Abstract
Background
This study supports a value-based approach to prostate cancer (PCa) treatment by systematically reviewing economic evaluations that compare the cost and cost-effectiveness of low-dose-rate brachytherapy (LDR-BT) with that of other treatment options for localised and locally advanced PCa.
Methods
Studies published between 2008 and 2023 were searched for in MEDLINE, EMBASE and Tufts Medical Center’s Cost-Effectiveness Analysis (CEA) Registry (Prospero protocol CRD42023-442027). Two reviewers independently screened the title and abstracts based on agreed inclusion and exclusion criteria, followed by full-text screening. The Drummond checklist was used to critically appraise the quality of the included studies.
Results
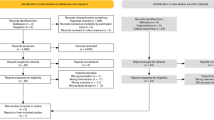
After screening 453 records, 36 were sought for retrieval and 14 eligible studies included. Of them, 11 compared treatments for low- and/or favourable intermediate-risk PCa, 2 compared options for unfavourable intermediate- and/or high-risk disease and 1 analysed treatments for both risk groups. Considerable heterogeneity was seen in the populations, perspectives, time horizons, costs and outcomes data used. If the oncological outcomes of standard treatment approaches are considered equivalent, LDR-BT was the most cost-effective type of radiation therapy (RT) in 9 (75%) of 12 studies, was more cost-effective than radical prostatectomy (RP) in 6 (67%) of 9 studies and, depending on the time horizon, was more cost-effective than active surveillance (AS) in 3 (60%) of 5 studies. LDR-BT was more cost-effective than high-dose-rate brachytherapy (HDR-BT) in all 4 (100%) of the studies that made this comparison and, overall, LDR-BT was the least costly of all active treatment options in 7 (50%) of the 14 studies.
Conclusion
The available health economic evidence suggests that LDR-BT has significant cost advantages and an important role to play in the delivery of value-based PCa care. In the future these advantages could be challenged if radiotherapy favours ultrahypofractionated strategies such as stereotactic body radiation therapy (SBRT) and reduced fractionation in HDR-BT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomatarum I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Sharma R. The burden of prostate cancer is associated with human development index: evidence from 87 countries, 1990-2016. EPMA J. 2019;10:137–52.
Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9:465–72.
Eastham J, Auffenberg G, Barocas D, Chou R, Crispino T, Davis J, et al. Clinically localized prostate cancer: AUA/ASTRO guideline, part I: introduction, risk assessment, staging, and risk-based management. J Urol. 2022;208:10–18.
Mottet N, Cornford P, van den Berg R, Briers E, De Santis M, Gillessen S, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62.
National Institute for Health and Care Excellence (2022). Prostate cancer: diagnosis and management (NG131). Last updated 15 December 2021.
King M, Keyes M, Frank S, Crook J, Butler W, Rossi P, et al. Low dose rate brachytherapy for primary treatment of localized prostate cancer: asystemic review and executive summary of an evidence-based consensus statement. Brachytherapy. 2021;20:1114–29.
Hamdy F, Donovan J, Lane J, Metcalfe C, David M, Turner E, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl J Med. 2023;388:1547–58.
Donovan J, Hamdy F, Athene Lane J, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl J Med. 2016;375:1425–37.
Skowronek J. Current status of brachytherapy in cancer treatment—a short overview. J Contemp Brachytherapy. 2017;9:581–9.
Ragde H, Grado G, Nadir B, Elgamal AA. Modern prostate brachytherapy. CA Cancer J Clin. 2000;50:380–93.
Schlussel Markovic E, Buckstein M, Stone N, Stock R. Outcomes and toxicities in patients with intermediate risk prostate cancer treated with brachytherapy alone or brachytherapy and supplemental external beam radiation therapy. BJU Int. 2018;121:774–80.
Chargari C, Deutsch E, Blanchard P, Gouy S, Martelli H, Guérin F, et al. Brachytherapy: an overview for clinicians. CA Cancer J Clin. 2019;69:386–401.
Oh J, Tyldesley S, Pai H, McKenzie M, Halperin R, Duncan G, et al. An updated analysis of the survival endpoints of ASCENDE-RT. Int J Radiat Oncol Biol Phys 2023;115:1061–70.
Vu C, Jawad M, Krauss D. The cost-effectiveness and value proposition of brachytherapy. Semin Radiat Oncol. 2020;30:87–93.
