Abstract
Background
Comparative effectiveness research (CER) using national registries influences cancer clinical trial design, treatment guidelines, and patient management. However, the extent to which treatment selection bias (TSB) affects overall survival (OS) in cancer CER remains poorly defined. We sought to quantify the TSB effect on OS in the setting of low-risk prostate cancer, where 10-year prostate cancer-specific survival (PCSS) approaches 100% regardless of treatment modality.
Methods
The Surveillance, Epidemiology, and End Results database was queried for patients with low-risk prostate cancer (cT1-T2a, PSA < 10, and Gleason 6) who received radical prostatectomy (RP), brachytherapy (BT), or external beam radiotherapy (EBRT) from 2005 to 2015. The TSB effect was defined as the unadjusted 10-year OS difference between modalities that was not due to differences in PCSS. Propensity score matching was used to estimate the TSB effect on OS due to measured confounders (variables present in the database and associated with OS) and unmeasured confounders.
Results
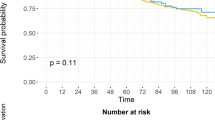
A total of 50,804 patients were included (8845 RP; 18,252 BT; 23,707 EBRT) with a median follow-up of 7.4 years. The 10-year PCSS for the entire cohort was 99%. The 10-year OS was 92.9% for RP, 83.6% for BT, and 76.9% for EBRT (p < 0.001). OS differences persisted after propensity score matching of RP vs. EBRT (7.4%), RP vs. BT (4.6%), and BT vs. EBRT (3.7%) (all p < 0.001). The TSB effect on 10-year OS was estimated to be 15.0% for RP vs. EBRT (8.6% measured, 6.4% unmeasured), 8.5% for RP vs. BT (4.8% measured, 3.7% unmeasured), and 6.5% for BT vs. EBRT (3.1% measured, 3.4% unmeasured).
Conclusions
Patients with low-risk prostate cancer selected for RP exhibited large OS differences despite similar PCSS compared to radiotherapy, suggesting OS differences are almost entirely driven by TSB. The quantities of these effects are important to consider when interpreting prostate cancer CER using national registries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jairam V, Park HS. Strengths and limitations of large databases in lung cancer radiation oncology research. Transl Lung Cancer Res. 2019;8(Suppl 2):S172–83.
Kunz R, Vist G, Oxman AD. Randomisation to protect against selection bias in healthcare trials. Cochrane Database Syst Rev. 2007;MR000012.
Pearlstein KA, Basak R, Chen RC. Comparative effectiveness of prostate cancer treatment options: limitations of retrospective analysis of cancer registry data. Int J Radiat Oncol, Biol, Phys. 2019;103:1053–7.
Soni PD, Hartman HE, Dess RT, Abugharib A, Allen SG, Feng FY, et al. Comparison of population-based observational studies with randomized trials in oncology. J Clin Oncol. 2019;37:1209–16.
Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–66.
Chen RC. Challenges of interpreting registry data in prostate cancer: interpreting retrospective results along with or in absence of clinical trial data. J Clin Oncol. 2018;36:1181–3.
National Comprehensive Cancer Network. Prostate cancer (Version 2.2020). National Comprehensive Cancer Network. 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24.
Scosyrev E, Messing J, Noyes K, Veazie P, Messing E. Surveillance Epidemiology and End Results (SEER) program and population-based research in urologic oncology: an overview. Urologic Oncol. 2012;30:126–32.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424.
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107.
Abdollah F, Schmitges J, Sun M, Jeldres C, Tian Z, Briganti A, et al. Comparison of mortality outcomes after radical prostatectomy versus radiotherapy in patients with localized prostate cancer: a population-based analysis. Int J Urol. 2012;19:836–44.
Sun M, Sammon JD, Becker A, Roghmann F, Tian Z, Kim SP, et al. Radical prostatectomy vs radiotherapy vs observation among older patients with clinically localized prostate cancer: a comparative effectiveness evaluation. BJU Int. 2014;113:200–8.
Marsh S, Walters RW, Silberstein PT. Survival outcomes of radical prostatectomy versus radiotherapy in intermediate-risk prostate cancer: a NCDB study. Clin Genitourin Cancer. 2017;S1558-7673(17)30239-2.
