Abstract
Background
The CARD study demonstrated superiority of cabazitaxel over abiraterone/enzalutamide in patients with metastatic castration-resistant prostate cancer (mCRPC) who received prior docetaxel and progressed ≤12 months on the alternative androgen-receptor-targeted agent (ARTA). The objective was to compare characteristics and treatment patterns of patients from a real-world dataset with the CARD population.
Methods
Real-world data were collected from Medimix Live TrackerTM, a retrospective, global oncology database of healthcare professional-reported electronic patient medical forms (2001–2019), with data from patients from Europe, USA, Brazil and Japan. The database contained patient, tumor and treatment information for 12,140 patients who received ≥1 line of treatment for mCRPC. A CARD-like cohort included patients treated with docetaxel, prior abiraterone/enzalutamide and cabazitaxel.
Results
A large proportion of patients received ≥2 lines of ARTA (35.1%) with 42% of patients who received a first-line ARTA receiving another ARTA in second line. Of the total patients, 452 were eligible for the CARD-like cohort. Median age of the CARD-like cohort was comparable to CARD (73 vs 70 years). The CARD-like cohort had unfavorable disease characteristics vs CARD: ECOG PS ≥ 2 (45% vs 4.7%); metastasis at diagnosis (46% vs 38%) and Gleason 8–10 (65% vs 57%). More patients in the CARD-like cohort received ARTA before docetaxel (48% vs 39%) and received the first ARTA for >12 months (30% vs 17%) compared with CARD. Despite more patients in the CARD-like cohort receiving the lower 20 mg/m2 dose of cabazitaxel (55% vs 21%), cabazitaxel treatment duration was similar (21.9 vs 22.0 weeks).
Conclusions
Sequential use of ARTA was frequent. Results indicate the CARD population is reflective of routine clinical practice and duration of response to cabazitaxel was similar in a real-world population.
Similar content being viewed by others
Introduction
Prostate cancer is the second most frequent cancer among men and the fifth leading cause of cancer-related death worldwide [1]. Management of advanced prostate cancer has dramatically evolved over the past 15 years. Treatments approved for metastatic castration-resistant prostate cancer (mCRPC) include taxanes (docetaxel, cabazitaxel), androgen-receptor-targeted agents (ARTAs; abiraterone, enzalutamide), radioisotopes (radium-223), poly-ADP ribose polymerase inhibitors (olaparib and rucaparib), and immunotherapy (sipuleucel-t and pembrolizumab for microsatellite instability high/deficient mismatch repair tumors) [2, 3]. Docetaxel was the first chemotherapy to demonstrate a significant survival benefit for patients with mCRPC based on the TAX-327 Phase III trial [4]. Cabazitaxel was approved for the treatment of patients with mCRPC previously treated with docetaxel, demonstrating overall survival benefits over mitoxantrone in the Phase III TROPIC trial [5]. Studies also demonstrated that cabazitaxel retains its activity in patients whose disease progressed with androgen-signaling-targeted inhibitors [6,7,8].
The optimal sequencing of available treatments for patients with mCRPC is unknown. Chemotherapy has routinely been reserved for later lines of treatment, and is sometimes not used at all, due to patient health status and patients’ preferences to avoid the side effect profile [9,10,11]. Thus, in clinical practice, many patients with prostate cancer often receive sequential ARTAs despite evidence that many patients may not benefit from a second alternative inhibitor [12]. Retrospective studies evaluating the sequential use of abiraterone and enzalutamide in patients with mCRPC after treatment with docetaxel have suggested cross-resistance between ARTAs, and two recent prospective studies demonstrate that patients who have progressed on an ARTA are unlikely to respond to a second alternative inhibitor [12,13,14,15,16]. Since multiple treatment options are available for patients with mCRPC, and the optimal sequence of these treatments remains unknown, it is critical to better understand patients’ and physicians’ preferences and how treatment choices may be influenced by patient characteristics, and treatment access [9, 17].