Williams V, Kahn J, Thaker N, Beriwal S, Nguyen P, Arthur D, et al. The case for brachytherapy: why it deserves a renaissance. Adv Radiat Oncol. 2021;6:100605.
Teisberg E, Wallace S, O’Hara S. Defining and implementing value-based health care: a strategic framework. Acad Med. 2020;95:682–5.
Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Prospero. Low-dose-rate brachytherapy as a primary treatment for localised and locally advanced prostate cancer: a systematic review of economic evaluations. National Institute for Health Research 2023. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023442027.
Mathes T, Walgenbach M, Antione S-L, Pieper D, Eikermann M. Methods for systematic reviews of health economic evaluations: a systematic review, comparison, and synthesis of method literature. Med Decis Mak. 2014;34:826–40.
Tufts Medical Center—Center for the Evaluation of Value and Risk in Health. https://cevr.tuftsmedicalcenter.org/databases/cea-registry.
National Institute for Health and Care Excellence (2022). NICE health technology evaluations: the manual (PMG36). Last updated 31 October 2023.
Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ. 1996;313:275–83.
Frederix G. Check your checklist: the dangers of over- and underestimating the quality of economic evaluations. Pharmacoecon Open. 2019;3:433–5.
Kato T, Yokomizo A, Matsumoto R, Tohi Y, Miyakawa J, Mitsuzuka K, et al. Comparison on the medical costs between active surveillance and other treatment for early prostate cancer in Japan: from the data of PRAIS-JAPAN study. Int J Urol. 2022;29:1271–8.
Eldefrawy A, Katkoori D, Abramowitz M, Soloway M, Manoharan M. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol. 2013;31:576–80.
Hayes J, Ollendorf D, Pearson S, Barry M, Kantoff P, Lee P, et al. Observation versus initial treatment for men with localised, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158:853–60.
Laviana A, Ilg A, Veruttipong D, Tan H-J, Burke M, Niedzwiecki D, et al. Utilising time-driven activity-based costing to understand the short- and long-term costs of treating localised, low-risk prostate cancer. Cancer. 2016;122:447–55.
Keegan K, Dall’Era M, Durbin-Johnson B, Evans C. Active surveillance for prostate cancer compared with immediate treatment: an economic analysis. Cancer. 2012;118:3512–8.
Weng X, Zhong L, Xiang P, Li Y, Paciorek A, Dong L, et al. Cost-effectiveness analysis of primary treatments for localised prostate cancer: a population-based Markov analysis using real-world evidence. Eur J Cancer Care. 2022;31:e13740.
Cooperberg M, Ramakrishna N, Duff S, Hughes K, Sadownik S, Smith J, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int. 2013;111:437–50.
Becerra Bachino V, Cots F, Guedea F, Pera J, Boladeras A, Aguiló F, et al. Cost comparison of three treatments for localised prostate cancer in Spain: radical prostatectomy, prostate brachytherapy and external 3D conformal radiotherapy. Gac Sanit. 2011;25:35–43.
Satoh T, Ishiyama H, Matsumoto K, Tabata K-I, Kitano M, Iwamura M, et al. Cost comparison of curative therapies for localised prostate cancer in Japan: a single-institution experience. Jpn J Radio. 2009;27:348–54.
Shah C, Lanni T, Ghilezan M, Gustafson G, Marvin K, Ye H, et al. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy. 2012;11:441–5.
Ilg A, Laviana A, Kamrava M, Veruttipong D, Steinberg M, Par S-J, et al. Time-driven activity-based costing of low-dose-rate and high-dose-rate brachytherapy for low-risk prostate cancer. Brachytherapy. 2016;15:760–7.
Helou J, Torres S, Musunuru H, Raphael J, Cheung P, Vesprini D, et al. Stereotactic body radiotherapy versus low dose rate brachytherapy for localised prostate cancer: a cost-utility analysis. Clin Oncol (R Coll Radio). 2017;29:718–31.
Kowalchuk R, Kim H, Harmsen W, Jeans E, Morris L, Mullikin T, et al. Cost effectiveness of treatment strategies for high risk prostate cancer. Cancer. 2022;128:3815–23.
Alyamani N, Song J, van Katwyk S, Thavorn K, Renaud J, Haddad A, et al. Cost-utility analysis of radiation treatment modalities for intermediate-risk prostate cancer. Curr Oncol. 2021;28:2385–98.
