Abstract
Background
To understand the value of multiparametric magnetic resonance imaging (mpMRI) and targeted biopsies at recruitment on active surveillance (AS) outcomes.
Materials and methods
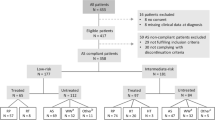
This retrospective single-center study enrolled two cohorts of 206 and 310 patients in AS. The latter group was submitted to mpMRI and targeted biopsies at recruitment. Kaplan–meier curves quantified progression-free survival (PFS) and Bioptic-PFS (B-PFS: no upgrading or >3 positive cores) in the two cohorts. Cox-regression analyses tested independent predictors of PFS and B-PFS. In patients submitted to radical prostatectomy (RP) after AS, significant cancer (csPCa) was defined as: GS ≥ 4 + 3 and/or pT ≥ 3a and/or pN+ . Logistic-regression analyses predicted csPCa at RP.
Results and limitations
Median time follow-up and median time of persistence in AS were 46 (24–70) and 36 (23–58) months, respectively. Patients submitted to mpMRI at AS begin, showed greater PFS at 1- (98% vs. 91%), 3- (80% vs. 57%), and 5-years (70% vs. 35%) follow-up, respectively (all p < 0.01). At Cox-regression analysis only confirmatory mpMRI± targeted biopsy (HR: 0.3; 95% CI 0.2–0.5; p < 0.01) at AS begin was an independent predictor of PFS. Globally, 50 (16%) vs. 128 (62%) and 26 (8.5%) vs. 64 (31%) [all p < 0.01] men in the two groups experienced any-cause and bioptic AS discontinuation, respectively. Patients submitted to confirmatory mpMRI experienced greater 1-(98% vs. 93%), 3-(90% vs. 75%), and 5-years (83% vs. 56%) B-PFS, respectively (all p < 0.01). At Cox-regression analysis, mpMRI±-targeted biopsy at AS begin was associated with B-PFS (HR: 0.3; 95% CI 0.2–0.6; p < 0.01). No differences were recorded in csPCa rates between the two groups (22% vs. 28%; p = 0.47). Limitations of the study are the single-center retrospective nature and the absence of long-term follow-up.
Conclusions
Confirmatory mpMRI±-targeted biopsies are associated with higher PFS and B-PFS during AS. However, a non-negligible percentage of patients experience csPCa after switching to active treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
13 December 2019
A Correction to this paper has been published: https://doi.org/10.1038/s41391-019-0198-2
References
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7.
Bokhorst LP, Valdagni R, Rannikko A, Kakehi Y, Pickles T, Bangma CH, et al. A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol. 2016;70:954–60.
Conti SL, Dall’Era M, Fradet V, Cowan JE, Simko J, Carroll PR. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628–33.
Lee MC, Dong F, Stephenson AJ, Jones JS, Magi-Galluzzi C, Klein EA. The Epstein criteria predict for organ-confined but not insignificant disease and a high likelihood of cure at radical prostatectomy. Int Braz J Urol. 2011;37:123.
Adamy A, Yee DS, Matsushita K, Maschino A, Cronin A, Vickers A, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011;185:477–82.
Wong LM, Alibhai SMH, Trottier G, Timilshina N, Van Der Kwast T, Zlotta A, et al. A negative confirmatory biopsy among men on active surveillance for prostate cancer does not protect them from histologic grade progression. Eur Urol. 2014;66:406–13.
Schoots IG, Nieboer D, Giganti F, Moore CM, Bangma CH, Roobol MJ. Is magnetic resonance imaging-targeted biopsy a useful addition to systematic confirmatory biopsy in men on active surveillance for low-risk prostate cancer? A systematic review and meta-analysis. BJU Int. 2018;122:946–58.
Gallagher KM, Christopher E, Cameron AJ, Little S, Innes A, Davis G, et al. 4-year outcomes from an MP-MRI based active surveillance programme—PSA dynamics and serial MRI scans allow omission of protocol biopsies. BJU Int. 2019;123:429–38.
Bryant RJ, Yang B, Philippou Y, Lam K, Obiakor M, Ayers J, et al. Does the introduction of prostate multiparametric magnetic resonance imaging into the active surveillance protocol for localized prostate cancer improve patient re-classification? BJU Int. 2018;122:794–800.
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radio. 2012;22:746–57.
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40.
Gandaglia G, Ploussard G, Isbarn H, Suardi N, De Visschere PJL, Futterer JJ, et al. What is the optimal definition of misclassification in patients with very low-risk prostate cancer eligible for active surveillance? Results from a multi-institutional series. Urol Oncol Semin Orig Investig. 2015;33:164.e1–e9.
Klotz L, Loblaw A, Sugar L, Moussa M, Berman DM, Van der Kwast T, et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur Urol. 2019;75:300–9.
Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MGM. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–50.
Marzouk K, Assel M, Ehdaie B, Vickers A. Long-term cancer specific anxiety in men undergoing active surveillance of prostate cancer: findings from a large prospective cohort. J Urol. 2018;200:1250–5.