To address the question of optimal sequencing of treatments for mCRPC, the prospective, randomized CARD study (NCT02485691) compared the efficacy and safety of cabazitaxel versus abiraterone or enzalutamide in patients with mCRPC who had previously received docetaxel and who progressed within 12 months on the alternative ARTA. Patients receiving cabazitaxel in this setting had significantly longer imaging-based progression-free survival and overall survival compared with patients receiving abiraterone or enzalutamide, as well as improvements in other secondary endpoints, including prostate-specific antigen response, pain and tumor responses, occurrence of symptomatic skeletal events, and quality of life as measured by the EQ-5D-5L utility index [18, 19]. The incidence of grade ≥3 adverse events was comparable between arms [19].
Prospective, randomized trials enroll highly selected patients with good performance status and well-controlled comorbidities, while patients with poor functional status and greater comorbidity burden are often under-represented. Real-world studies offer the possibility of validating the results of randomized clinical trials by including patient populations reflective of those in routine clinical practice and characterizing trends in healthcare service utilization. Here, we report the findings of a real-world study assessing the treatment sequences received by patients with mCRPC in daily clinical practice. We also evaluated the characteristics of patients receiving cabazitaxel who satisfied the main CARD enrollment criteria (i.e. prior treatment with docetaxel and one ARTA, in any order) to determine whether the population of patients in the CARD study is reflective of the patients with mCRPC seen in routine clinical practice.
Materials and methods
Study description
Real-world data available in the Medimix (now Evidera) LiveTrackerTM database were used in this study. The LiveTrackerTM contains data from electronic patient medical forms entered into the database by a global network of medical oncologists and urologists who were the treating physicians of the respective patients. This retrospective, observational, cohort study focused on patient data entered into the database over the period of January 2019 to December 2019. Treatment sequence for all patients in the dataset was evaluated. We also assessed the characteristics of patients receiving cabazitaxel after docetaxel and one ARTA to compare patient characteristics and the treatment duration of cabazitaxel with the CARD clinical study population. In the CARD study (NCT02485691), cabazitaxel treatment duration was defined as Last dose date - first dose date +21 days [15]. In the cohort analysis, treatment duration was not collected, but patients were classed as on treatment if they received it for ≥1 day and calculated as treatment end date—treatment start date. A new line of therapy was defined as a switch to a new treatment or if the physician had entered the same treatment in two or more consecutive, distinct lines.
Study patients
The database contains patient characteristics, prescribing physician/institution information, and tumor and treatment information for patients who received at least one line of active treatment for mCRPC between 2001 and 2019. For this analysis, patients with mCRPC treated in France, Germany, Italy, Spain, United Kingdom, USA, Japan and Brazil were included. Data were collected following a cross-sectional retrospective methodology, with no patient identifiers collected. Collected data were in total respect of General Data Protection Regulation and Health Insurance Portability and Accountability Act compliance guidelines. The CARD study was approved by the institutional review board at each center and was conducted in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
CARD-like cohort of patients
In a second step, we restricted the population to patients satisfying the main inclusion criteria of the CARD study to define the CARD-like cohort. This cohort comprised patients with mCRPC who had previously been treated with three or more cycles of docetaxel, had previously received abiraterone or enzalutamide treatment for any duration of exposure (≥1 day), before or after docetaxel therapy, and received cabazitaxel after all previous conditions were met. The use of docetaxel or abiraterone in the context of metastatic hormone-sensitive disease and docetaxel rechallenge were allowed. Patients were excluded from the CARD-like cohort if they had received more than one ARTA prior to cabazitaxel or if they had received prior chemotherapy other than docetaxel for prostate cancer except estramustine. Data analysis was performed on patients with documented treatment durations.
The following data were collected: patient and disease characteristics, history of prostate cancer, baseline laboratory outcomes, details of previous therapies and treatment exposure.
