol. Life Sci. 79, 53 (2021).
Breyer, M. D. & Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Disco. 15, 568–588 (2016).
Lewis, E. J. et al. Pyridorin in type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 23, 131–136 (2012).
Sedeek, M. et al. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin. Sci. (Lond.) 124, 191–202 (2013).
Gorin, Y. et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol. 308, F1276–F1287 (2015).
Reutens, A. T. et al. A physician-initiated double-blind, randomised, placebo-controlled, phase 2 study evaluating the efficacy and safety of inhibition of NADPH oxidase with the first-in-class Nox-1/4 inhibitor, GKT137831, in adults with type 1 diabetes and persistently elevated urinary albumin excretion: Protocol and statistical considerations. Contemp. Clin. Trials 90, 105892 (2020).
Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011).
de Zeeuw, D. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 (2013).
Rush, B. M. et al. Genetic or pharmacologic Nrf2 activation increases proteinuria in chronic kidney disease in mice. Kidney Int. 99, 102–116 (2021).
Chertow, G. M. et al. Effects of selonsertib in patients with diabetic kidney disease. J. Am. Soc. Nephrol. 30, 1980–1990 (2019).
Tang, P. M., Nikolic-Paterson, D. J. & Lan, H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158 (2019).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 10, 493–503 (2014).
Wen, Y. et al. KLF4 in macrophages attenuates TNFα-mediated kidney injury and fibrosis. J. Am. Soc. Nephrol. 30, 1925–1938 (2019).
Ai, K. et al. Methyl-CpG-binding domain protein 2 contributes to renal fibrosis through promoting polarized M1 macrophages. Cell Death Dis. 13, 125 (2022).
Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 589, 281–286 (2021).
Tang, P. M. et al. The proto-oncogene tyrosine protein kinase Src is essential for macrophage-myofibroblast transition during renal scarring. Kidney Int. 93, 173–187 (2018).
Tang, P. M. et al. Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition. Proc. Natl Acad. Sci. USA 117, 20741–20752 (2020).
Nikolic-Paterson, D. J. CD4+ T cells: a potential player in renal fibrosis. Kidney Int. 78, 333–335 (2010).
Sato, Y. & Yanagita, M. Immunology of the ageing kidney. Nat. Rev. Nephrol. 15, 625–640 (2019).
Tapmeier, T. T. et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 78, 351–362 (2010).
Liu, L. et al. CD4+ T Lymphocytes, especially Th2 cells, contribute to the progress of renal fibrosis. Am. J. Nephrol. 36, 386–396 (2012).
Chen, A. et al. Bowman's capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. J. Clin. Invest. 128, 3413–3424 (2018).
Kleczko, E. K. et al. CD8(+) T cells modulate autosomal dominant polycystic kidney disease progression. Kidney Int. 94, 1127–1140 (2018).
Kurts, C. & Meyer-Schwesinger, C. Protecting the kidney against autoimmunity and inflammation. Nat. Rev. Nephrol. 15, 66–68 (2019).
Dorrington, M. G. & Fraser, I. D. C. NF-κB signaling in macrophages: dynamics, crosstalk, and signal integration. Front Immunol. 10, 705 (2019).
Zhang, H. & Sun, S. C. NF-κB in inflammation and renal diseases. Cell Biosci. 5, 63 (2015).
Sanz, A. B. et al. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 21, 1254–1262 (2010).
Song, N., Thaiss, F. & Guo, L. NFκB and kidney injury. Front Immunol. 10, 815 (2019).
Li, L. X. et al. Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J. Clin. Invest. 127, 2751–2764 (2017).
Yang, H. et al. Tim-3 aggravates podocyte injury in diabetic nephropathy by promoting macrophage activation via the NF-κB/TNF-α pathway. Mol. Metab. 23, 24–36 (2019).
Cordido, A. et al. TWEAK signaling pathway blockade slows cyst growth and disease progression in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 32, 1913–1932 (2021).
Lv, W., Booz, G. W., Wang, Y., Fan, F. & Roman, R. J. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharm. 820, 65–76 (2018).
Valiño-Rivas, L. et al. NIK as a druggable mediator of tissue injury. Trends Mol. Med. 25, 341–360 (2019).
Chen, A. et al. Soluble RARRES1 induces podocyte apoptosis to promote glomerular disease progression. J. Clin. Invest. 130, 5523–5535 (2020).
