Abstract
Background
Enzalutamide and abiraterone acetate plus prednisone (AAP) have similar efficacy in metastatic castration-resistant prostate cancer (mCRPC), but different mechanisms of action. The aim was to compare patient-reported health-related quality of life (HRQoL) in men treated with enzalutamide vs AAP for mCRPC.
Methods
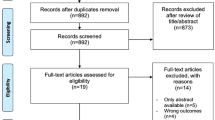
We systematically reviewed the literature in June 2020 according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. Patient-reported outcomes (PROs) until the last follow-up were summarised in a narrative synthesis. Short-term changes (12 weeks) in HRQoL, measured by the Functional Assessment of Cancer Therapy-Prostate total score (FACT-P), were compared between treatment groups and were analysed for enzalutamide and AAP in separate meta-analyses. Higher FACT-P scores indicate better HRQoL.
Results
Eight studies were included in the systematic review, four of which were randomised clinical trials (RCTs) eligible for the meta-analyses. The meta-analyses showed mean within-subject FACT-P changes from baseline to week 12 of −1.3 points (95% confidence interval [CI] −2.7; 0.1) for enzalutamide and 4.7 points (95% CI −0.1; 9.6) for AAP.
One RCT and three non-randomised studies directly compared enzalutamide with AAP. The RCT showed better short-term HRQoL for AAP (6.8 FACT-P-points, 95% CI 1.7; 11.8) and better long-term HRQoL for AAP in men ≥75 years (7.35 FACT-P-points, 95% CI 2.59; 12.11). The non-randomised studies showed no difference in long-term HRQoL but had all a serious risk of bias.
Limitations of the included studies include that the PRO in the included trials were inconsistently reported and that only one study defined the HRQoL measures in their published protocol.
Conclusions
AAP seems to be associated with better short-term HRQoL than enzalutamide. This difference was not apparent at longer follow-up, but the long-term studies had serious risks of bias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019. https://doi.org/10.1016/S1470-2045(19)30688-6.
Ryan C, Wefel JS, Morgans AK. A review of prostate cancer treatment impact on the CNS and cognitive function. Prostate Cancer Prostatic Dis. 2019. https://doi.org/10.1038/s41391-019-0195-5.
Moreira RB, Debiasi M, Francini E, Nuzzo P V., De Velasco G, Maluf FC, et al. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget 2017;8:84572–8.
Auchus RJ, Yu MK, Nguyen S, Mundle SD. Use of prednisone with abiraterone acetate in metastatic castration‐resistant prostate cancer. Oncologist. 2014. https://doi.org/10.1634/theoncologist.2014-0167.
Venkitaraman R, Lorente D, Murthy V, Thomas K, Parker L, Ahiabor R, et al. A randomised phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol. 2015;67:673–9.
Fosså SD, Brausi SM, Horenblas S, Hall RR, Hetherington JW, Aaronson N et al. Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European Organization for Research and Treatment of Cancer Genitourinary Group. J Clin Oncol. 2001;19:62–71.
Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, Rider W. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol. 1989;7:590–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009. https://doi.org/10.1136/bmj.b2535.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - Inconsistency. J Clin Epidemiol. 2011. https://doi.org/10.1016/j.jclinepi.2011.03.017.
Higgins JPTGS. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011. www.handbook.cochrane.org.
Graff JN, Baciarello G, Armstrong AJ, Higano CS, Iversen P, Flaig TW, et al. Efficacy and safety of enzalutamide in patients 75 years or older with chemotherapy-naive metastatic castrationresistant prostate cancer: results from PREVAIL. Ann Oncol. 2016;27:286–94.
Shore ND, Saltzstein D, Sieber P, Mehlhaff B, Gervasi L, Phillips J, et al. Results of a real-world study of enzalutamide and abiraterone acetate with prednisone tolerability (REAAcT). Clin Genitourin Cancer. 2019;17:457–63. e6.
Thiery-Vuillemin A, Hvid Poulsen M, Lagneau E, Ploussard G, Birtle A, Dourthe L-M, et al. Impact of abiraterone acetate plus prednisone or enzalutamide on patient-reported outcomes in patients with metastatic castration-resistant prostate cancer: final 12-mo analysis from the observational AQUARiUS Study. Eur Urol. 2019. https://doi.org/10.1016/j.eururo.2019.09.019.
Gotto G, Fradet V, Drachenberg D, Sabbagh R, Rendon RA, Shayegan B, et al. Real-world evidence in patient-related outcomes (PROs) of metastatic castrate-resistant prostate cancer (mCRPC) patients treated with abiraterone acetate plus prednisone (AA+P). J Clin Oncol. 2018;36:196.
Thiery-Vuillemin A, Hvid Poulsen M, Lagneau E, Ploussard G, Birtle A, Dourthe LM, et al. Impact of abiraterone acetate plus prednisone or enzalutamide on fatigue and cognition in patients with metastatic castration-resistant prostate cancer: Initial results from the observational AQUARiUS study. ESMO Open. 2018;3. https://doi.org/10.1136/esmoopen-2018-000397.
Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509–21.
Cella D, Li S, Li T, Kheoh T, Todd MB, Basch E. Repeated measures analysis of patient-reported outcomes in prostate cancer after abiraterone acetate. J Community Support Oncol. 2016;14:148–54.