Results
Overall treatment trends for mCRPC
A total of 12,140 patients received at least one line of active treatment for mCRPC between 2001 and 2019; Table 1. The number of patients with available data for each line decreased with advancing lines of treatment (Fig. 1). The proportion of patients receiving ARTAs decreased after the second line of treatment for mCRPC, whereas the proportion of patients receiving cabazitaxel increased (Fig. 1). A large proportion of patients received at least two lines of ARTA treatment (3142 [35.1%]; Fig. 2A). Of the patients who received an ARTA in first line (n = 5118), 42% received the same or an alternative inhibitor in the second line. There were 75 patients who received abiraterone in both first and second line. The median break between lines of abiraterone was 30 days (range 0–577 days). There were 125 patients who received enzalutamide in both first and second line. The median break between lines of enzalutamide was 30 days (range 0–394 days). Of the patients who received an ARTA in the second line (n = 2890), 29% received the same or an alternative inhibitor in the third line and 16% in fourth line.
aTreatment may include addition of denosumab. bTreatment may include addition of denosumab or zoledronic acid. cTreatment may include addition of denosumab, active surveillance, ADT, ADT + bicalutamide, ADT + pembrolizumab, ADT + mitoxantrone + prednisone, ADT + sipuleucel-T, ADT + steroids, ADT + radium-223, LHRH + bicalutamide, LHRH agonist + flutamide, pain management, palliative care, abiraterone + radium-223, clinical trial or was unspecified. ADT androgen deprivation therapy, LHRH luteinizing hormone-releasing hormone, mCRPC metastatic castration-resistant prostate cancer.
A Treatment sequence of patients in the database who received at least two ARTAs by line of therapya and B most frequent treatment sequences in the CARD-like cohort. aTotal number of patients provided represents the number of patients included in the database who have data for the given lines of therapy. ARTA androgen-receptor targeted agent, CAB cabazitaxel, DOC docetaxel, ENZA enzalutamide, L line.
One hundred and thirteen patients (0.9%) received ARTAs consecutively in first, second and third lines. At some point over the course of their treatment, 2546 (21%) patients received cabazitaxel, predominantly in third and fourth lines of therapy (Fig. 1). Of the 695 patients who received fourth-line cabazitaxel, 78% had already received both abiraterone and enzalutamide.
Treatment trends for patients with DNA repair mutant mCRPC
Although the number of patients in the database tested for DNA repair mutations (BRCA1, BRCA2 and ATM) is unknown, mutations were reported in 649, of which 177 received cabazitaxel. In these patients, median duration of treatment with cabazitaxel was longer than with docetaxel, both in second-line setting (26.1 weeks vs 17.4 weeks) and third-line setting (19.6 weeks vs 8.7 weeks; Table 2).
Patient characteristics in the CARD-like cohort
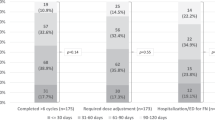
Of the 12,140 patients with mCRPC included in the database, 452 patients received cabazitaxel after progressing on docetaxel and one ARTA, in any order, and had documented treatment durations. Patient baseline characteristics are summarized in Table 1. The median age for the CARD-like cohort was similar to the CARD study (73 vs 70 years). More patients in the CARD-like cohort had unfavorable disease characteristics compared with the CARD study patients including Eastern Cooperative Oncology Group (ECOG) performance status (PS) score ≥ 2 (45% vs 4.7%), M1 disease at diagnosis (46% vs 38%) and Gleason 8–10 (65% vs 57%). The proportion of patients with visceral metastases compared with CARD was 12% vs 16%. More patients in the CARD-like cohort received a prior ARTA before docetaxel compared with the CARD study (48% vs 39%). Additionally, 57% of patients received abiraterone as a prior ARTA compared with 43% in the CARD study. In the CARD-like cohort, 297 patients (66%) received prior ARTA treatment for ≤12 months compared with 136 patients (30%) who received ARTA treatment for >12 months, which is higher than in the CARD study (17%).
Treatment sequence and duration in the CARD-like cohort
More patients in the CARD-like cohort received ≥1 cycle of the lower 20 mg/m2 dose of cabazitaxel instead of the 25 mg/m2 dose used in the CARD study (55% vs 21%; Table 3). The lower dose may have been used as the starting dose in the CARD-like cohort, while the starting dose in the CARD trials was 25 mg/m2 for all patients. Despite the larger proportion of patients receiving a lower dose of cabazitaxel in the CARD-like cohort compared with the CARD study, the duration of cabazitaxel treatment received was comparable (21.9 vs 22.0 weeks). In the CARD-like cohort, the proportion of patients receiving cabazitaxel after first-line ARTA and second-line docetaxel was similar to those receiving first-line docetaxel and second-line ARTA (Fig. 2B). Use of granulocyte-colony stimulating factor and other supportive care regimens were not documented in the database.