Cantero-Navarro, E. et al. Renin-angiotensin system and inflammation update. Mol. Cell Endocrinol. 529, 111254 (2021).
Esteban, V. et al. Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J. Am. Soc. Nephrol. 15, 1514–1529 (2004).
Ke, G. et al. Receptor activator of NF-κB mediates podocyte injury in diabetic nephropathy. Kidney Int. 100, 377–390 (2021).
Wu, L. et al. Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 100, 107–121 (2021).
Li, H. et al. The cell membrane repair protein MG53 modulates transcription factor NF-κB signaling to control kidney fibrosis. Kidney Int. 101, 119–130 (2022).
Zhong, F. et al. Tyro3 is a podocyte protective factor in glomerular disease. JCI Insight 3, e123482 (2018).
Zhong, F. et al. Protein S protects against podocyte injury in diabetic nephropathy. J. Am. Soc. Nephrol. 29, 1397–1410 (2018).
Lu, Q. et al. Circulating miR-103a-3p contributes to angiotensin II-induced renal inflammation and fibrosis via a SNRK/NF-κB/p65 regulatory axis. Nat. Commun. 10, 2145 (2019).
Liu, E. et al. METTL3/N6-methyladenosine/ miR-21-5p promotes obstructive renal fibrosis by regulating inflammation through SPRY1/ERK/NF-κB pathway activation. J. Cell Mol. Med. 25, 7660–7674 (2021).
Lv, L. L. et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 27, 210–226 (2020).
Mansell, A. et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7, 148–155 (2006).
Sun, D. et al. MiR-802 causes nephropathy by suppressing NF-κB-repressing factor in obese mice and human. J. Cell Mol. Med. 23, 2863–2871 (2019).
Yang, H., Liao, D., Tong, L., Zhong, L. & Wu, K. MiR-373 exacerbates renal injury and fibrosis via NF-κB/MatrixMetalloproteinase-9 signaling by targeting Sirtuin1. Genomics 111, 786–792 (2019).
Fu, H. X., Fan, X. P., Li, M., Liu, M. J. & Sun, Q. L. MiR-146a relieves kidney injury in mice with systemic lupus erythematosus through regulating NF-κB pathway. Eur. Rev. Med. Pharm. Sci. 23, 7024–7032 (2019).
Yu, H. Y. et al. Protective effect of miR-146 against kidney injury in diabetic nephropathy rats through mediating the NF-κB signaling pathway. Eur. Rev. Med. Pharm. Sci. 24, 3215–3222 (2020).
Villarino, A. V., Kanno, Y. & O'Shea, J. J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 18, 374–384 (2017).
Brosius, F. C. III & He, J. C. JAK inhibition and progressive kidney disease. Curr. Opin. Nephrol. Hypertens. 24, 88–95 (2015).
Fragiadaki, M. et al. STAT5 drives abnormal proliferation in autosomal dominant polycystic kidney disease. Kidney Int. 91, 575–586 (2017).
Sellmayr, M. et al. Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J. Am. Soc. Nephrol. 31, 2773–2792 (2020).
Tao, J. et al. JAK-STAT activity in peripheral blood cells and kidney tissue in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 15, 973–982 (2020).
Tao, J. et al. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int. 94, 795–808 (2018).
Tang, S. C. W. & Yiu, W. H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 16, 206–222 (2020).
Zhang, H. et al. Podocyte-specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int. 92, 909–921 (2017).
Jin, Q. et al. Overexpression of E3 ubiquitin ligase Cbl attenuates endothelial dysfunction in diabetes mellitus by inhibiting the JAK2/STAT4 signaling and Runx3-mediated H3K4me3. J. Transl. Med. 19, 469 (2021).
Li, J. et al. Bixin protects against kidney interstitial fibrosis through promoting STAT6 degradation. Front Cell Dev. Biol. 8, 576988 (2020).
Yan, J., Zhang, Z., Yang, J., Mitch, W. E. & Wang, Y. JAK3/STAT6 stimulates bone marrow-derived fibroblast activation in renal fibrosis. J. Am. Soc. Nephrol. 26, 3060–3071 (2015).
Johnson, D. E., O'Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Chen, W. et al. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation. Theranostics 9, 3980–3991 (2019).