Berg S, Cole AP, Krimphove MJ, Nabi J, Marchese M, Lipsitz SR, et al. Comparative effectiveness of radical prostatectomy versus external beam radiation therapy plus brachytherapy in patients with high-risk localized prostate cancer. Eur Urol. 2019;75:552–5.
Merglen A, Schmidlin F, Fioretta G, Verkooijen HM, Rapiti E, Zanetti R, et al. Short- and long-term mortality with localized prostate cancer. Arch Intern Med. 2007;167:1944–50.
Degroot JM, Brundage MD, Lam M, Rohland SL, Heaton J, Mackillop WJ, et al. Prostate cancer-specific survival differences in patients treated by radical prostatectomy versus curative radiotherapy. Can Urol Assoc J. 2013;7:E299–305.
Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Investigators ftCotPSURE.Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116:5226–34.
Hoffman RM, Koyama T, Fan KH, Albertsen PC, Barry MJ, Goodman M, et al. Mortality after radical prostatectomy or external beam radiotherapy for localized prostate cancer. J Natl Cancer Inst. 2013;105:711–8.
Ladjevardi S, Sandblom G, Berglund A, Varenhorst E. Tumour grade, treatment, and relative survival in a population-based cohort of men with potentially curable prostate cancer. Eur Urol. 2010;57:631–40.
Rice KR, Colombo ML, Wingate J, Chen Y, Cullen J, McLeod DG, et al. Low risk prostate cancer in men >/= 70 years old: to treat or not to treat. Urol Oncol. 2013;31:755–60.
Sooriakumaran P, Nyberg T, Akre O, Haendler L, Heus I, Olsson M, et al. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: observational study of mortality outcomes. BMJ. 2014;348:g1502.
Wallis CJD, Saskin R, Choo R, Herschorn S, Kodama RT, Satkunasivam R, et al. Surgery versus radiotherapy for clinically-localized prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:21–30.
Zaid HB, Karnes RJ. A house divided: the irradiation versus prostatectomy debate continues. Int J Radiat Oncol, Biol, Phys. 2017;99:512–4.
Weiner AB, Matulewicz RS, Schaeffer EM, Liauw SL, Feinglass JM, Eggener SE. Contemporary management of men with high-risk localized prostate cancer in the United States. Prostate Cancer Prostatic Dis. 2017;20:283–8.
Williams SB, Huo J, Chamie K, Smaldone MC, Kosarek CD, Fang JE, et al. Discerning the survival advantage among patients with prostate cancer who undergo radical prostatectomy or radiotherapy: the limitations of cancer registry data. Cancer. 2017;123:1617–24.
O’Donnell H, Parker C. What is low-risk prostate cancer and what is its natural history? World J Urol. 2008;26:415–22.
Collaborative Stage Data Collection System, published online: https://seer.cancer.gov/tools/collabstaging/. 2020.
Zaorsky NG, Zhang Y, Tuanquin L, Bluethmann SM, Park HS, Chinchilli VM. Suicide among cancer patients. Nat Commun. 2019;10:207.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HSP reports honoraria from RadOnc Questions, LLC. JBY reports personal fees from Boston Scientific and Galera Pharmaceuticals. PLN reports honoraria from Janssen, research grants from Janssen, Astellas, Bayer, consultation for Astellas, COTA, and Boston Scientific, and advisory board for Astellas. MTK has received research grants from Bayer and Palette Life Sciences. All of these conflicts of interest are outside of the submitted work and no other conflicts of interest were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Miccio, J.A., Talcott, W.J., Jairam, V. et al. Quantifying treatment selection bias effect on survival in comparative effectiveness research: findings from low-risk prostate cancer patients. Prostate Cancer Prostatic Dis 24, 414–422 (2021). https://doi.org/10.1038/s41391-020-00291-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-00291-3
This article is cited by
-
Uncovering interpretable potential confounders in electronic medical records
Nature Communications (2022)
-
Current issues in medical epistemology and statistics: a view from the frontline of medicine
Synthese (2022)