Discussion
This real-world evidence study, based on patient data entered into the database over the period of 2001 to 2019, demonstrated that sequential use of ARTAs before chemotherapy initiation is common practice. Here we present a cohort of patients with mCRPC who received cabazitaxel after an ARTA, the same as patients in the cabazitaxel arm of the CARD study. More patients in the CARD-like cohort had aggressive disease characteristics, worse ECOG PS, and received a lower dose of cabazitaxel compared with the CARD population. Despite this, the duration of cabazitaxel treatment was similar for the CARD-like cohort and that reported in the CARD study. This suggests that, although the CARD population may not be completely reflective of patients in routine clinical practice, the results of the CARD study are still relevant to patients in routine clinical practice who have received an ARTA.
According to previously published retrospective analysis from the community practice and Veterans Affairs settings in the US, many patients with mCRPC receive ARTAs in sequence [12, 20]. This may be driven by patient health status or patient and clinician preferences to avoid the expected adverse events related to chemotherapy [9, 11]. Recent evidence from several prospective randomized studies demonstrates that progression on one ARTA is associated with a poor response to a second ARTA, likely due to similar mechanisms of resistance [13, 14, 19, 21]. The CARD study demonstrated that cabazitaxel significantly improved imaging-based progression-free survival and overall survival, as well as quality of life and other clinical outcomes, compared with abiraterone or enzalutamide in patients with mCRPC who had previously received docetaxel and progressed within 12 months on the alternative ARTA. In the CARD study, granulocyte-colony stimulating factor was administered at every cabazitaxel cycle and the incidence of grade ≥3 adverse events was comparable between patients treated with cabazitaxel and ARTAs.
Although clinical trials provide valuable evidence for novel treatments, RWE studies are needed to bridge the data reported in clinical trials and how they translate to routine clinical practice. In randomized clinical trials, patients are selected based on strict eligibility criteria. As a result, patients in routine clinical practice may be older, have more advanced or aggressive disease or comorbidities, any of which could subsequently affect the efficacy and tolerability of treatment [22]. In this study, the CARD-like cohort did have more aggressive disease features. However, the duration of treatment with cabazitaxel was comparable to the CARD study. This suggests that the CARD study, although more selective, is representative of patients in routine clinical practice [19].
RWE studies also help identify treatment patterns [22]. In this study, analysis of treatment patterns suggests that in the recent past, sequential use of ARTAs was common in daily practice. This supports the design of the CARD study and that the practice-changing results of the CARD trial are applicable to real-world clinical practice and support the recent changes in clinical practice guidelines [2, 3, 23, 24].
The database was used to perform a small exploratory analysis of treatment patterns among patients with DNA damage repair mutations. Median treatment duration of cabazitaxel was longer than docetaxel in patients with DNA repair abnormalities in the second- and third-line setting, which may be a result of greater activity in this subgroup of patients known to have a poor prognosis, though our study could not specifically test this possibility. The data surrounding the use of taxanes in patients with DNA-repair mutations are conflicted. Some studies have reported no impact, whereas other studies suggest that these patients do have worse outcomes [22, 25, 26]. Although olaparib has recently been approved in the US for this population, there is growing evidence that these patients may benefit from the use of taxanes at some point during their course of treatment [2, 25]. In two large retrospective, international, observational studies, patients with mCRPC tested for germline DNA damage repair mutations had similar progression-free survival and response rate with docetaxel regardless of whether they did or did not have germline DNA damage repair mutations [26, 27]. In this small, exploratory, real-world evidence analysis, the improved treatment duration of cabazitaxel compared with docetaxel in the second- or third-line treatment of patients with rare mutation subtypes may suggest greater activity of cabazitaxel in these patients with aggressive disease, though the data should still be considered hypothesis generating due to limited patient numbers and our inability to track progression risk. The potentially greater activity of cabazitaxel over docetaxel may be due to greater intratumoral drug concentrations of cabazitaxel, especially in patients who were intrinsically resistant to docetaxel [28, 29]. However, these findings are hypothesis generating, and prospective randomized trials are needed to confirm these data.