Brosius, F. C., Tuttle, K. R. & Kretzler, M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 59, 1624–1627 (2016).
Liau, N. P. D. et al. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 9, 1558 (2018).
Ortiz-Muñoz, G. et al. Suppressors of cytokine signaling abrogate diabetic nephropathy. J. Am. Soc. Nephrol. 21, 763–772 (2010).
Estrada, C. C. et al. Krüppel-like factor 4 is a negative regulator of STAT3-induced glomerular epithelial cell proliferation. JCI Insight 3, e98214 (2018).
Pace, J. A. et al. Podocyte-specific KLF4 is required to maintain parietal epithelial cell quiescence in the kidney. Sci. Adv. 7, eabg6600 (2021).
Li, P. & Chang, M. Roles of PRR-mediated signaling pathways in the regulation of oxidative stress and inflammatory diseases. Int. J. Mol. Sci. 22, 7688 (2021).
Komada, T. & Muruve, D. A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 15, 501–520 (2019).
Xiong, W., Meng, X. F. & Zhang, C. NLRP3 inflammasome in metabolic-associated kidney diseases: an update. Front Immunol. 12, 714340 (2021).
Mangan, M. S. J. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Disco. 17, 588–606 (2018).
Cuevas, S. & Pelegrín, P. Pyroptosis and redox balance in kidney diseases. Antioxid. Redox Signal 35, 40–60 (2021).
Shi, J., Gao, W. & Shao, F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 42, 245–254 (2017).
Chi, H. H. et al. IL-36 signaling facilitates activation of the NLRP3 inflammasome and IL-23/IL-17 axis in renal inflammation and fibrosis. J. Am. Soc. Nephrol. 28, 2022–2037 (2017).
Zewinger, S. et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 21, 30–41 (2020).
Li, Y. et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death Differ. 28, 2333–2350 (2021).
Wyczanska, M. & Lange-Sperandio, B. DAMPs in unilateral ureteral obstruction. Front Immunol. 11, 581300 (2020).
Wu, M. et al. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism 118, 154748 (2021).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
Han, Y. et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 16, 32–46 (2018).
Hou, Y. et al. CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis. 12, 523 (2021).
Fu, R. et al. Pim-1 as a therapeutic target in lupus nephritis. Arthritis Rheumatol. 71, 1308–1318 (2019).
Ding, H. et al. MicroRNA-10 negatively regulates inflammation in diabetic kidney via targeting activation of the NLRP3 inflammasome. Mol. Ther. 29, 2308–2320 (2021).
Gan, X. G., Wang, Z. H. & Xu, H. T. Mechanism of miRNA-141-3p in calcium oxalate-induced renal tubular epithelial cell injury via NLRP3-mediated pyroptosis. Kidney Blood Press. Res. 47, 300–308 (2022).
Zhang, C. et al. Long noncoding RNA Kcnq1ot1 promotes sC5b-9-induced podocyte pyroptosis by inhibiting miR-486a-3p and upregulating NLRP3. Am. J. Physiol. Cell Physiol. 320, C355–c364 (2021).
Fitzgerald, K. A. & Kagan, J. C. Toll-like receptors and the control of immunity. Cell 180, 1044–1066 (2020).
Lin, M. & Tang, S. C. Toll-like receptors: sensing and reacting to diabetic injury in the kidney. Nephrol. Dial. Transplant. 29, 746–754 (2014).
Casanova, J. L., Abel, L. & Quintana-Murci, L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev. Immunol. 29, 447–491 (2011).
Gluba, A. et al. The role of Toll-like receptors in renal diseases. Nat. Rev. Nephrol. 6, 224–235 (2010).
Wada, J. & Makino, H. Innate immunity in diabetes and diabetic nephropathy. Nat. Rev. Nephrol. 12, 13–26 (2016).
Singh, M. V. et al. Angiotensin II-induced hypertension and cardiac hypertrophy are differentially mediated by TLR3- and TLR4-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 316, H1027–h1038 (2019).
Milanesi, S. et al. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J. Cell Physiol. 234, 10868–10876 (2019).
Xu, Z. et al. Inhibition of STAT3 activation mediated by toll-like receptor 4 attenuates angiotensin II-induced renal fibrosis and dysfunction. Br. J. Pharm. 176, 2627–2641 (2019).
