Abstract
Background
The phase III SPARTAN study demonstrated that apalutamide significantly improves metastasis-free survival and overall survival vs. placebo in patients with non-metastatic castration-resistant prostate cancer (nmCRPC). However, patients receiving apalutamide experienced falls more frequently vs. those receiving placebo (15.6% vs. 9.0%).
Methods
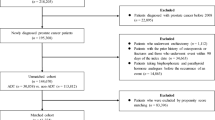
806 patients with nmCRPC randomized to apalutamide in SPARTAN and treated with apalutamide in addition to ongoing androgen deprivation therapy (ADT) were included in this post-hoc analysis investigating clinical variables associated with a subsequent fall. Time to a fall was assessed with Cox proportional-hazards models adjusted for baseline characteristics and time-varying factors. Statistical inference was based on final multivariable models.
Results
Falls were reported for 125/803 (15.6%) patients treated with apalutamide and ADT. Most falls were grade 1 or 2 and did not require hospitalization. Median time from randomization to first fall was 9.2 months (range 0.1–25.3 months). In the final multivariable model of both baseline and after-baseline covariates, baseline patient characteristics (older age, poor Eastern Cooperative Oncology Group performance status, history of neuropathy, and α-blocker use before study treatment) remained significantly associated with fall; after-baseline clinical characteristics significantly associated with time to fall were development of neuropathy, arthralgia, and weight loss before fall.
Conclusions
This analysis identified risk factors for fall among nmCRPC patients treated with apalutamide. Clinical management can minimize these identified risks while enhancing patient outcomes. Preventive interventions should be considered when the identified baseline conditions and post-treatment neuropathy, arthralgia, or weight decrease are present, to reduce risk of fall.
Trial registration
ClinicalTrials.gov: NCT01946204
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Change history
24 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41391-023-00683-1
References
ERLEADA (apalutamide) [prescribing information]. Janssen Pharmaceutical Companies, Horsham, PA; 2020.
ERLEADA (apalutamide). [Summary of Product Characteristics]. The electronic Medicines Compendium (eMC) Web site (https://www.ema.europa.eu/en/documents/product-information/erleada-epar-product-information_en.pdf) and https://www.ema.europa.eu/en/medicines/human/EPAR/erleada.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79:150–8.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl J Med. 2018;378:1408–18.
Saad F, Cella D, Basch E, Hadaschik BA, Mainwaring PN, Oudard S, et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1404–16.
Oudard S, Hadaschik BA, Saad F, Cella D, Basch E, Mainwaring PN, et al. Health-related quality of life (HRQoL) at final analysis of the SPARTAN study of apalutamide (APA) vs placebo (PBO) in patients (pts) with non-metastatic castration-resistant prostate cancer (nmCRPC) receiving androgen deprivation therapy (ADT). Ann Oncol. 2020;31:S521. 632P
Myint ZW, Momo HD, Otto DE, Yan D, Wang P, Kolesar JM. Evaluation of fall and fracture risk among men with prostate cancer treated with androgen receptor inhibitors: A systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2025826.
Hussain S, Breunis H, Timilshina N, Alibhai S. Falls in men on androgen deprivation therapy for prostate cancer. J Geriatr Oncol. 2010;1:32–39.
Winters-Stone KM, Moe E, Graff JN, Dieckmann NF, Stoyles S, Borsch C, et al. Falls and frailty in prostate cancer survivors: current, past, and never users of androgen deprivation therapy. J Am Geriatr Soc. 2017;65:1414–9.
Wu FJ, Sheu SY, Lin HC, Chung SD. Increased fall risk in patients receiving androgen deprivation therapy for prostate cancer. Urology. 2016;95:145–50.
Small EJ, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann Oncol. 2019;30:1813–20.
Panel on Prevention of Falls in Older Persons, American Geriatrics Society, British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59:148–57.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
Burns E, Kakara R. Deaths from falls among persons aged ≥65 years—United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509–14.
Toomey A, Friedman L. Mortality in cancer patients after a fall-related injury: the impact of cancer spread and type. Injury. 2014;45:1710–6.
Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116–9.
Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993–8.
Centers for Disease Control and Prevention. Older adult falls: important facts about falls. https://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html.
Tinetti ME, Gordon C, Sogolow E, Lapin P, Bradley EH. Fall-risk evaluation and management: challenges in adopting geriatric care practices. Gerontologist. 2006;46:717–25.
Wildes TM, Dua P, Fowler SA, Miller JP, Carpenter CR, Avidan MS, et al. Systematic review of falls in older adults with cancer. J Geriatr Oncol. 2015;6:70–83.