Devlin N, Herdman M, Pavesi M, Phung D, Naidoo S, Beer TM, et al. Health-related quality of life effects of enzalutamide in patients with metastatic castration-resistant prostate cancer: an in-depth post hoc analysis of EQ-5D data from the PREVAIL trial. Health Qual Life Outcomes 2017;15. https://doi.org/10.1186/s12955-017-0704-y.
Siemens DR, Klotz L, Heidenreich A, Chowdhury S, Villers A, Baron B, et al. Efficacy and safety of enzalutamide vs bicalutamide in younger and older patients with metastatic castration resistant prostate cancer in the TERRAIN trial. J Urol. 2018;199:147–54.
Shore ND, Chowdhury S, Villers A, Klotz L, Robert Siemens D, Phung D, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17:153–63.
Salem S, Komisarenko M, Timilshina N, Martin L, Grewal R, Alibhai S, et al. Impact of abiraterone acetate and enzalutamide on symptom burden of patients with chemotherapy-naive metastatic castration-resistant prostate cancer. Clin Oncol. 2017;29:601–8.
Basch E, Autio K, Ryan CJ, Mulders P, Shore N, Kheoh T, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: Patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013. https://doi.org/10.1016/S1470-2045(13)70424-8.
Heidenreich A, Chowdhury S, Klotz L, Siemens DR, Villers A, Ivanescu C, et al. Impact of enzalutamide compared with bicalutamide on quality of life in men with metastatic castration-resistant prostate cancer: additional analyses from the TERRAIN Randomised Clinical Trial. Eur Urol. 2017;71:534–42.
Khalaf DJ, Sunderland K, Eigl BJ, Kollmannsberger CK, Ivanov N, Finch DL, et al. Health-related quality of life for abiraterone plus prednisone versus enzalutamide in patients with metastatic castration-resistant prostate cancer: results from a phase II randomized trial. Eur Urol. 2019;75:940–7.
Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66:815–25.
Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof 2005. https://doi.org/10.1177/0163278705275340.
Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the functional assessment of cancer therapy-prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12:124–9.
Dores GM, Bryant-Genevier M, Perez-Vilar S. Adverse events associated with the use of Sipuleucel-T reported to the US food and drug administration’s adverse event reporting system 2010–2017. JAMA Netw open. 2019;2:e199249.
De Santis M, Saad F. Practical guidance on the role of corticosteroids in the treatment of metastatic castration-resistant prostate cancer. Urology. 2016. https://doi.org/10.1016/j.urology.2016.02.010.
Armstrong AJ, Lin P, Higano CS, Iversen P, Sternberg CN, Tombal B, et al. Prognostic association of prostate-specific antigen decline with clinical outcomes in men with metastatic castration-resistant prostate cancer treated with enzalutamide in a randomized clinical trial. Eur Urol Oncol. 2019;2:677–84.
Smith MR, Rathkopf DE, Mulders PFA, Carles J, Van Poppel H, Li J, et al. Efficacy and safety of abiraterone acetate in elderly (75 Years or Older) chemotherapy naïve patients with metastatic castration resistant prostate cancer. J Urol. 2015;194:1277–84.
Cherrier MM, Higano CS. Impact of androgen deprivation therapy on mood, cognition, and risk for AD. Urol. Oncol. Semin. Orig. Investig. 2019. https://doi.org/10.1016/j.urolonc.2019.01.021.
Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020. https://doi.org/10.1016/S1474-4422(19)30368-0.
Coens C, Pe M, Dueck AC, Sloan J, Basch E, Calvert M, et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. 2020;21:e83–96.
Kretschmer A, Ploussard G, Heidegger I, Tsaur I, Borgmann H, Surcel C, et al. Health-related quality of life in patients with advanced prostate cancer: a systematic review. Eur Urol Focus. 2020. https://doi.org/10.1016/j.euf.2020.01.017.
Acknowledgements
Support: No external grants or funds were used to support this research. Following statement refers to the COU-AA-302 data, used in the meta-analysis, obtained via the Yale University Open Data Access (YODA) project: “This study, carried out under YODA Project # 2019-3990, used data obtained from the Yale University Open Data Access Project, which has an agreement with JANSSEN RESEARCH & DEVELOPMENT, L.L.C. The interpretation and reporting of research using this data are solely the responsibility of the authors and does not necessarily represent the official views of the Yale University Open Data Access Project or JANSSEN RESEARCH & DEVELOPMENT, L.L.C.”.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of study and interpretation of the results as well as read, reviewed and approved the final manuscript. KKT and ABN screened full text publications. KKT and OB assessed risk of bias and evidence graded the included studies. KKT and TWK made the statistical analyses. KKT and PBO drafted the manuscript. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MF is an advisor and speaker for Ferring and Astellas. PBO has been a speaker for Astellas, Ipsen and Ferring. HL is an advisor for Roche, Janssen, Astellas, Bayer and Sanofi-Aventis. OB has been speaker and moderator at non-product specific meetings arranged by AstraZeneca, Janssen, Amgen, Astellas, Ipsen and Bayer. KKT, ABN, CK, GP, and JS and TWK declare no potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ternov, K.K., Nolsøe, A.B., Bratt, O. et al. Quality of life in men with metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 24, 948–961 (2021). https://doi.org/10.1038/s41391-021-00359-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00359-8