Upadhyay, R. et al. Free light chains injure proximal tubule cells through the STAT1/HMGB1/TLR axis. JCI Insight 5, e137191 (2020).
Lakhia, R. et al. Interstitial microRNA miR-214 attenuates inflammation and polycystic kidney disease progression. JCI Insight 5, e133785 (2020).
Liu, H. et al. H19 promote calcium oxalate nephrocalcinosis-induced renal tubular epithelial cell injury via a ceRNA pathway. EBioMedicine 50, 366–378 (2019).
Wang, Y. et al. Blockade of myeloid differentiation 2 attenuates diabetic nephropathy by reducing activation of the renin-angiotensin system in mouse kidneys. Br. J. Pharm. 176, 2642–2657 (2019).
Poluzzi, C. et al. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 95, 540–562 (2019).
Raby, A. C. et al. Toll-like receptors 2 and 4 are potential therapeutic targets in peritoneal dialysis-associated fibrosis. J. Am. Soc. Nephrol. 28, 461–478 (2017).
Raby, A. C. et al. Targeting Toll-like receptors with soluble Toll-like receptor 2 prevents peritoneal dialysis solution-induced fibrosis. Kidney Int. 94, 346–362 (2018).
Hopfner, K. P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799.e785 (2019).
Bai, J. et al. DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc. Natl Acad. Sci. USA 114, 12196–12201 (2017).
Li, X. et al. DsbA-L mediated renal tubulointerstitial fibrosis in UUO mice. Nat. Commun. 11, 4467 (2020).
Wu, J. et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J. Clin. Invest. 131, e136329 (2021).
Davis, S. E., Khatua, A. K. & Popik, W. Nucleosomal dsDNA stimulates APOL1 expression in human cultured podocytes by activating the cGAS/IFI16-STING signaling pathway. Sci. Rep. 9, 15485 (2019).
Puthumana, J. et al. Biomarkers of inflammation and repair in kidney disease progression. J. Clin. Invest. 131, e139927 (2021).
Li, H. et al. IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J. Clin. Invest. 131, e142428 (2021).
Kim, D. H. et al. IL-10 deficiency aggravates renal inflammation, fibrosis and functional failure in high-fat dieted obese mice. Tissue Eng. Regen. Med. 18, 399–410 (2021).
Kim, S. R. et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 11, 2127 (2020).
Yaribeygi, H., Butler, A. E., Atkin, S. L., Katsiki, N. & Sahebkar, A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: possible molecular pathways. J. Cell Physiol. 234, 223–230 (2018).
Heerspink, H. J. L. et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 62, 1154–1166 (2019).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Ridker, P. M. et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397, 2060–2069 (2021).
Ridker, P. M. et al. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J. Am. Coll. Cardiol. 71, 2405–2414 (2018).
Tuttle, K. R. et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol. Dial. Transplant. 33, 1950–1959 (2018).
Lei, Y. et al. A multicenter blinded preclinical randomized controlled trial on Jak1/2 inhibition in MRL/MpJ-Fas(lpr) mice with proliferative lupus nephritis predicts low effect size. Kidney Int. 99, 1331–1341 (2021).
Zhong, Y. et al. Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat. Commun. 10, 4523 (2019).
Voelkl, J. et al. Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. J. Am. Soc. Nephrol. 29, 1636–1648 (2018).
Trachtman, H. et al. Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group. BMC Nephrol. 16, 111 (2015).
Mertelj, T., Smrekar, N., Kojc, N., Lindič, J. & Kovač, D. IgA nephropathy in a patient treated with adalimumab. Case Rep. Nephrol. Dial. 11, 233–240 (2021).
Graziano, F., Busè, M., Cassata, N., Lentini, V. L. & Citrano, M. IgA nephropathy in a child: Crohn's disease-associated or adalimumab induced? Curr. Med. Res. Opin. 38, 139–143 (2022).
Bhagat Singh, A. K., Jeyaruban, A. S., Wilson, G. J. & Ranganathan, D. Adalimumab-induced IgA nephropathy. BMJ Case Rep. 12, e226442 (2019).
Zhang, L. et al. Connectivity mapping identifies BI-2536 as a potential drug to treat diabetic kidney disease. Diabetes 70, 589–602 (2021).