Huang MH, Blackwood J, Godoshian M, Pfalzer L. Factors associated with self-reported falls, balance or walking difficulty in older survivors of breast, colorectal, lung, or prostate cancer: results from Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey linkage. PLoS One. 2018;13:e0208573.
Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29:1458–64.
Guerard EJ, Deal AM, Williams GR, Jolly TA, Nyrop KA, Muss HB. Falls in older adults with cancer: evaluation by oncology providers. J Oncol Pr. 2015;11:470–4.
Centers for Disease Control and Prevention. Algorithm for fall risk screening, assessment, and intervention. https://www.cdc.gov/steadi/pdf/STEADI-Algorithm-508.pdf.
Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30:2128–33.
Vande Walle N, Kenis C, Heeren P, Van Puyvelde K, Decoster L, Beyer I, et al. Fall predictors in older cancer patients: a multicenter prospective study. BMC Geriatr. 2014;14:135.
Williams GR, Deal AM, Nyrop KA, Pergolotti M, Guerard EJ, Jolly TA, et al. Geriatric assessment as an aide to understanding falls in older adults with cancer. Support Care Cancer. 2015;23:2273–80.
Acknowledgements
Editorial assistance was provided by Tamara Fink, PhD, and Gwendolyn Elphick, PhD, of Parexel.
Funding
The SPARTAN study was funded by Janssen Research & Development. Funding for editorial assistance was provided by Janssen Global Services, LLC.
Author information
Authors and Affiliations
Contributions
Conception, design, and drafting of the manuscript: YP, EJS, AL, SDM, SB-M, PDP, BR; Conception, design, acquisition of data, statistical analysis, and drafting of the manuscript: AB. All authors contributed to analysis and interpretation of the data and critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
MRS reports travel expenses from and an advisory role for Amgen; research funding and travel expenses from and an advisory role for Bayer; research funding and travel expenses from and an advisory role for Janssen; travel expenses and an advisory role for Lilly; and an advisory role for Novartis and Pfizer. FS reports honoraria from AbbVie and Amgen; consultancy for and research funding and honoraria from Astellas, AstraZeneca/MedImmune, Bayer, Janssen, and Sanofi; and research funding from Bristol Myers Squibb and Pfizer. SC reports consultancy and speakers bureau participation for and honoraria and travel expenses from Astellas Pharma, Bayer, Beigene, Clovis Oncology, Janssen-Cilag, Johnson & Johnson, Novartis, and Sanofi; and research funding from Clovis Oncology. SO reports consultancy for and honoraria from Astellas; consultancy for and travel expenses and honoraria from Bayer, Bristol Myers Squibb, Eisai Merck, Sharp & Dohme, Novartis, and Pfizer; research funding from Ipsen; consultancy for and honoraria from Janssen; and consultancy for and research funding and honoraria from Sanofi. BH reports research funding from German Cancer Aid and the German Research Foundation; consultancy for and research funding and travel expenses from Janssen; consultancy for and travel expenses from Astellas and Bayer; research funding and royalties from Uromed; and consultancy for ABX, Pfizer, and Lightpoint Medical, Inc. DO reports advisory roles for AstraZeneca, Bayer, Clovis Oncology, Daiichi-Sankyo, Janssen, MSD, and Genentech/Roche; compensation for travel from Bayer, Ipsen, Janssen, and Genentech/Roche; honoraria from Bayer, Janssen, and Sanofi; and research funding from Astellas, AstraZeneca, Bayer, Genentech/Roche, Janssen, Pfizer, Medivation, MSD, and Pfizer. HU reports honoraria and travel expenses from Janssen; honoraria from Astellas; consultancy for and honoraria and travel expenses from Takeda; honoraria and travel expenses from Sanofi and Bayer; and consultancy for AstraZeneca. EJS reports an advisory role for and stock in Fortis; stock in Harpoon Therapeutics; honoraria for speaking engagements and an advisory role for Janssen; honoraria from Johnson & Johnson for speaking engagements; and consultancy or as an advisory board member for Teon Therapeutics, Ultragenyx, Beigene, and Tolero. AB, AL, BR, SB-M, PD, and SDM report that they are employees of Janssen and hold stock in Johnson & Johnson. JYL and YP have no conflicts to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the affiliation details for Author Ji Youl Lee were incorrectly given as St. 'Mary’s Hospital of Catholic University, Seoul, South Korea.' but should have been 'Department of Urology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.' The original article has been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pollock, Y., Smith, M.R., Saad, F. et al. Clinical characteristics associated with falls in patients with non-metastatic castration-resistant prostate cancer treated with apalutamide. Prostate Cancer Prostatic Dis 26, 156–161 (2023). https://doi.org/10.1038/s41391-022-00592-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00592-9