Abstract
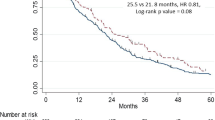
It is unclear whether cancer patients enrolled in clinical trials have improved outcomes compared with non-study patients. We compared prostate cancer-specific mortality (PCSM) in patients in a real-world setting (SEER-Medicare database) versus on a trial (NRG/RTOG 0521). The 7-year freedom from PCSM was superior in trial patients (92.4% vs. 88.1%, sHR = 1.77 [95% CI 1.05–2.97], P = 0.03). Black trial patients had significantly superior freedom from PCSM than Black real-world patients (sHR 6.52, 95% CI 1.43–29.72, P = 0.02), which was not seen among non-Black patients. Trial patients may have improved outcomes, and racial disparities are accentuated in the real world.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data for these analyses were made available to the authors through agreement with the NRG/Radiation Therapy Oncology Group (RTOG) and the Surveillance, Epidemiology, and End Results (SEER)-Medicare database. As such, the authors cannot make these data publicly available due to data use agreement.
References
Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363:263–70.
Rosenthal SA, Hu C, Sartor O, Gomella LG, Amin MB, Purdy J, et al. Effect of chemotherapy with docetaxel with androgen suppression and radiotherapy for localized high-risk prostate cancer: the randomized phase III NRG oncology RTOG 0521 trial. J Clin Oncol. 2019;37:1159–68.
Sandler HM, Karrison T, Sartor AO, Gomella LG, Amin MB, Purdy J, et al. Adjuvant docetaxel for high-risk localized prostate cancer: Update of NRG oncology/RTOG 0521. J Clin Oncol. 2020;38:333.
Chowdhury-Paulino IM, Ericsson C, Vince R Jr., Spratt DE, George DJ, Mucci LA. Racial disparities in prostate cancer among black men: epidemiology and outcomes. Prostate Cancer Prostatic Dis. 2022;25:397–402.
Dess RT, Hartman HE, Mahal BA, Soni PD, Jackson WC, Cooperberg MR, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5:975–83.
Ma TM, Romero T, Nickols NG, Rettig MB, Garraway IP, Roach M 3rd, et al. Comparison of response to definitive radiotherapy for localized prostate cancer in black and white men: a meta-analysis. JAMA Netw Open. 2021;4:e2139769.
Acknowledgements
We acknowledge that this study used the linked SEER-Medicare database and the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
Funding
Funding support for this study comes from the Prostate Cancer Foundation and ASTRO to AUK. AUK also thanks generous donations from the DeSilva, McCarrick, and Bershad families.
Author information
Authors and Affiliations
Contributions
Conception: TMM, MX, AUK Data curation: TMM, MX, AUK. Formal Analysis: TMM, MX, AUK. Funding acquisition: AUK Investigation: TMM, FYF, SAR, MBR, ACR, DES, MX, AUK. Project administration: MX, AUK. Supervision: MX, AUK. Writing—original draft: TMM, MX, AUK. Writing—review & editing: TMM, FYF, SAR, MBR, ACR, DES, MX, AUK.
Corresponding author
Ethics declarations
Competing interests
AUK reports funding support from grant P50CA09213 from the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence and grant W81XWH-22-1-0044 from the Department of Defense, as well as grant RSD1836 from the Radiological Society of North America, the STOP Cancer organization, the Jonsson Comprehensive Cancer Center, and the Prostate Cancer Foundation. ACR reports research grant from Intelligent Automation Inc. and Viewray Inc., consulting work for Viewray Inc and Clarity PSO/RO-ILS RO-HAC, honoraria from Clarity PSO/RO-ILS RO-HAC and as a rectal cancer panel member of the Veteran’s Health Administration Radiation Oncology Quality Surveillance Program Services, outside the submitted work. All other authors have no conflict of interest to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, T.M., Feng, F.Y., Rosenthal, S.A. et al. Race-dependent association of clinical trial participation with improved outcomes for high-risk prostate cancer patients treated in the modern era. Prostate Cancer Prostatic Dis 26, 625–627 (2023). https://doi.org/10.1038/s41391-023-00663-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-023-00663-5