Abstract
Background
While the addition of androgen receptor signaling inhibitors (ARSIs) to androgen deprivation therapy (ADT) results in better of overall survival in patients with metastatic hormone-sensitive prostate cancer (mHSPC), information regarding health related quality of life (HR-QoL) is sparse. We aimed at summarizing current evidence on the impact of ARSIs on HR-QoL.
Methods
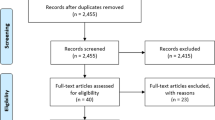
We performed a systematic review of the published literature on PubMed/EMBASE, Web of Science, SCOPUS, and the Cochrane libraries between January 2011 and April 2022. We included only phase III randomized controlled trials (RCT), which were selected according to the PRISMA guidelines. We aimed at evaluating differences in HR-QoL, assessed by validated patient reported outcomes instruments. We analyzed global scores and sub-domains such as sexual functioning, urinary symptoms, bowel symptoms, pain/fatigue, emotional and social/family wellbeing. We reported data descriptively.
Results
Six RCTs were included: two used enzalutamide with ADT as intervention arms (ARCHES, ENZAMET); one used apalutamide with ADT (TITAN); two abiraterone acetate and prednisone (AAP) with ADT (STAMPEDE, LATITUDE); and one darolutamide with ADT (ARASENS). Enzalutamide or AAP with ADT increase overall HR-QoL in comparison with ADT alone, ADT with first generation nonsteroideal anti-androgens or ADT with docetaxel, whereas apalutamide and darolutamide with ADT maintain HR-QoL similarly to ADT alone or ADT with docetaxel, respectively. Time to first deterioration of pain was longer with combination therapy with enzalutamide, AAP or darolutamide, but not with apalutamide. No worsening of emotional wellbeing was reported from the addition of ARSIs to ADT than ADT alone.
Conclusions
The addition of ARSIs to ADT in mHSPC tends to increase overall HR-QoL and prolong time to first deterioration of pain/fatigue compared with ADT alone, ADT with first generation nonsteroideal anti-androgens, and ADT with docetaxel. ARSIs show a complex interaction with remaining HR-QoL domains. We advocate a standardization of HR-QoL measurement and reporting to allow further comparisons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity - United States, 2001-2017. MMWR Morb Mortal Wkly Rep. 2020;69:1473–80.
Desai MM, Cacciamani GE, Gill K, Zhang J, Liu L, Abreu A, et al. Trends in incidence of metastatic prostate cancer in the US. JAMA Netw Open. 2022;5:e222246.
Kinsey EN, Zhang T, Armstrong AJ. Metastatic hormone-sensitive prostate cancer: a review of the current treatment landscape. Cancer J. 2020;26:64–75.
Ng K, Smith S, Shamash J. Metastatic hormone-sensitive prostate cancer (mHSPC): advances and treatment strategies in the first-line setting. Oncol Ther. 2020;8:209–30.
Tagawa ST, Ramaswamy K, Huang A, Mardekian J, Schultz NM, Wang L, et al. Survival outcomes in patients with chemotherapy-naive metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone acetate. Prostate Cancer Prostatic Dis. 2021;24:1032–40.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–86.
Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29:1235–48.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–31.
Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386:1132–42.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. part ii-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–82.
Saad F, Bögemann M, Suzuki K, Shore N. Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors. Prostate Cancer Prostatic Dis. 2021;24:323–34.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
van Andel G, Bottomley A, Fosså SD, Efficace F, Coens C, Guerif S, et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer. 2008;44:2418–24.
Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–8.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36.
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–38.
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96.
Stenzl A, Dunshee C, De Giorgi U, Alekseev B, Iguchi T, Szmulewitz RZ, et al. Effect of enzalutamide plus androgen deprivation therapy on health-related quality of life in patients with metastatic hormone-sensitive prostate cancer: an analysis of the ARCHES randomised, placebo-controlled, phase 3 study. Eur Urol. 2020;78:603–14.
Stockler MR, Martin AJ, Davis ID, Dhillon HM, Begbie SD, Chi KN, et al. Health-related quality of life in metastatic, hormone-sensitive prostate cancer: ENZAMET (ANZUP 1304), an international, randomized phase III trial led by ANZUP. J Clin Oncol. 2022;40:837–46.
Agarwal N, McQuarrie K, Bjartell A, Chowdhury S, Pereira de Santana Gomes AJ, Chung BH, et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20:1518–30.
Chi KN, Protheroe A, Rodríguez-Antolín A, Facchini G, Suttman H, Matsubara N, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol. 2018;19:194–206.
Rush HL, Murphy L, Morgans AK, Clarke NW, Cook AD, Attard G, et al. Quality of life in men with prostate cancer randomly allocated to receive docetaxel or abiraterone in the STAMPEDE trial. J Clin Oncol. 2022;40:825–36.
Gupta D, Braun DP, Staren ED. Prognostic value of changes in quality of life scores in prostate cancer. BMC Urol. 2013;13:32.
Koo KC, Park SU, Kim KH, Rha KH, Hong SJ, Yang SC, et al. Predictors of survival in prostate cancer patients with bone metastasis and extremely high prostate-specific antigen levels. Prostate Int. 2015;3:10–5.
Author information
Authors and Affiliations
Contributions
Conceptualization: LA, ML, FE; study design: LA, ML, FE, MM, GG, RC; data collection: LA, ML, FE; data analysis: LA, ML; data interpretation: LA, ML; writing: ML, LA; revision: ML, LA, MM, GG, AKM, RC, AM, AB, RMS, RP, CDN, FE; final approval: ML, LA, MM, GG, AKM, RC, AM, AB, RMS, RP, CDN, FE; accountability: ML, LA, MM, GG, AKM, RC, AM, AB, RMS, RP, CDN, FE.
Corresponding author
Ethics declarations
Competing interests
RC: Advisory role for Astellas, Janssen, Bayer, Sanofi, MSD, Roche, BMS, Merck, Pfizer, Ipsen; honoraria from Astellas, Janssen, Merck. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afferi, L., Longoni, M., Moschini, M. et al. Health-related quality of life in patients with metastatic hormone-sensitive prostate cancer treated with androgen receptor signaling inhibitors: the role of combination treatment therapy. Prostate Cancer Prostatic Dis (2023). https://doi.org/10.1038/s41391-023-00668-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-023-00668-0