Pace, J., Paladugu, P., Das, B., He, J. C. & Mallipattu, S. K. Targeting STAT3 signaling in kidney disease. Am. J. Physiol. Ren. Physiol. 316, F1151–f1161 (2019).
da Costa, M. O. L. et al. Nifuroxazide as JAK2 inhibitor: a binding mode proposal and Hel cell proliferation assay. Eur. J. Pharm. Sci. 162, 105822 (2021).
Said, E., Zaitone, S. A., Eldosoky, M. & Elsherbiny, N. M. Nifuroxazide, a STAT3 inhibitor, mitigates inflammatory burden and protects against diabetes-induced nephropathy in rats. Chem. Biol. Interact. 281, 111–120 (2018).
Hassan, N. M. E., Said, E. & Shehatou, G. S. G. Nifuroxazide suppresses UUO-induced renal fibrosis in rats via inhibiting STAT-3/NF-κB signaling, oxidative stress and inflammation. Life Sci. 272, 119241 (2021).
Chen, D. et al. JAK/STAT pathway promotes the progression of diabetic kidney disease via autophagy in podocytes. Eur. J. Pharm. 902, 174121 (2021).
Gasparitsch, M. et al. Tyrphostin AG490 reduces inflammation and fibrosis in neonatal obstructive nephropathy. PLoS One 14, e0226675 (2019).
Coll, R. C. et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 15, 556–559 (2019).
Tapia-Abellán, A. et al. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 15, 560–564 (2019).
Fu, R. et al. Podocyte activation of NLRP3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol. 69, 1636–1646 (2017).
Yu, Q., Zhang, M., Qian, L., Wen, D. & Wu, G. Luteolin attenuates high glucose-induced podocyte injury via suppressing NLRP3 inflammasome pathway. Life Sci. 225, 1–7 (2019).
Yang, S. R. et al. Xenon blunts NF-κB/NLRP3 inflammasome activation and improves acute onset of accelerated and severe lupus nephritis in mice. Kidney Int. 98, 378–390 (2020).
Wu, R. et al. IL-6 receptor blockade ameliorates diabetic nephropathy via inhibiting inflammasome in mice. Metabolism 83, 18–24 (2018).
Anders, H. J. et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 93, 656–669 (2018).
Tajima, T. et al. β-hydroxybutyrate attenuates renal ischemia-reperfusion injury through its anti-pyroptotic effects. Kidney Int. 95, 1120–1137 (2019).
Mulay, S. R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 96, 58–66 (2019).
Shahzad, K. et al. Caspase-1, but not Caspase-3, promotes diabetic nephropathy. J. Am. Soc. Nephrol. 27, 2270–2275 (2016).
Jheng, H. F. et al. Albumin stimulates renal tubular inflammation through an HSP70-TLR4 axis in mice with early diabetic nephropathy. Dis. Model Mech. 8, 1311–1321 (2015).
Lin, M. et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 83, 887–900 (2013).
Chen, S. et al. β-catenin-controlled tubular cell-derived exosomes play a key role in fibroblast activation via the OPN-CD44 axis. J. Extracell. Vesicles 11, e12203 (2022).
Chen, L. et al. Relaxin abrogates renal interstitial fibrosis by regulating macrophage polarization via inhibition of Toll-like receptor 4 signaling. Oncotarget 8, 21044–21053 (2017).
Falke, L. L., Gholizadeh, S., Goldschmeding, R., Kok, R. J. & Nguyen, T. Q. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat. Rev. Nephrol. 11, 233–244 (2015).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Gewin, L. S. Renal fibrosis: primacy of the proximal tubule. Matrix Biol. 68-69, 248–262 (2018).
Robertson, I. B. et al. Latent TGF-β-binding proteins. Matrix Biol. 47, 44–53 (2015).
Huang, X. R., Chung, A. C., Wang, X. J., Lai, K. N. & Lan, H. Y. Mice overexpressing latent TGF-beta1 are protected against renal fibrosis in obstructive kidney disease. Am. J. Physiol. Ren. Physiol. 295, F118–F127 (2008).
Shi, M. et al. Latent TGF-beta structure and activation. Nature 474, 343–349 (2011).
Robertson, I. B. et al. Latent TGF-beta-binding proteins. Matrix Biol. 47, 44–53 (2015).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Yan, X. & Chen, Y. G. Smad7: not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J. 434, 1–10 (2011).