The CARD study enrolled patients who had disease progression within 12 months of starting an ARTA [19]. An important consideration is whether or not this is reflective of daily clinical practice. The real-world dataset found that a majority of patients treated with cabazitaxel in similar conditions as CARD received less than 12 months of ARTA therapy. These results suggest that these patients experienced disease progression within 12 months of receiving the first ARTA, though specific progression data were not recorded in the database. This finding is also in agreement with findings of prospective randomized studies. Phase III randomized studies conducted with abiraterone and enzalutamide in chemotherapy-naive patients with mCRPC demonstrated a median time until prostate-specific antigen progression of less than 12 months [30, 31]. In a randomized cross-over study of abiraterone followed by enzalutamide (or the inverse sequence) in patients with newly diagnosed mCRPC, time to progression with the first ARTA was less than 8 months [13]. Lastly, in the PLATO study, median duration of first-line enzalutamide in chemo-naïve mCRPC patients was 9.1 months [14]. However, not surprisingly, the number of patients receiving prior ARTA treatment for a duration >12 months was higher in the real-world dataset compared with the CARD study due to the eligibility criteria of CARD requiring that participants have disease progression within 12 months of treatment with one ARTA. Progression was defined by biochemical and/or radiologic and/or clinical progression. In daily clinical practice, patients may continue to receive ARTAs despite prostate-specific antigen progression until unambiguous progression, i.e. radiologic and/or pain progression. Since many physicians do not regularly image their patients at this stage of the disease, ARTAs may be discontinued when severe pain develops rather than at first radiographic progression, a point at which patients frequently experience deterioration of performance status. This may contribute to the higher rate of poor performance status (ECOG PS ≥ 2: 45%) in the CARD-like cohort compared with the CARD study (ECOG PS ≥ 2: 4.7%).
A small proportion of patients received the same ARTA for both first- and second-line treatment. This is not recommended by ESMO or NCCN, either presently or before the practice changing results of the CARD study [15, 21, 23]. Given the short median time between first and second line in this population (30 days), and that some patients had an interval of 0 days, it may be that the change in line was recorded in error. Alternatively, it may be that a new line was recorded as treatment was continued despite indicators of disease progression. There are many factors that could be involved in this observation and due to the limitations of the database, further studies would be needed to investigate further. There are limitations to the study that should be considered when interpreting the results. First, the data available were provided by the clinician and no data audit was performed. This is a common limitation of real-world studies. Additionally, confirmation of progressive disease on the therapy preceding cabazitaxel was not always possible due to the input method used in the database. Although the time to treatment switch was recorded in the database, this was not equivalent to time to progression as was recorded in the CARD study. ECOG PS was recorded at patient data entry only (not necessarily at initiation of cabazitaxel), which is also different to the CARD study that recorded ECOG PS at randomization. As such, the proportion of patients with poor ECOG PS at the time of treatment with cabazitaxel may be greater than what is reported, making an even greater disparity between the CARD-like cohort and the actual CARD study. In light of this, the similar treatment duration between the CARD-like cohort and the actual CARD study is even more noteworthy. Poor ECOG PS due to cancer or other comorbidities could not be differentiated in the database, while only patients with ECOG PS 2 due to prostate cancer could be enrolled in the CARD study. The population of the CARD-like cohort is also not a direct copy of the CARD population as the treatment landscape has changed since CARD enrolment to include PARP inhibitors (olaparib, and rucaparib) for patients with DNA repair abnormalities. Randomized clinical trials are needed to further clarify the place of cabazitaxel in such patients. Differences exist between the clinic and clinical trials in patients’ assessment and treatment. For example, in the real-world cohort, imaging was collected less frequently compared with the CARD study and no data on imaging timing were available, the use of granulocyte-colony stimulating factor was not recorded, and no details were provided on pain management. Finally, this study focused on patient baseline characteristics and treatment durations, but efficacy data were not reported.