Filson C. Quality of care and economic considerations of active surveillance of men with prostate cancer. Transl Androl Urol. 2018;7:203–13.
Noble S, Garfield K, Athene Lane J, Metcalfe C, Davis M, Walsh E, et al. The ProtecT randomised trial cost-effectiveness analysis comparing active monitoring, surgery, or radiotherapy for prostate cancer. Br J Cancer. 2020;123:1063–70.
Degeling K, Corcoran N, Pereira-Salgado A, Hamid A, Siva S, IJzerman M. Lifetime health and economic outcomes of active surveillance, radical prostatectomy, and radiotherapy for favourable-risk localised prostate cancer. Value Health. 2021;24:1737–45.
Grummet J, Gorin M, Popert R, O’Brien T, Lamb A, Hadaschik B, et al. “TREXIT 2020”: why the time to abandon transrectal prostate biopsy starts now. Prostate Cancer Prostatic Dis 2020;23:62–65.
Cheung D, Finelli A. Magnetic resonance imaging diagnosis of prostate cancer: promise and caution. CMAJ. 2019;191:E1177–E1178.
Richstone L, Bianco F, Shah H, Kattan M, Eastham J, Scardino P, et al. Radical prostatectomy in men aged >or=70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101:541–6.
Zhang P, Qian B, Shi J, Xiao Y. Radical prostatectomy versus brachytherapy for clinically localized prostate cancer on oncological and functional outcomes: a meta-analysis. Transl Androl Urol 2020;9:332–43.
Crew B. A closer look at a revered robot. Nat Suppl. 2020;580:S5–S7.
Correa R, Loblaw A. Stereotactic body radiotherapy: hitting harder, faster, and smarter in high-risk prostate cancer. Front Oncol. 2022;12:889132.
Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–95.
Yu T, Zhang Q, Zheng T, Shi H, Liu Y, Feng S, et al. The effectiveness of intensity modulated radiation therapy versus three-dimensional radiation therapy in prostate cancer: a meta-analysis of the literatures. PLoS One. 2016;11:e0154499.
Martinez A, Demanes J, Vargas C, Schour L, Ghilezan M, Gustafson G. High-dose-rate prostate brachytherapy: an excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol. 2010;33:481–8.
Skowronek J. Low-dose-rate or high-dose-rate brachytherapy in treatment of prostate cancer—between options. J Contemp Brachytherapy. 2013;5:33–41.
Schaeffer E, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. Prostate cancer, version 4.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2023;21:1067–96.
Moideen N, Crook J, Araujo C, Batchelar D, Canovas F, Halperin R, et al. A randomised phase III trial comparing health-related quality of life after low dose rate (LDR) or high dose rate (HDR) prostate brachytherapy boost combined with external beam pelvic radiotherapy (EBRT). Int J Radiat Oncol Biol Phys. 2022;174:S4.
Acknowledgements
We thank Beata Coffey (BC), Information Specialist at the Royal Society of Medicine, for her assistance in developing a search strategy and performing the literature search. We also thank Alka Singh (AS), Associate Consultant at Mtech Access, and Jodie Worrall (JW), Associate Director at Mtech Access, who in their capacity as second reviewers independently screened reports, assessed them for eligibility and helped select reports for inclusion by consensus with the authors.
Funding
The study was funded by Becton, Dickinson and Company but they took no part in the design of the study, the collection and analysis of data, the preparation of the manuscript or the decision to publish.
Author information
Authors and Affiliations
Contributions
Benedict Stanberry (BS) jointly conceived the design of the study with Nikki Webber-Jones (NWJ). BS then developed and submitted the protocol. Together with BC he jointly developed the search strategy and BC performed the literature search. BS, AS and JW independently screened reports, assessed them for eligibility and selected reports for inclusion by consensus. BS wrote the manuscript and NWJ critically reviewed it.
Corresponding author
Ethics declarations
Competing interests
BS declares that he has received compensation from Becton, Dickinson and Company for participating in conferences and workshops. NWJ works for Becton, Dickinson and Company.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stanberry, B., Webber-Jones, N. Low-dose-rate brachytherapy as a primary treatment for localised and locally advanced prostate cancer: a systematic review of economic evaluations. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00817-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00817-z