Gu, Y. Y., Liu, X. S., Huang, X. R., Yu, X. Q. & Lan, H. Y. TGF-β in renal fibrosis: triumphs and challenges. Future Med. Chem. 12, 853–866 (2020).
Wilson, H. M. et al. Effect of transforming growth factor-beta 1 on plasminogen activators and plasminogen activator inhibitor-1 in renal glomerular cells. Exp. Nephrol. 1, 343–350 (1993).
Zhang, X. et al. TIMP-1 promotes age-related renal fibrosis through upregulating ICAM-1 in human TIMP-1 transgenic mice. J. Gerontol. A Biol. Sci. Med. Sci. 61, 1130–1143 (2006).
Zhou, T. et al. Runt-related transcription factor 1 (RUNX1) promotes TGF-β-induced renal tubular epithelial-to-mesenchymal transition (EMT) and renal fibrosis through the PI3K subunit p110δ. EBioMedicine 31, 217–225 (2018).
Chen, Y. T. et al. Endoplasmic reticulum protein TXNDC5 promotes renal fibrosis by enforcing TGF-β signaling in kidney fibroblasts. J. Clin. Invest. 131, e143645 (2021).
Wang, P. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-β/Smad3 pathway.Sci. Transl. Med. 10, eaat2039 (2018).
Sharma, K. et al. Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 22, 1144–1151 (2011).
Vincenti, F. et al. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int. Rep. 2, 800–810 (2017).
Voelker, J. et al. Anti-TGF-β1 antibody therapy in patients with diabetic nephropathy. J. Am. Soc. Nephrol. 28, 953–962 (2017).
Song, M. K. et al. Bardoxolone ameliorates TGF-β1-associated renal fibrosis through Nrf2/Smad7 elevation. Free Radic. Biol. Med. 138, 33–42 (2019).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Doi, S. et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 286, 8655–8665 (2011).
Yuan, Q. et al. A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling. Nat. Commun. 13, 438 (2022).
Ma, T. T. & Meng, X. M. TGF-β/Smad and renal fibrosis. Adv. Exp. Med. Biol. 1165, 347–364 (2019).
Ai, J. et al. GQ5 hinders renal fibrosis in obstructive nephropathy by selectively inhibiting TGF-β-induced Smad3 phosphorylation. J. Am. Soc. Nephrol. 26, 1827–1838 (2015).
Nusse, R. & Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Clevers, H. & Nusse, R. Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 (2012).
Li, Z. et al. (Pro)renin receptor is an amplifier of Wnt/β-catenin signaling in kidney injury and fibrosis. J. Am. Soc. Nephrol. 28, 2393–2408 (2017).
Zhou, D., Tan, R. J., Fu, H. & Liu, Y. Wnt/β-catenin signaling in kidney injury and repair: a double-edged sword. Lab. Invest. 96, 156–167 (2016).
Meng, P., Zhu, M., Ling, X. & Zhou, L. Wnt signaling in kidney: the initiator or terminator? J. Mol. Med. (Berl.) 98, 1511–1523 (2020).
Xiao, L. et al. Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J. Am. Soc. Nephrol. 27, 1727–1740 (2016).
Zhou, L., Li, Y., Zhou, D., Tan, R. J. & Liu, Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 24, 771–785 (2013).
Tan, R. J., Zhou, D., Zhou, L. & Liu, Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int. Suppl. (2011) 4, 84–90 (2014).
Zhou, L. et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 26, 107–120 (2015).
Feng, Y. et al. Wnt/β-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J. Am. Soc. Nephrol. 29, 182–193 (2018).
Luo, C. et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 29, 1238–1256 (2018).
Zhou, D. et al. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J. Am. Soc. Nephrol. 28, 2322–2336 (2017).
He, W., Kang, Y. S., Dai, C. & Liu, Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 22, 90–103 (2011).
Hao, S. et al. Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 22, 1642–1653 (2011).
Schunk, S. J., Floege, J., Fliser, D. & Speer, T. WNT-β-catenin signalling – a versatile player in kidney injury and repair. Nat. Rev. Nephrol. 17, 172–184 (2021).
Zuo, Y. & Liu, Y. New insights into the role and mechanism of Wnt/β-catenin signalling in kidney fibrosis. Nephrology (Carlton) 23(Suppl 4), 38–43 (2018).