In summary, these results indicate that the CARD study population is reflective of patients receiving care in the real-world setting. Additionally, cabazitaxel treatment duration in the CARD study is similar to that observed in daily practice, despite patients in the real world having more aggressive disease characteristics and poorer performance status at baseline. These data highlight the frequent use of sequential ARTAs in this real-world population, despite evidence of shared mechanisms of resistance. As recommended by international guidelines, treatment with cabazitaxel should be the preferred choice in patients with mCRPC with progression of disease after treatment with docetaxel and an ARTA in lieu of treatment with a subsequent ARTA.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Prostate Cancer (Version 2.2020). 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Mottet N, van den Bergh RCN, Briers E, Cornford P, De Santis M, Fanti S, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2020. 2020. http://uroweb.org/guideline/prostate-cancer/.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54.
van Soest RJ, de Morree ES, Kweldam CF, de Ridder CM, Wiemer EA, Mathijssen RH, et al. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol. 2015;67:981–5.
van Soest RJ, Nieuweboer AJ, de Morree ES, Chitu D, Bergman AM, Goey SH, et al. The influence of prior novel androgen receptor targeted therapy on the efficacy of cabazitaxel in men with metastatic castration-resistant prostate cancer. Eur J Cancer. 2015;51:2562–9.
Delanoy N, Hardy-Bessard AC, Efstathiou E, Le Moulec S, Basso U, Birtle A, et al. Sequencing of taxanes and new androgen-targeted therapies in metastatic castration-resistant prostate cancer: results of the International Multicentre Retrospective CATS database. Eur Urol Oncol. 2018;1:467–75.
Eliasson L, de Freitas HM, Dearden L, Calimlim B, Lloyd AJ. Patients’ preferences for the treatment of metastatic castrate-resistant prostate cancer: a discrete choice experiment. Clin Ther. 2017;39:723–37.
Carter N, Bryant-Lukosius D, DiCenso A, Blythe J, Neville AJ. The supportive care needs of men with advanced prostate cancer. Oncol Nurs Forum. 2011;38:189–98.
Puts MT, Tapscott B, Fitch M, Howell D, Monette J, Wan-Chow-Wah D, et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat Rev. 2015;41:197–215.
Oh WK, Cheng WY, Miao R, Vekeman F, Gauthier-Loiselle M, Duh MS, et al. Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor-targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting. Urol Oncol. 2018;36:500.e501–500.e509.
Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730–9.
Attard G, Borre M, Gurney H, Loriot Y, Andresen-Daniil C, Kalleda R, et al. Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol. 2018;36:2639–46.
Loriot Y, Bianchini D, Ileana E, Sandhu S, Patrikidou A, Pezaro C, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol. 2013;24:1807–12.
Maines F, Caffo O, Veccia A, Trentin C, Tortora G, Galligioni E, et al. Sequencing new agents after docetaxel in patients with metastatic castration-resistant prostate cancer. Crit Rev Oncol Hematol. 2015;96:498–506.
Andrews JR, Ahmed ME, Karnes RJ, Kwon E, Bryce AH. Systemic treatment for metastatic castrate resistant prostate cancer: does seqence matter? Prostate. 2020;80:399–406.
Fizazi K, Kramer G, Eymard J-C, Sternberg C, de Bono J, Castellano D, et al. Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol. 2020;21:1513–25.
de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–18.
Freedland SJ, De Hoedt AM, DerSarkissian M, Chang R, Satija A, Nguyen C, et al. Veterans affairs prostate cancer treatment sequencing (VAPCaTS): a real-world evidence study of men with metastatic prostate cancer. Urol Pract. 2021;8:112–8.
de Bono JS, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102.
Di Maio M, Perrone F, Conte P. Real-world evidence in oncology: opportunities and limitations. Oncologist. 2020;25:e746–e752.
Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1119–34.
Lowrance W, Breau R, Chou R, Chapin BF, Crispino T, Dreicer R, et al. Advanced prostate cancer: AUA/ASTRO/SUO Guideline 2020. 2020. https://www.auanet.org/guidelines/advanced-prostate-cancer.