Kaye DR, Qi J, Morgan TM, Linsell S, Lane BR, Montie JE, et al. Association between early confirmatory testing and the adoption of active surveillance for men with favorable-risk prostate cancer. Urology. 2018;118:127–33.
Eineluoto JT, Järvinen P, Kenttämies A, Kilpeläinen TP, Vasarainen H, Sandeman K, et al. Repeat multiparametric MRI in prostate cancer patients on active surveillance. PLoS ONE. 2017;12:e0189272.
Rais-Bahrami S, Türkbey B, Rastinehad AR, Walton-Diaz A, Hoang AN, Minhaj Siddiqui M, et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: recommendations for interval imaging follow-up. Diagn Inter Radio. 2014;20:293–8.
Olivier J, Kasivisvanathan V, Drumez E, Fantoni JC, Leroy X, Puech P, et al. Low-risk prostate cancer selected for active surveillance with negative MRI at entry: can repeat biopsies at 1 year be avoided? A pilot study. World J Urol. 2019;37:253–9.
Alberts AR, Roobol MJ, Drost FJH, van Leenders GJ, Bokhorst LP, Bangma CH, et al. Risk-stratification based on magnetic resonance imaging and prostate-specific antigen density may reduce unnecessary follow-up biopsy procedures in men on active surveillance for low-risk prostate cancer. BJU Int. 2017;120:511–9.
Schoots IG, Osses DF, Drost F-JH, Verbeek JFM, Remmers S, van Leenders GJLH, et al. Reduction of MRI-targeted biopsies in men with low-risk prostate cancer on active surveillance by stratifying to PI-RADS and PSA-density, with different thresholds for significant disease. Transl Androl Urol. 2018;7:132–44.
Bloom JB, Hale G, Gold SA, Rayn K, Smith C, Mehralivand S, et al. Predicting gleason group progression for men on prostate cancer active surveillance: the role of a negative confirmatory MRI-US fusion biopsy. J Urol. 2019;201:84–90.
Kearns JT, Faino AV, Newcomb LF, Brooks JD, Carroll PR, Dash A, et al. Role of surveillance biopsy with no cancer as a prognostic marker for reclassification: results from the canary prostate active surveillance study[formula presented]. Eur Urol. 2018;73:706–12.
Luzzago S, Rannikko A, Laajala TD, Vasarainen H, Musi G, Mirtti T, et al. Cumulative cancer locations is a novel metric for predicting active surveillance outcomes: a multicenter study. Eur Urol Oncol. 2018;1:268–75.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29.
Robertson NL, Hu Y, Ahmed HU, Freeman A, Barratt D, Emberton M. Prostate cancer risk inflation as a consequence of image-targeted biopsy of the prostate: a computer simulation study. Eur Urol. 2014;65:628–34.
Gandaglia G, van den Bergh RCN, Tilki D, Fossati N, Ost P, Surcel CI, et al. How can we expand active surveillance criteria in patients with low- and intermediate-risk prostate cancer without increasing the risk of misclassification? Development of a novel risk calculator. BJU Int. 2018;122:823–30.
Moore CM, Robertson NL, Jichi F, Damola A, Ambler G, Giganti F, et al. The effect of dutasteride on magnetic resonance imaging defined prostate cancer: MAPPED—a randomized, placebo controlled, double-blind clinical trial. J Urol. 2017;197:1006–13.
Wegelin O, van Melick HHE, Hooft L, Bosch JLHR, Reitsma HB, Barentsz JO, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. 2017;71:517–31.
Elkjær MC, Andersen MH, Høyer S, Pedersen BG, Borre M. Prostate cancer: in-bore magnetic resonance guided biopsies at active surveillance inclusion improve selection of patients for active treatment. Acta Radio. 2018;59:619–26.
Park JJ, Park BK. Role of PI-RADSv2 with multiparametric MRI in determining who needs active surveillance or definitive treatment according to PRIAS. J Magn Reson Imaging. 2016;45:1753–9.
Moldovan PC, Van den Broeck T, Sylvester R, Marconi L, Bellmunt J, van den Bergh RCN, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017;72:250–66.
Luzzago S, Petralia G, Musi G, Catellani M, Alessi S, Di Trapani E, et al. Multiparametric magnetic resonance imaging second opinion may reduce the number of unnecessary prostate biopsies: time to improve radiologists’ training program? Clin Genitourin Cancer. 2018;17:88–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luzzago, S., Catellani, M., Di Trapani, E. et al. Confirmatory multiparametric magnetic resonance imaging at recruitment confers prolonged stay in active surveillance and decreases the rate of upgrading at follow-up. Prostate Cancer Prostatic Dis 23, 94–101 (2020). https://doi.org/10.1038/s41391-019-0160-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0160-3
This article is cited by
-
Association between previous negative biopsies and lower rates of progression during active surveillance for prostate cancer
World Journal of Urology (2022)
-
Active surveillance for prostate cancer: comparison between incidental tumors vs. tumors diagnosed at prostate biopsies
World Journal of Urology (2022)