Abstract
Background
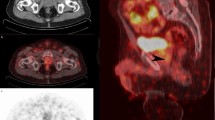
High-risk prostate cancer is associated with adverse pathology and unfavorable outcomes after radical prostatectomy. 68Ga-PSMA PET/CT is more accurate than conventional imaging for preoperative staging. We aimed to evaluate whether lymph node involvement on 68Ga-PSMA PET/CT prior to radical prostatectomy in patients with high-risk prostate cancer is associated with worse short-term oncologic outcomes.
Methods
We retrospectively reviewed 149 patients with high-risk localized or locoregional prostate cancer who underwent 68Ga-PSMA PET/CT prior to radical prostatectomy between 2015 and 2020. None of the patients received neoadjuvant or adjuvant treatment. The study endpoints were PSA persistence and biochemical recurrence. Logistic regression models were used to identify preoperative predictors of PSA persistence. Kaplan–Meier analyses were used to estimate biochemical recurrence-free survival.
Results
Of 149 identified patients, 19 (13%) were found to have lymph node involvement on preoperative 68Ga-PSMA PET/CT. The sensitivity, specificity, and accuracy of 68Ga-PSMA PET/CT for identifying pathologic lymph node involvement were 68%, 95%, and 92%, respectively. PSA persistence rate was lower among patients with PET-negative lymph nodes than those with PET-positive nodes (15 vs. 84%, p < 0.001). Positive nodes on imaging (OR = 41.03, p < 0.001) and clinical T2c–T3 stage (OR = 6.96, p = 0.002) were associated with PSA persistence on multivariable analysis. Among patients with PET-negative nodes the 1- and 2-year biochemical recurrence-free survival rates were 87% and 76%, respectively.
Conclusions
Preoperative staging with 68Ga-PSMA PET/CT may identify a subgroup of high-risk prostate cancer patients with favorable short-term outcomes after radical prostatectomy without adjuvant treatment. Future studies will evaluate whether these results are sustained during long-term follow-up.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chang AJ, Autio KA, Roach M 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014;11:308–323.
Imnadze M, Sjoberg DD, Vickers AJ. Adverse pathologic features at radical prostatectomy: effect of preoperative risk on oncologic outcomes. Eur Urol. 2016;69:143–8.
Joniau S, Briganti A, Gontero P, Gandaglia G, Tosco L, Fieuws S, et al. Stratification of high-risk prostate cancer into prognostic categories: a European multi-institutional study. Eur Urol. 2015;67:157–64.
Yossepowitch O, Eggener SE, Serio AM, Carver BS, Bianco FJ Jr., Scardino PT, et al. Secondary therapy, metastatic progression, and cancer-specific mortality in men with clinically high-risk prostate cancer treated with radical prostatectomy. Eur Urol. 2008;53:950–9.
Moris L, Cumberbatch MG, Van den Broeck T, Gandaglia G, Fossati N, Kelly B, et al. Benefits and risks of primary treatments for high-risk localized and locally advanced prostate cancer: an International Multidisciplinary Systematic Review. Eur Urol. 2020;77:614–27.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–43.
van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119:209–15.
Dekalo S, Kuten J, Mabjeesh NJ, Beri A, Even-Sapir E, Yossepowitch O. 68Ga-PSMA PET/CT: does it predict adverse pathology findings at radical prostatectomy? Urologic Oncol. 2019;37:574 e519–574 e524.
van Kalmthout LWM, van Melick HHE, Lavalaye J, Meijer RP, Kooistra A, de Klerk JMH, et al. Prospective validation of gallium-68 prostate specific membrane antigen-positron emission tomography/computerized tomography for primary staging of prostate cancer. J Urol. 2020;203:537–45.
Luiting HB, van Leeuwen PJ, Busstra MB, Brabander T, van der Poel HG, Donswijk ML, et al. Use of gallium-68 prostate-specific membrane antigen positron-emission tomography for detecting lymph node metastases in primary and recurrent prostate cancer and location of recurrence after radical prostatectomy: an overview of the current literature. BJU Int. 2020;125:206–14.
Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77:403–17.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16.
van Leeuwen PJ, Donswijk M, Nandurkar R, Stricker P, Ho B, Heijmink S, et al. Gallium-68-prostate-specific membrane antigen ((68) Ga-PSMA) positron emission tomography (PET)/computed tomography (CT) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate- and high-risk prostate cancer. BJU Int. 2019;124:62–68.
Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate cancer, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:479–505.
Bahler CD, Green M, Hutchins GD, Cheng L, Magers MJ, Fletcher J, et al. Prostate specific membrane antigen targeted positron emission tomography of primary prostate cancer: assessing accuracy with whole mount pathology. J Urol. 2020;203:92–99.
Moreira DM, Presti JC Jr., Aronson WJ, Terris MK, Kane CJ, Amling CL, et al. Definition and preoperative predictors of persistently elevated prostate-specific antigen after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2010;105:1541–7.
Ploussard G, Staerman F, Pierrevelcin J, Saad R, Beauval JB, Roupret M, et al. Predictive factors of oncologic outcomes in patients who do not achieve undetectable prostate specific antigen after radical prostatectomy. J Urol. 2013;190:1750–6.
Preisser F, Chun FKH, Pompe RS, Heinze A, Salomon G, Graefen M, et al. Persistent prostate-specific antigen after radical prostatectomy and its impact on oncologic outcomes. Eur Urol. 2019;76:106–14.
Haseebuddin M, Dehdashti F, Siegel BA, Liu J, Roth EB, Nepple KG, et al. 11C-acetate PET/CT before radical prostatectomy: nodal staging and treatment failure prediction. J Nucl Med. 2013;54:699–706.
Delporte G, Henon F, Ploussard G, Briganti A, Rizk J, Rozet F, et al. Radical prostatectomy for locally advanced and high-risk prostate cancer: A systematic review of the literature. Prog Urol. 2018;28:875–89.
Kneebone A, Fraser-Browne C, Duchesne GM, Fisher R, Frydenberg M, Herschtal A, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020;21:1331–40.
Sargos P, Chabaud S, Latorzeff I, Magné N, Benyoucef A, Supiot S, et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. Lancet Oncol. 2020;21:1341–52.
Parker CC, Clarke NW, Cook AD, Kynaston HG, Petersen PM, Catton C, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. The Lancet. 2020;396:1413–21.
Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. The Lancet. 2020;396:1422–31.
Bastian PJ, Gonzalgo ML, Aronson WJ, Terris MK, Kane CJ, Amling CL, et al. Clinical and pathologic outcome after radical prostatectomy for prostate cancer patients with a preoperative Gleason sum of 8 to 10. Cancer. 2006;107:1265–72.
Carver BS, Bianco FJ Jr., Scardino PT, Eastham JA. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol. 2006;176:564–8.
Yossepowitch O, Eggener SE, Bianco FJ Jr., Carver BS, Serio A, Scardino PT, et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol. 2007;178:493–9. discussion 499
Nguyen K, Eltz S, Drouin SJ, Comperat E, Audenet F, Renard-Penna R, et al. Oncologic outcome after radical prostatectomy in men with PSA values above 20 ng/ml: a monocentric experience. World J Urol. 2009;27:653–8.
Briganti A, Karnes RJ, Gandaglia G, Spahn M, Gontero P, Tosco L, et al. Natural history of surgically treated high-risk prostate cancer. Urologic Oncol. 2015;33:163 e167–113.
Acknowledgements
We would like to thank Ms. Elizabeta Davidov for her assistance in compiling the data for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dekalo, S., Kuten, J., Mintz, I. et al. Preoperative 68Ga-PSMA PET/CT defines a subgroup of high-risk prostate cancer patients with favorable outcomes after radical prostatectomy and lymph node dissection. Prostate Cancer Prostatic Dis 24, 910–916 (2021). https://doi.org/10.1038/s41391-021-00347-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00347-y
This article is cited by
-
Development and validation of [18 F]-PSMA-1007 PET-based radiomics model to predict biochemical recurrence-free survival following radical prostatectomy
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Using PSMA imaging for prognostication in localized and advanced prostate cancer
Nature Reviews Urology (2023)
-
Next-generation imaging in localized high-risk prostate cancer
Prostate Cancer and Prostatic Diseases (2021)