Bronimann S, Lemberger U, Bruchbacher A, Shariat SF, Hassler MR. Poly(ADP-ribose) polymerase inhibitors in prostate and urothelial cancer. Curr Opin Urol. 2020;30:519–26.
Mateo J, Cheng HH, Beltran H, Dolling D, Xu W, Pritchard CC, et al. Clinical outcome of prostate cancer patients with germline DNA repair mutations: retrospective analysis from an international study. Eur Urol. 2018;73:687–93.
Aldea M, Lam L, Llacer Perez C, Saint-Ghislain M, Mescam GG, Fléchon A, et al. Cabazitaxel (CBZ) activity in men with metastatic castration resistant prostate cancer (mCRPC) with and without DNA damage repair (DDR) defects. Abstracts of the ESMO Virtual Congress 2020. Ann Oncol. 2020;31:614MO.
Azarenko O, Smiyun G, Mah J, Wilson L, Jordan MA. Antiproliferative mechanism of action of the novel taxane cabazitaxel as compared with the parent compound docetaxel in MCF7 breast cancer cells. Mol Cancer Ther. 2014;13:2092–103.
de Morree E, van Soest R, Aghai A, de Ridder C, de Bruijn P, Ghobadi Moghaddam-Helmantel I, et al. Understanding taxanes in prostate cancer; importance of intratumoral drug accumulation. Prostate. 2016;76:927–36.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Acknowledgements
This study was funded by Sanofi. The authors would like to thank Henry Gazay for the creation of the LiveTracker and assistance in the data collection. The authors would like to thank Raphael Ognar for overseeing the chart collection. The authors received editorial support from Danielle Walsh and Amber Wood of MediTech Media, funded by Sanofi.
Author information
Authors and Affiliations
Contributions
Conceptualization: GM, PC, AC, RP, AO. Data curation: GM, PC, AC. Formal analysis: GM, PC, AC. Funding acquisition: RP, AO. Methodology: GM, PC, AC. Validation: RdW, SF, SO, GM, PC, AC, RP, AO, AM. Writing-original draft preparation, RdW, SF, SO, GM, PC, AC, RP, AO, AM. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
RdW has provided a consulting or advisory role for Sanofi, Merck Sharp & Dohme, Roche/Genetech, Janssen, Bayer and Clovis Oncology, and received travel/accommodation/expenses from Lilly. RdW has received honoraria and/or research funding from Sanofi, Merck Sharp & Dohme and Bayer. SF received research funding from Pfizer and received personal fees from Pfizer, Astellas, Janssen, Bayer, Myovant, AstraZeneca, Merck, Dendreon and Sanofi. SO received honoraria from Pfizer, Bayer, Novartis, BMS, Merck, Janssen and Astellas, and received travel/accommodation/expenses from Pfizer, Bayer, Novartis, BMS, Merck, Janssen, Astellas and Sanofi. GM has received personal fees (contracted by Sanofi) from Sanofi. PC has received fees (contracted by Sanofi) from Sanofi and is employed by Medimix. AC has received fees (contracted by Sanofi) from Sanofi and as a data provider for Medimix. RP is employed by Sanofi and AO is a former employee of Sanofi. AO owns stock in Sanofi. AM has received personal fees from Janssen, Astellas, Seattle Genetics and Genentech, and received personal fees and research funding from Bayer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Wit, R., Freedland, S.J., Oudard, S. et al. Real-world evidence of patients with metastatic castration-resistant prostate cancer treated with cabazitaxel: comparison with the randomized clinical study CARD. Prostate Cancer Prostatic Dis 26, 67–73 (2023). https://doi.org/10.1038/s41391-021-00487-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00487-1
This article is cited by
-
Real-world effectiveness of third-line cabazitaxel in patients with metastatic castration-resistant prostate cancer: CARD-like analysis of data from a post-marketing surveillance in Japan
BMC Cancer (2023)
-
A US real-world study of treatment patterns and outcomes in localized or locally advanced prostate cancer patients
World Journal of Urology (2023)