Lin, C. L. et al. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J. Am. Soc. Nephrol. 21, 124–135 (2010).
Sprinzak, D. & Blacklow, S. C. Biophysics of Notch signaling. Annu. Rev. Biophys. 50, 157–189 (2021).
Edeling, M., Ragi, G., Huang, S., Pavenstädt, H. & Susztak, K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 12, 426–439 (2016).
Murea, M. et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int. 78, 514–522 (2010).
Bielesz, B. et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Invest. 120, 4040–4054 (2010).
Djudjaj, S. et al. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J. Pathol. 228, 286–299 (2012).
Liu, M. et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat. Commun. 8, 413 (2017).
Li, L. et al. Aberrant activation of Notch1 signaling in glomerular endothelium induces albuminuria. Circ. Res. 128, 602–618 (2021).
Roscioni, S. S., Heerspink, H. J. & de Zeeuw, D. The effect of RAAS blockade on the progression of diabetic nephropathy. Nat. Rev. Nephrol. 10, 77–87 (2014).
Forrester, S. J. et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 98, 1627–1738 (2018).
Ruiz-Ortega, M., Rayego-Mateos, S., Lamas, S., Ortiz, A. & Rodrigues-Diez, R. R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 16, 269–288 (2020).
Kalaitzidis, R. G. & Elisaf, M. S. Treatment of hypertension in chronic kidney disease. Curr. Hypertens. Rep. 20, 64 (2018).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
Hou, F. F. et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N. Engl. J. Med. 354, 131–140 (2006).
Georgianos, P. I. & Agarwal, R. Mineralocorticoid receptor antagonism in chronic kidney disease. Kidney Int Rep. 6, 2281–2291 (2021).
Barrera-Chimal, J., Girerd, S. & Jaisser, F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int. 96, 302–319 (2019).
Bakris, G. L. et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 314, 884–894 (2015).
Bakris, G. L. et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 383, 2219–2229 (2020).
Kim, H. J., Moradi, H., Yuan, J., Norris, K. & Vaziri, N. D. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am. J. Physiol. Ren. Physiol. 296, F1297–F1306 (2009).
Westenfeld, R. et al. Fetuin-A protects against atherosclerotic calcification in CKD. J. Am. Soc. Nephrol. 20, 1264–1274 (2009).
Zuo, Y. et al. Renal dysfunction potentiates foam cell formation by repressing ABCA1. Arterioscler Thromb. Vasc. Biol. 29, 1277–1282 (2009).
Yin, Q. H. et al. Exendin-4 ameliorates lipotoxicity-induced glomerular endothelial cell injury by improving ABC transporter A1-mediated cholesterol efflux in diabetic apoE knockout mice. J. Biol. Chem. 291, 26487–26501 (2016).
de Zeeuw, D. et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 3, 687–696 (2015).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81961138007, 82100729, 81974096, 81770711, 81974097, 81873602), the National Key Research and Development Program (2018YFC1314000), the Program for HUST Academic Frontier Youth Team (2017QYTD20), and the program for China postdoctoral science foundation (2021M691160).
Author information
Authors and Affiliations
Contributions
Q.Y. and C.Z. conceptualized this review. Q.Y. and B.T. drafted the manuscript. Q.Y. drew the figures. C.Z. revised the manuscript. All authors have read and approved the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, Q., Tang, B. & Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Sig Transduct Target Ther 7, 182 (2022). https://doi.org/10.1038/s41392-022-01036-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-01036-5
This article is cited by
-
Fisetin ameliorates fibrotic kidney disease in mice via inhibiting ACSL4-mediated tubular ferroptosis
Acta Pharmacologica Sinica (2024)
-
Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases
Signal Transduction and Targeted Therapy (2024)
-
The role of a novel mineralocorticoid receptor antagonist, finerenone, in chronic kidney disease: mechanisms and clinical advances
Clinical and Experimental Nephrology (2024)
-
Functional analysis reveals calcium-sensing receptor gene regulating cell–cell junction in renal tubular epithelial cells
International Urology and Nephrology (2024)
-
TLR7 activation by miR-21 promotes renal fibrosis by activating the pro-inflammatory signaling pathway in tubule epithelial cells
Cell Communication and Signaling (2023)